Serotonin signaling mediates protein valuation and aging
Figures
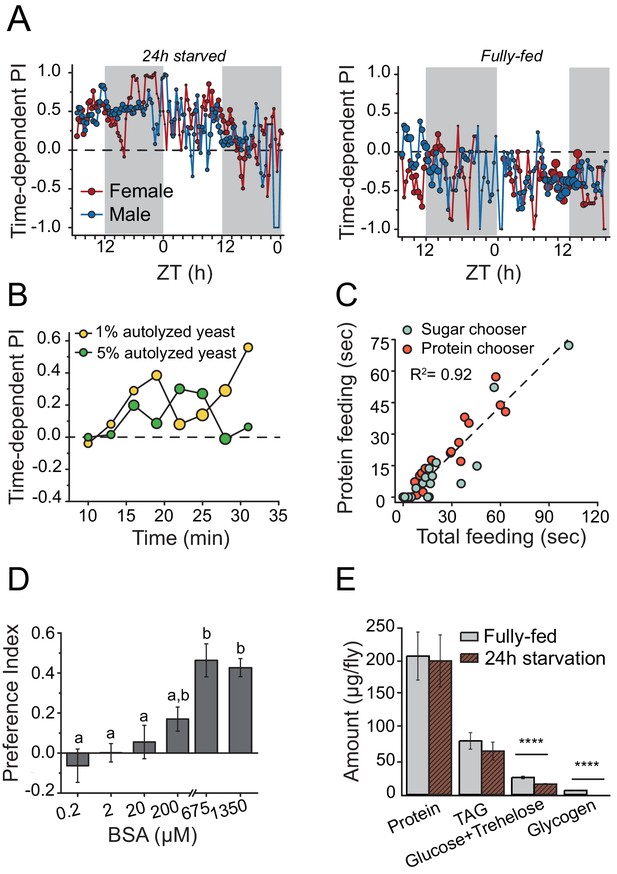
Drosophila demonstrates energy-state dependent protein feeding preference.
(A) Male and female Canton-S flies' real-time feeding preference over 24 hr. The choice was given as 2% autolyzed yeast (w/v) +1% sucrose (w/v) vs. 1% sucrose (w/v). Gray shades on the graphs indicate "light-off" periods. The size of the symbols is proportional to the number of flies that were feeding during the given time period (fully-fed female flies N=17; fully-fed male flies N=21; starved female flies N=10; starved male flies N=11). A Preference Index (PI) = 1 indicates complete preference for the yeast-containing food. (B) Time-dependent PI plot from flies given a choice between 1% sucrose vs. 1% autolyzed yeast or 1% sucrose vs. 5% autolyzed yeast. Similar yeast preference is observed in both experiments, and in the first experiment the diets are isocaloric. (C) Flies that had increased total food consumption during the choice assay were likely to eat more protein meal. From the continuous FLIC data, we identified, for each fly, its first meal choice (sugar or protein chooser), time spent on protein feeding, and total feeding time at the end of a 30 min choice experiment. Flies were given a choice between isocaloric 1% sucrose vs 1% autolyzed yeast. Linear regression analysis revealed that total feeding time positively correlated with the protein feeding time (F(1,32)= 173.7, P<1.8E−14). (D) 24 hr-starved female flies' BSA preference was dose-dependent. Bars indicate the mean and the standard error of the mean (SEM). (N= 8–14 per each concentration treatment. Letters differentiate groups that are significantly different from one another as determined by Tukey's multiple-comparison at α=0.05) (E) Quantification of stored nutrient levels in fully-fed or 24 hr-starved female flies. Flies lost a significant amount of carbohydrate reserves after 24 hr of starvation. (P values determined by two-way ANOVA, followed by Tukey's multiple-comparison test. ***P≤0.0001).
