New learning while consolidating memory during sleep is actively blocked by a protein synthesis dependent process
Figures
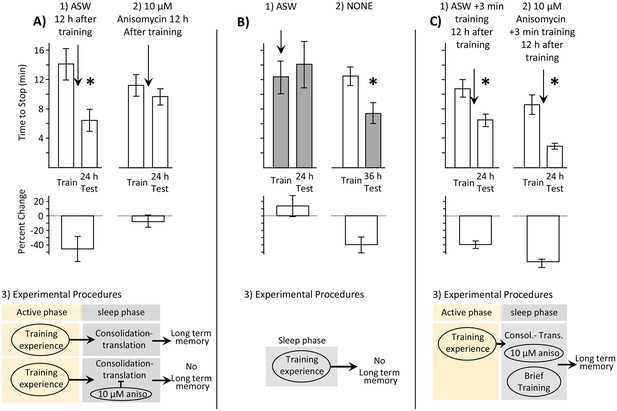
Memory is consolidated, but training is ineffective, during the sleep phase.
In this and in subsequent figures, values from measurements obtained during the inactive and active phases are respectively shaded and unshaded. In this and subsequent figures, upper bars show the time to stop responding. Lower bars show the percent change in the response (-((Train-Test)/Train)*100), which is a measure of memory. In all figures standard errors are shown. Experiments in this figure were performed on the diurnally active A. californica. (A) Blocking protein synthesis during the sleep phase blocks 24 hr memory. Animals were trained during their active phase (day), and were tested 24 hr later. 12 hr after training, during the sleep phase, animals were injected with ASW (N = 9) or with 10 µM anisomycin (N = 12). (1) There is a significant decrease in the time to stop responding to the food during the 24 test (p=0.02, t = 2.76, df = 8; two tailed paired t-test), indicating memory. (2) There was no significant difference between time to stop responding during the original training, and the test 24 hr later (p=0.21, t = 1.34, df = 11; two tailed paired t-test), indicating that block of protein synthesis in the sleep phase following training blocks memory consolidation. (3) In addition to consolidation that follows training (not shown), a second phase of consolidation occurs during sleep. This phase is blocked by the translation blocker anisomycin, preventing the expression of long-term memory. (B) Training during the sleep phase is ineffective in causing long-term memory. (1) Training animals during the sleep phase (N = 5) did not lead to long-term memory, as shown by a lack of savings when animals were tested 24 hr later (p=0.3, t = 1.12, df = 4; two-tailed paired t-test). In this experiment, animals were treated with ASW just before the training. (2) Memory after training during the active phase is expressed during the sleep phase (N = 11), as shown by a significant reduction in the time to stop 36 hr after training, when animals are tested during the inactive phase (p=0.013, t = 3.03, df = 10; two-tailed paired t-test with Bonferroni correction). (3) A diagram showing that training during the sleep phase is ineffective in producing memory. (C) Effect of a brief recall during the sleep phase. (1) A 3 min training during the sleep phase (N = 7), which is an effective means of recalling a memory, leaves the memory intact (p=0.001, t(6) = 6.02, two-tailed paired t-test). (2) A 3 min training paired with 10 µM anisomycin (N = 6) rescues the memory that would have been blocked by the anisomycin alone (p=0.004, t(5) = 5.01, two-tailed paired t-test). (3) A flow diagram showing that the anisomycin does not block memory formation when followed by a brief training.
-
Figure 1—source data 1
Memory is consolidated, but training is ineffective, during the sleep phase.
- https://doi.org/10.7554/eLife.17769.004
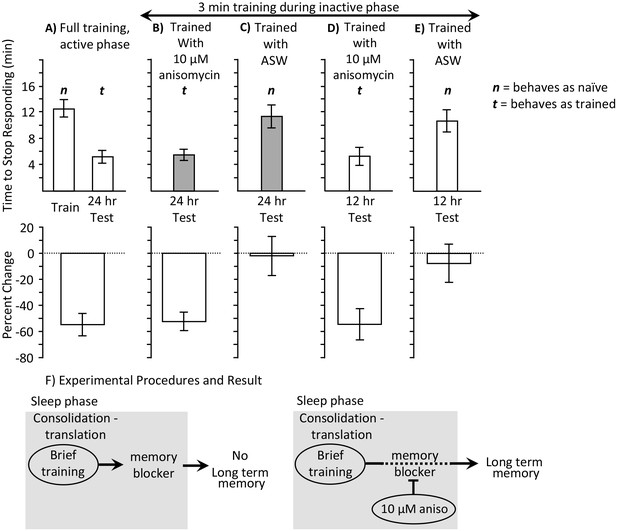
3 min training with anisomycin during the inactive phase produces memory.