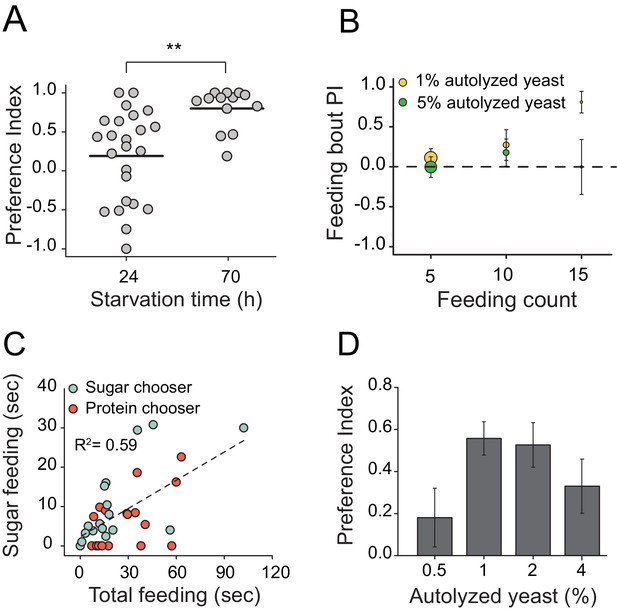
Characteristics of protein feeding behavior in flies.
(A) Protein choice is energy state-dependent. Canton S. females starved for longer periods of time (e.g., 24 hr vs. 70 hr) had stronger protein preference (Student’s t-test, **P≤0.01). (B) Feeding bout PI plot using the same data set shown in Figure 1B. There is no difference in preference when choice is given as 1% sucrose vs. 1% autolyzed yeast or 1% sucrose vs. 5% autolyzed yeast. (C) When given a choice between isocaloric 1% autolyzed yeast vs 1% sucrose choice, 24 hr-starved Canton S. female flies show modest positive correlation between total feeding time during the choice test and time spent consuming the sugar meal (F(1,32)=17.2, P<2.4E-4). Individual flies' first meal choice did not affect relationship between these correlates. (D) Starved female flies preferred wide concentrations of autolyzed yeast. The choice was given to 24 hr starved Canton S. female as 1% sucrose vs. 1% sucrose with different concentrations of autolyzed yeast. Bars indicate mean and standard error of mean (SEM). (N=6 per each concentration treatment; one-way ANOVA).
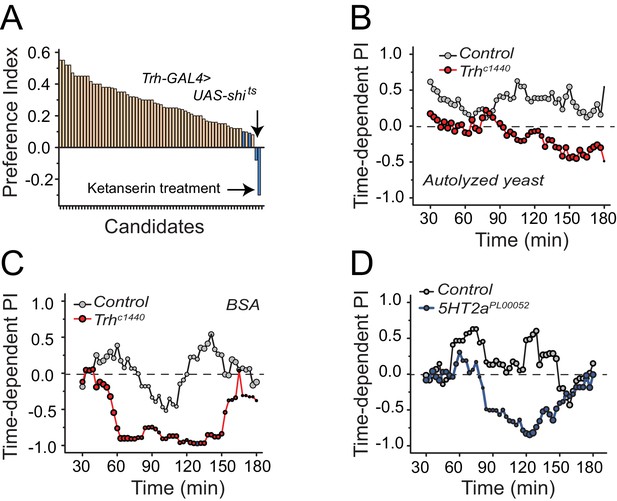
Serotonin signaling through receptor 2a modulates protein preference.
(A) Summary results from a candidate reverse genetic screen. We found that disruption of serotonin signaling consistently abrogates protein preference (See Supplementary file 1 for listed candidates). We used BSA as the protein source. Blue bars indicate manipulations of serotonin signaling that strongly disrupted protein preference. (B-C) Time-dependent PI plot from 24 hr-starved Trh mutant and control flies given a choice between sucrose-only or sucrose plus autolyzed yeast or BSA. When the protein source was autolyzed yeast, the cumulative preference index for Canton S files was 0.43 ± 0.1 (Mean ± SEM) whereas Trh mutant flies was −0.18 ± 0.06 (Student's t-test; P≤0.001). When the protein source was BSA, cumulative PI for Canton S. files was 0 ± 0.1 whereas Trh mutant flies was −0.43 ± 0.2 (Student's t-test; P≤0.05). (D) Time-dependent PI plot from 24 hr-starved flies with 5HT2aPL00052 mutant allele and Canton S., control flies given a choice between sucrose-only or sucrose plus BSA. Cumulative PI for the control was 0.2 ± 0.2 and for the mutant was −0.21 ± 0.2 (Student's t-test).