Experiments were on A. californica. (A) Time to stop responding during training in the active phase, and during a test of memory 24 hr later (N = 10). These data provide comparisons for data in B-E on the time to stop in a naïve, previously untrained animals, and during a test of memory after successful training. (B) Animals were trained for 3 min during the inactive phase just after treatment with anisomycin, and memory was tested 24 hr later (N = 15). (C) As a control, animals were trained for 3 min during the inactive phase just after treatment with ASW, and memory was tested 24 hr later (N = 7). (D, E) To test whether memory is expressed during the active phase, animals were trained for 3 min during the inactive phase just after treatment with anisomycin (N = 9 D), or ASW (N = 11 E), and memory was tested 12 hr later. There were significant differences between the six groups tested (training and testing in part A, and the four tests of memory in parts B–E) (p=0.00005, F(5,54) = 6.95; one-way analysis of variance). A post hoc- test (Student-Newman-Keuls, α = 0.05) showed no significant difference between naïve animals trained during the day and either group of animals trained for 3 min with ASW (marked by an n for behaving as if naive). Thus, a 3 min training during the inactive phase with ASW produces no memory. These 3 groups were significantly different from the other three groups (marked by a t for behaving as if trained), which were not significantly different from one another. Thus, a 3 min training during the inactive phase after anisomycin treatment produced memory 12 and 24 hr later. (F) The data are explained by the effects of 10 µM anisomycin on a process initiated by sleep phase training that blocks memory. The anisomycin prevents the action of the blocker, allowing the formation of long-term memory.
-
Figure 2—source data 1
3 min training with anisomycin during the inactive phase produces memory.
- https://doi.org/10.7554/eLife.17769.006
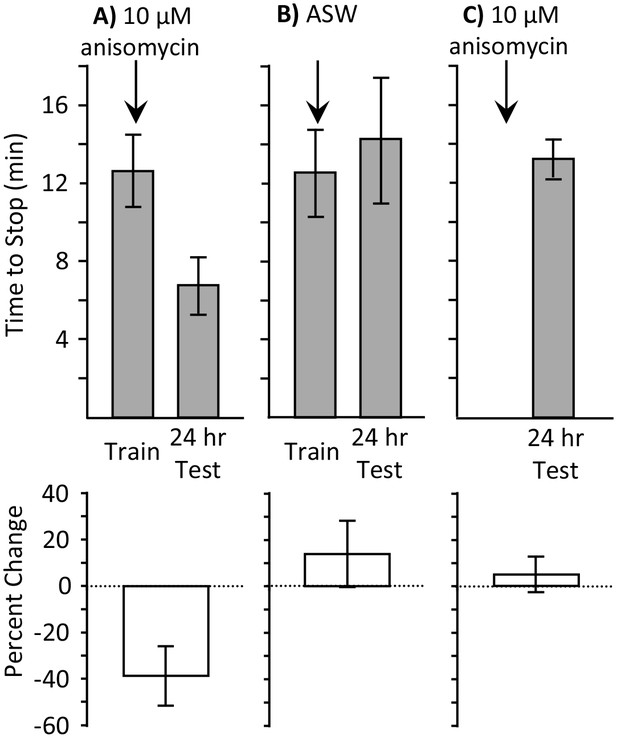
Full training during the sleep phase with anisomycin causes long-term memory.