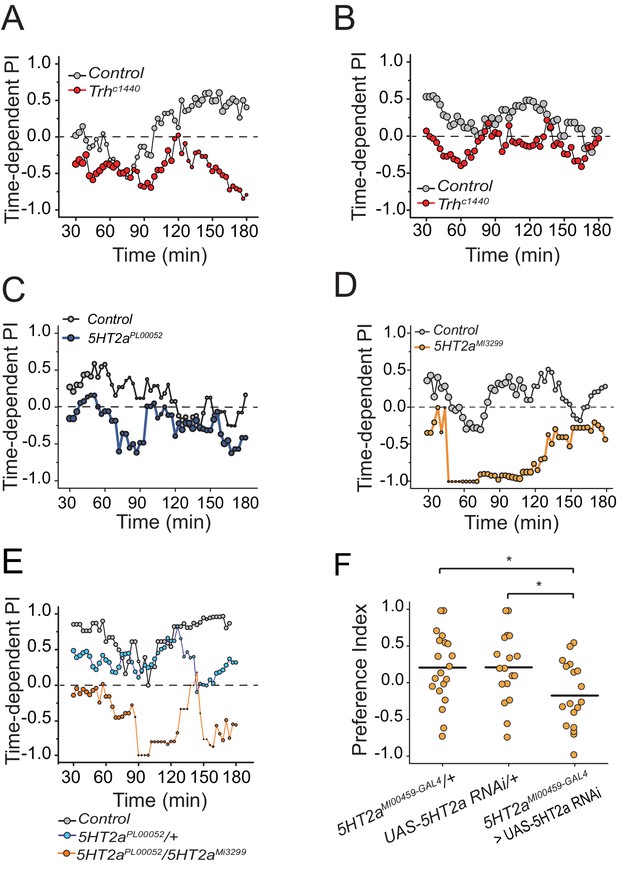
The effect of serotonin manipulations on protein choice behavior is robust and reproducible.
(A-B) Replicate experiments that serve as companions to Figure 2B. Time-dependent PI plot of control and Trh mutant flies on 1% sucrose vs. 1% sucrose + 2% autolyzed yeast. (C) A replicate experiment that serves as a companion to Figure 2D. Time-dependent PI plot of control and 5HT2aPL00052 mutant flies on 1% sucrose vs 1% sucrose + BSA. (D) Time-dependent preference plot of flies carrying another mutant allele of 5HT2a (5HT2aMI3299). The preference was abrogated as seen in the other 5HT2a mutant. The choice was given as 1% sucrose vs. 1% sucrose + BSA. (E) Time-dependent PI plot showing protein preference of starved flies heterozygous for 5HT2aPL00052 and trans-heterozygous for 5HT2aPL00052/ 5HT2aMI3299. The choice was given as 1% sucrose vs 1% sucrose + 2% autolyzed yeast. The protein preference is abrogated in the trans-heterozygote mutants. (F) We genetically knocked-down 5HT2a transcript level in the 5HT2a-positive neurons using 5HT2aMi00459-GAL4> UAS-5HT2a RNAi and tested protein preference of female flies. The choice was given as 2% autolyzed yeast +1% sucrose vs. 1% sucrose. Genotype effect was tested with one-way ANOVA followed by Tukey's multiple comparison (*P≤0.05).