Experiments were performed on A. californica. (A) Training animals until they stop responding during the inactive phase 10 min after treatment with anisomycin (N = 7) leads to memory when animals are tested 24 hr later, as shown by a decrease in the time stop responding (p=0.05, t = 2.49, df = 6; two-tailed paired t-test). (B) Training animals until they stop responding during the inactive phase 10 min after treatment with ASW (N = 5) did not lead to long-term memory, as shown by a lack of savings when animals were tested 24 hr later (p=0.3, t = 1.12, df = 4; two-tailed paired t-test). (C) Anisomycin treatment alone during the inactive phase (N = 7) does not produce memory 24 hr later (p=0.74, t = 0.34, df = 16 – data after anisomycin treatment were compared to data for naïve animals trained during the day in Figure 1B2, which were trained along with these animals.
-
Figure 3—source data 1
Full training during the sleep phase with anisomycin causes long-term memory.
- https://doi.org/10.7554/eLife.17769.008
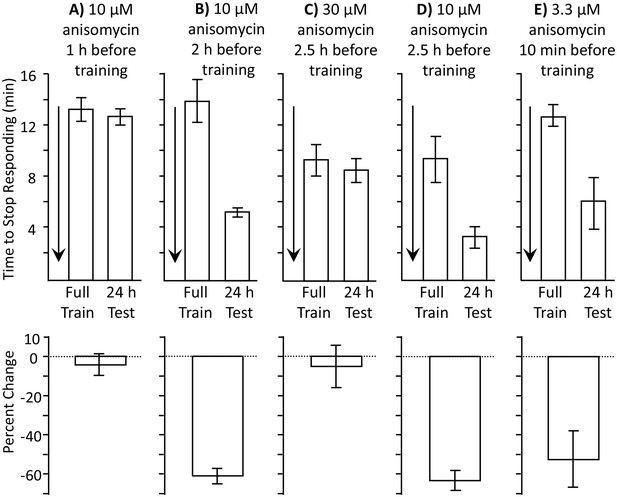
Length of protein synthesis inhibition is concentration dependent.
Experiments in parts A and B were performed on A. fasciata; Experiments in parts C-E were performed on A. californica. (A) 10 µM anisomycin injected 1 hr before training (N = 5) blocks 24 hr memory, as shown by no significant change in the time to stop between the training session and the 24 hr test of memory (p=0.66, t = 0.48, df = 4; two-tailed paired t-test) (B) 10 µM anisomycin injected 2 hr before training (N = 6) does not block 24 hr memory, as shown by a significant change in the time to stop between the training session and the 24 hr test of memory (p=0.006, t = 4.66, df = 5; two-tailed paired t-test). Thus, the actions of 10 µM anisomycin are short-acting, and this concentration is no longer effective after 2 hr. (C) 30 µM anisomycin injected 2.5 hr before training (N = 6) blocks memory, as shown by no significant change in the time to stop between the training session and the 24 hr test of memory (p=0.37, t = 0.99, df = 5; two-tailed paired t-test). (D) In a group of animals collected and tested along with those in C, 10 µM anisomycin 2.5 hr before training (N = 3) did not block memory (p=0.3, t = 5.45, df = 2). Thus, 30 µM anisomycin produces a longer-lasting inhibition than does 10 µM anisomycin. For parts C and D, note that the initial training time is shorter than in parts A and B. This is because the animals were young and collected from the ocean during the beginning of the season and were also trained and tested at a different time. Baseline values often change in different batches of animals collected at different times. (E) 3.3 µM anisomycin injected 10 min before training (N = 7) is ineffective in blocking memory, as shown by a significant decrease in the time to stop between the training session and the 24 hr test of memory (p=0.01, t = 3.53, df = 6, two-tailed paired t-test).
-
Figure 4—source data 1
Length of protein synthesis inhibition is concentration dependent.
- https://doi.org/10.7554/eLife.17769.010
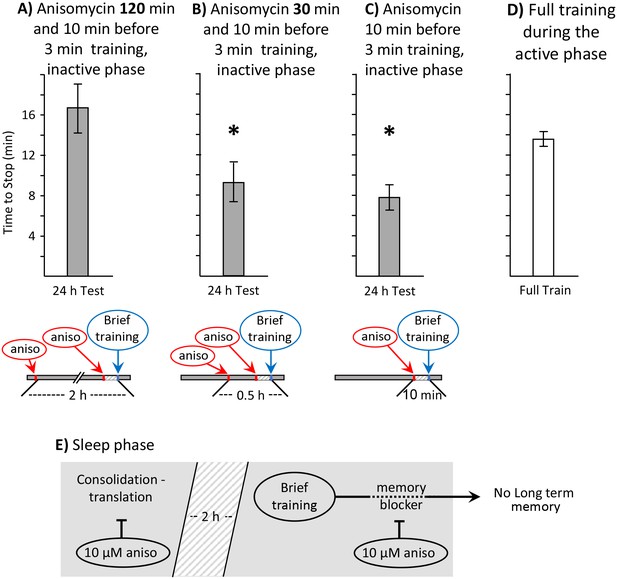
Block of sleep-time expression of proteins blocks memory formation.