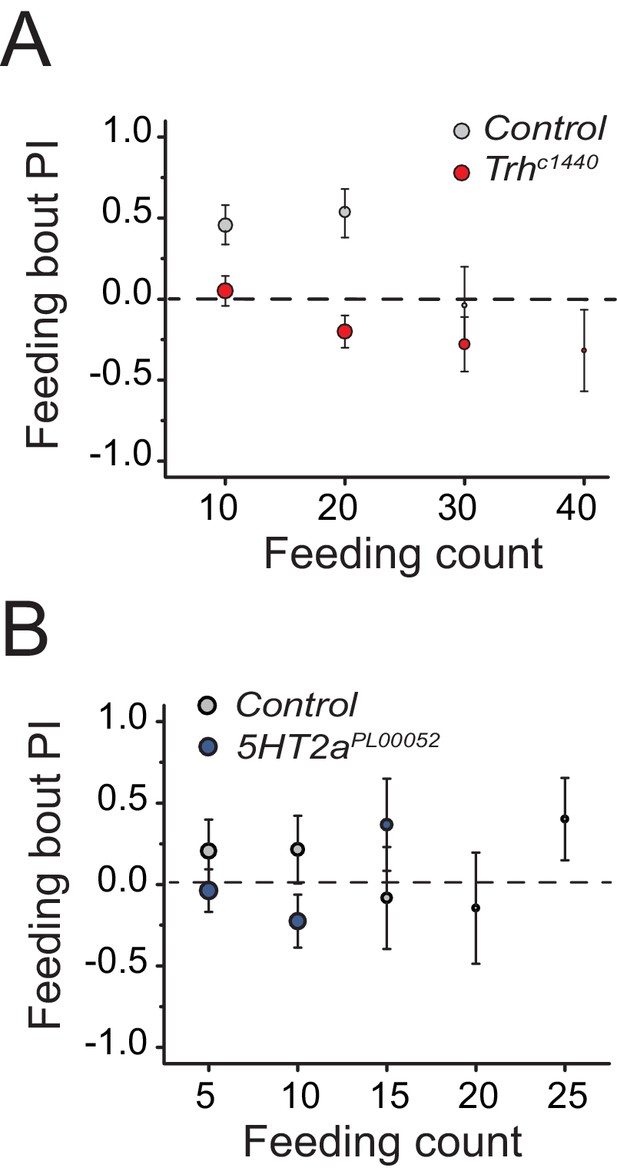
Serotonin signaling influences food early food choice.
We present feeding bout PIs for the same data sets represented in Figure 2B and D into (A) and (B), respectively. Mutation in (A) Trh and (B) 5HT2a suppresses protein preference in early feeding bouts.
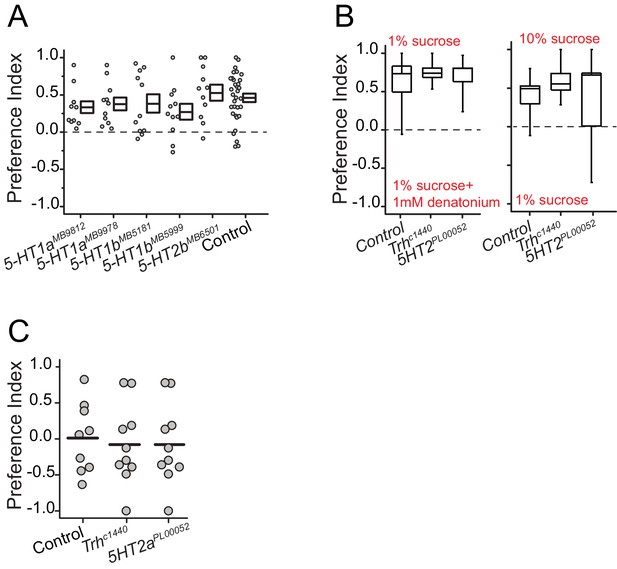
Serotonin receptor 2a is required for protein preference and mutation in Trh or 5HT2a does not affect other taste modality-dependent choice behavior.
(A) Protein preference of three serotonin receptor mutants. We tested 2 mutant alleles for 5HT1a and 5HT1b mutation. We used BSA as a protein source. Individual symbols indicate average PI from three female flies. The choice experiments were conducted using the CAFÉ assay. Boxes denote mean and SEM among biological replicates. Statistical significance for the genotype effect was determined using one-way ANOVA. (B) When given a choice between sweet vs. bitter food, Trh and 5HT2a mutant flies correctly distinguish foods and choose the sweet food like Canton S., control animals. (C) Protein preference of fully-fed Trh and 5HT2a mutant flies is not different than control flies. (N= 9–10 per genotype; one-way ANOVA, P>0.05).
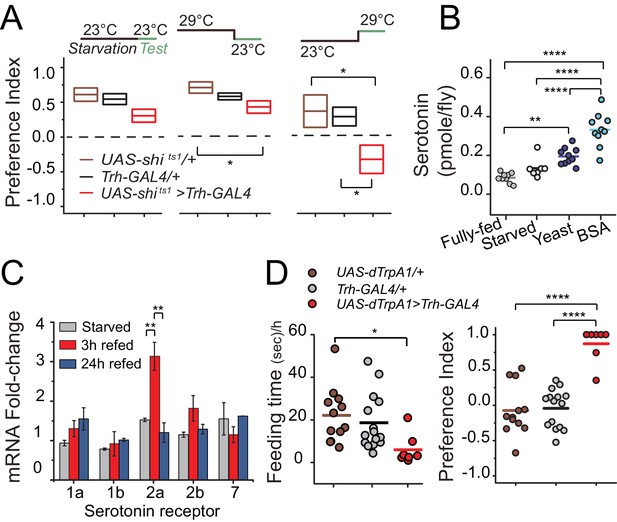
Temporal dynamics of serotonin signaling during protein choice behavior.
(A) Serotonin signaling is required during the choice test to develop protein preference. Flies were placed in either 23°C or 29°C during starvation or choice test as noted in the diagram above each plot. The box plots indicate mean and SEM. Statistical significance for genotype effect within each temperature-shift experiment was determined using one-way ANOVA followed by Fisher’s multiple-comparison, N=8–11/genotype (*P≤0.05). (B) Serotonin abundance in the heads of flies following specified diet treatment. Serotonin significantly increased when animals were allowed to refeed on autolyzed yeast or BSA for three hours after 24 hr starvation. Individual symbols represent measures based on 5 female fly heads, and lines denote the mean. Statistical significance for diet effect was determined using one-way ANOVA followed by Tukey’s multiple-comparison; N=7–10/treatment (**P≤0.01, ****P≤0.0001). (C) Neuronal mRNA abundance of five serotonin receptors. Abundance of 5HT2a transcript acutely increased during 3 hr protein refeeding after starvation. Statistical significance for the treatment effect was determined using one-way ANOVA followed by Tukey’s multiple-comparison (**P≤0.01). (D) Hyper-activation of serotonergic neurons is sufficient to suppress feeding behavior (left) and induce protein preference even in the absence of starvation (right). We observed feeding behaviors in 7 out of 14 flies during the choice test. Individual symbols indicate measures from single flies, and lines denote the mean value among biological replicates. Flies were fed standard 10% sugar/yeast medium prior to testing. Statistical significance for the genotype effect was determined using one-way ANOVA followed by Tukey's multiple-comparison (*P≤0.05, ****P≤0.0001).
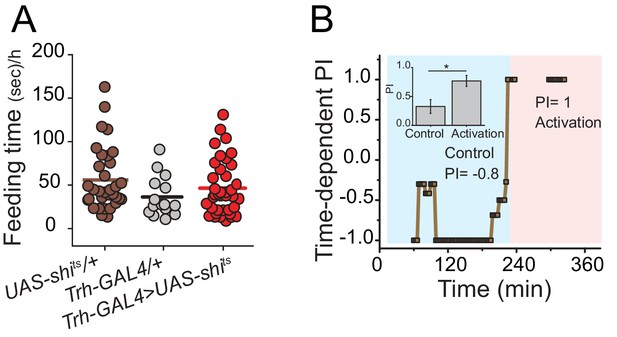
Effect of neuronal inhibition or activation of central serotonergic neurons on feeding behavior.
(A) To determine whether silencing serotonergic neurons disrupts normal feeding activity, we calculated the total time that flies spent interacting with the food during putative feeding bouts during the choice test and found no difference between control and test genotypes. Symbols indicate individual flies, and bars indicate mean among biological replicates (one-way ANOVA). (B) Hyperactivation of serotonergic neurons converts sugar-liking flies to protein-likers. A representative time-dependent PI plot of a single fully-fed Trh-GAL4>UAS-dTrpA1 female fly is shown. The choice was given as 2% autolyzed yeast +1% sucrose vs. 1% sucrose. The choice behavior was recorded for approximately 4 hr at 23°C then temperature was shift to 29°C (blue and red shades indicate respective temperatures). Mean PI and SEM of 13 individual flies' protein preference before and after serotonin activation is represented in the inset (Student's t-test, *P≤0.05).
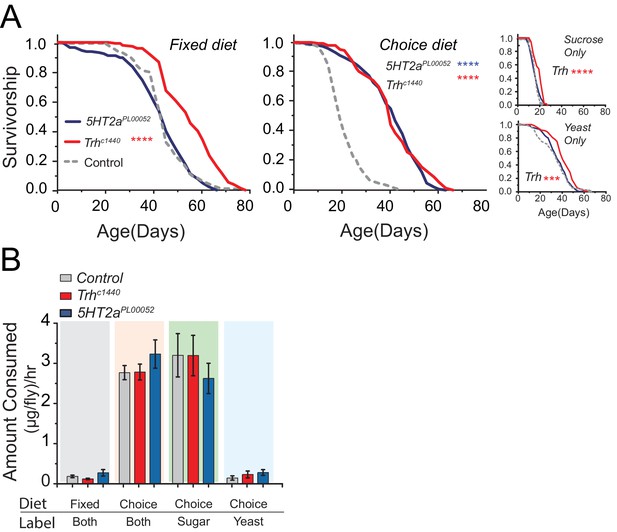
Serotonin modulates lifespan.
(A) Trh mutants live significantly longer than control flies when aged on the fixed diet (log-rank test), as well as on the choice diet (long-rank test). 5HT2a mutants live significantly longer than control flies on the choice diet (log-rank test). Trh mutants also live significantly longer in the sucrose-only and yeast-only diets. (***P≤0.001, ****P≤0.0001) (B) The amounts of total food (both fixed and choice diets) and individual nutrients (choice diet only) consumed by Trh and 5HT2a mutant flies were statistically indistinguishable from control flies regardless of the diet environment (two-way ANOVA, all P>0.05).
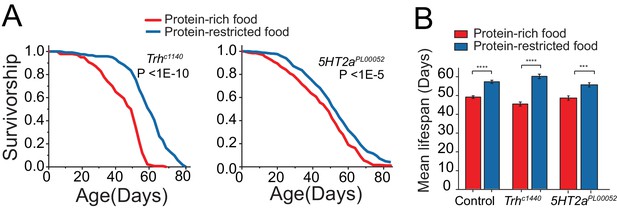
Lifespan analysis of Trh and 5HT2a mutants in the conventional protein restriction diet.
(A) Trh and 5HT2a mutants showed a modest, but statistically significant, life extension under protein restriction. (B) For mean lifespan, statistical significance was determined by one-way ANOVA followed by Tukey’s multiple-comparison(***P≤0.001). For diet-dependent survival, significance was determined using log-rank test. Based on cox-regression analysis, we found significant interaction between genotype and diet effect for Trh mutant (P<0.01) and 5HT2a mutant (P<0.05).
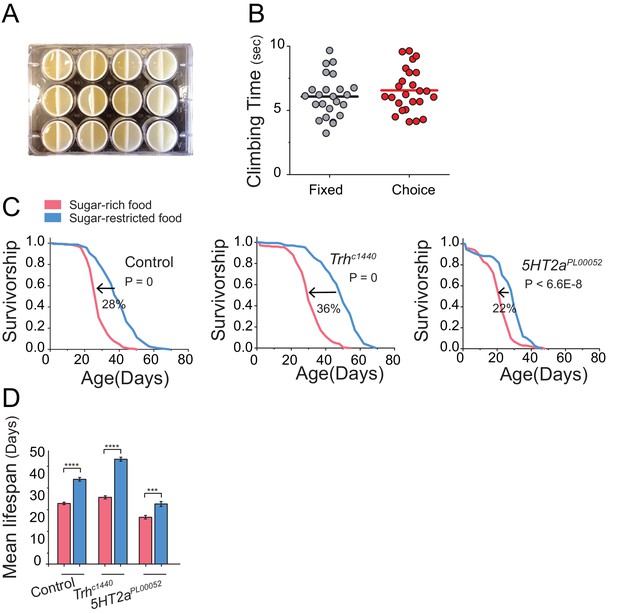
Health and Lifespan of Trh and 5HT2a mutants in various diet conditions.
(A) We printed plastic vial dividers using a Form 1+ 3-D printer to retrofit standard 12-well cell culture plates. These were then placed underneath acrylic chambers to create conditions in which aging flies were able to choose from two different feeding wells throughout their lifespan. These wells were then filled with identical (control) or nutrient-specific diets. (B) Analysis of total time taken to reach the top of the chamber in the negative geotaxis assay of Canton S. female flies kept on fixed or choice diets (Mann-Whitney U-test, P>0.05). (C) The high sucrose diet significantly reduces lifespan of control (w1118) flies and flies with mutation in Trh and 5HT2a (log-rank test). (D) Mean lifespan of flies exposed to either sugar-rich or -restricted diets. For mean lifespan, statistical significance was determined by one-way ANOVA followed by Tukey’s multiple-comparison (***P≤0.001, ****P≤0.0001). Based on cox-regression analysis, we found significant interaction between genotype and diet effect for Trh mutant (P=1.1E-1) and 5HT2a mutant (P=3.1E-2).
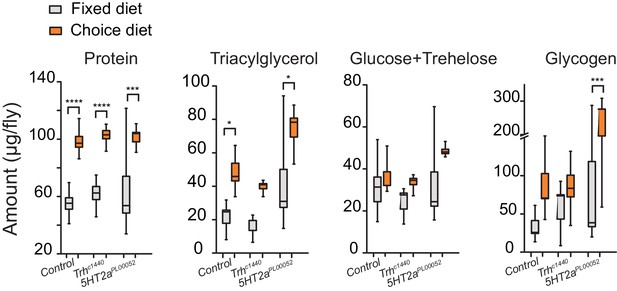
Stored nutrient levels in flies kept in fixed or choice diets.
Box indicates median and 1 SEM with whiskers showing 10–90% quantile. Significance of the diet effect within the genotype per metabolite was determined by Tukey's multiple-comparison after two-way ANOVA (*P≤0.05, ***P≤0.001, ****P≤0.0001).
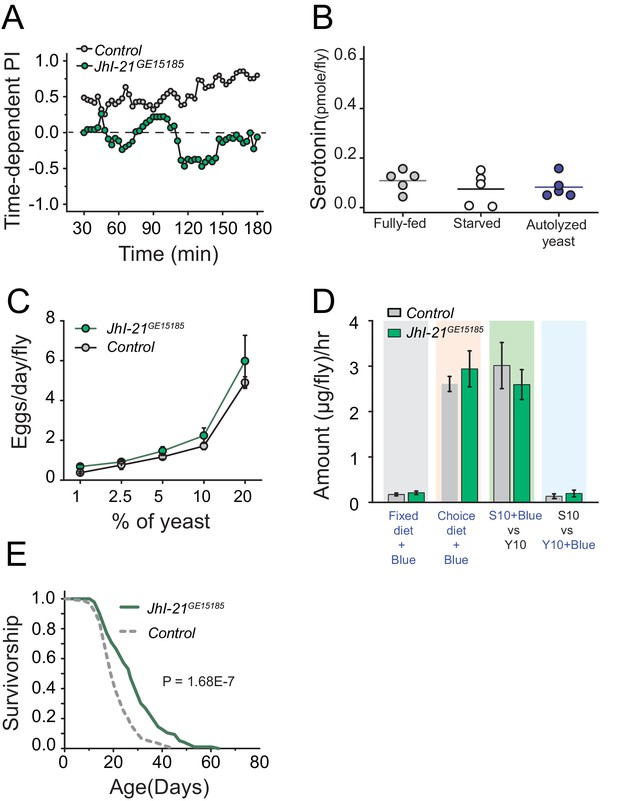
JhI-21 functions upstream of serotonin to modulate protein preference.
(A) Time-dependent protein preference of flies with mutation in one of the Drosophila SLC7A5 proteins, JhI-21. Mutation in JhI-21, abolished protein preference. The choice was given as 1% sucrose vs 1% sucrose+ 2% autolyzed yeast. Cumulative preference index for Canton S files was 0.6 ± 0.08 whereas JhI-21 mutant flies was −0.02 ± 0.1 (Student's t-test; P≤0.001). (B) Serotonin abundance in the heads of JhI-21 mutant flies after specified diet treatments. There was no change in serotonin abundance following 24 hr starvation or 3 hr of autolyzed yeast refeeding after starvation. Individual symbols represent measures based on 10 female fly heads, and lines denote the mean (one-way ANOVA; N=5 biological replicates/treatment). (C) JhI-21 mutant flies increase reproductive output normally as concentration of dietary protein increases. (D) JhI-21 mutant flies consume the same amount of sucrose and yeast as control flies regardless of the diet environment (two-way ANOVA). (E) Survivorship of JhI-21 mutants aged on the choice diet. Mutants live significantly longer in these conditions compared with the control flies (log-rank test).
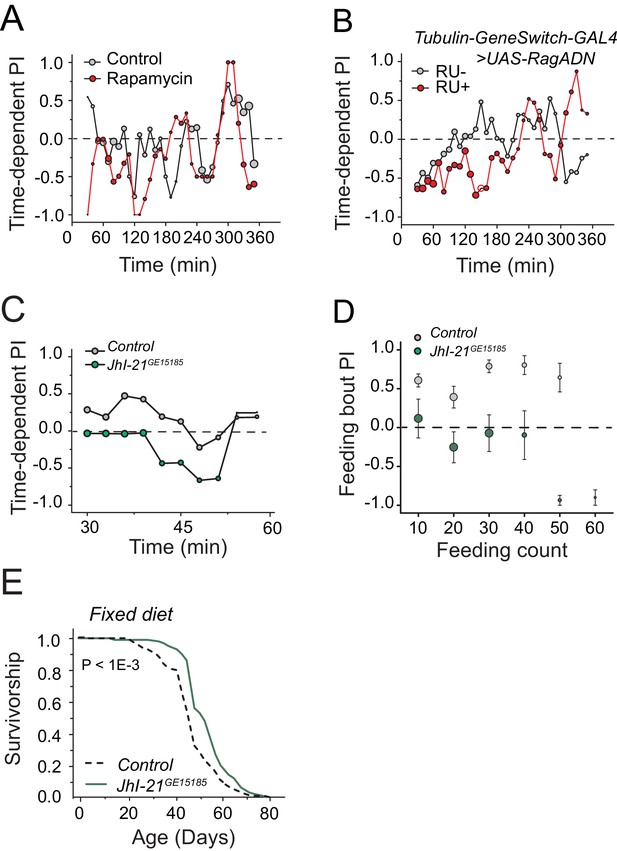
A role of TOR signaling and JhI-21 in protein preference and diet-dependent lifespan.
We tested the effect of down-regulation of TOR signaling on protein preference in fully-fed flies in the FLIC assay by (A) treating flies with Rapamycin, or (B) overexpressing a dominant negative form of RagA. We used autolyzed yeast as the protein source in these experiments. Down-regulation of TOR signaling using either manipulation was not sufficient to mimic starvation and induce protein preference in fully-fed animals. A replicate experiment to Figure 6A. Time-dependent PI plot of control and JhI-21 mutants in 1% sucrose vs 1% sucrose + 2% autolyzed yeast. (D) Feeding bout PI plot using the same data set shown in Figure 6D. JhI-21 mutants do not show any preference to the protein food throughout of their feeding bouts. (E) JhI-21 mutants live significantly (13% ) longer than control flies when aged on a fixed 10% sugar and yeast (w/v) diet (log-rank test).
Additional files
-
Supplementary file 1
A list of candidates used in the reverse genetic screen of protein preference.
The list is showing the genotype or treatment of candidates in descending orders of average preference index (PI). The summary of PI and candidate is graphically depicted in Figure 2A.
- https://doi.org/10.7554/eLife.16843.017
-
Supplementary file 2
Summary statistics of samples represented in Time-dependent preference index (PI) plots.
The list is showing genotype, cumulative PI, standard error of mean (SEM), and sample size (N) of the flies used in figures as noted.
- https://doi.org/10.7554/eLife.16843.018