These experiments were on A. fasciata. In addition to injecting animals with 10 µM anisomycin treatment 10 min before a 3 min training, the animals were also injected with 10 µM anisomycin A) 2 hr before the training (N = 8), or B) 0.5 hr before the training (N = 8). Values 24 hr after these procedures were compared to values in C) 24 hr after a 3 min training with a single injection of 10 µM anisomycin 10 min before training (N = 15). A one-way analysis of variance between the three groups showed significant differences (p=0.004, F(2,28) = 6.90). (A) Injecting 10 µM anisomycin 2 hr before training blocked memory formation (p=0.003, t = 3.62, df = 21; two-tailed t-test with Bonferroni correction – comparison of A to C), presumably because proteins that accompany consolidation during sleep were blocked. (B) 10 µM anisomycin treatment 0.5 hr before a 3 min training plus 10 µM anisomycin did NOT block memory formation (p=0.52, t = 0.66, df = 21; two-tailed t-test – comparison of B to C), presumably because sleep-phase proteins having a role in consolidation are still present at the time of the training. (C) As shown in the previous experiments, a 3 min training during the inactive phase10 min after 10 µM anisomycin produced 24 hr memory. (D) To provide a comparisons for data in B–D, the time to stop responding during the active phase in naïve, previously untrained animals was also measured (N = 6). (E) A diagram showing that translation relevant to consolidation during sleep is necessary for the expression of long-term memory after the brief training with anisomycin.
-
Figure 5—source data 1
Block of sleep-time expression of proteins blocks memory formation.
- https://doi.org/10.7554/eLife.17769.012
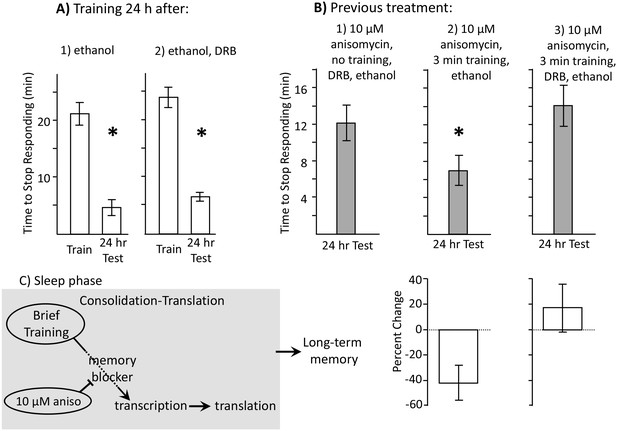
Learning during the inactive phase is transcription dependent.
Experiments were on A. californica. (A) Ethanol is benign. Animals were treated with (1) ethanol alone (N = 3), or with (2) DRB dissolved in ethanol (N = 4), and were trained 24 hr later. Memory was then tested after 24 hr (48 hr after ethanol or DRB treatment). Neither treatment affected the ability of animals to learn or remember that food was inedible 24 hr later (For animals tested with ethanol: p=0.009, t = 10.22, df = 2; for animals tested with DRB: p=0.03, t = 3.57, df = 3). (B) DRB blocks memory. (1) Control: Animals were not trained. They were treated with anisomycin and then treated with DRB dissolved in ethanol, and then tested after 24 hr (N = 6). (2) Vehicle: Animals were treated with anisomycin, and then trained for 3 min. After training, the animals were injected with ethanol, with no DRB. Memory was then tested 24 hr later (N = 7). (3) DRB: Animals were treated with anisomycin, and then trained for 3 min, and then injected with DRB dissolved in ethanol. Memory was then tested 24 hr later (N = 8). There were significant differences between the 3 groups tested (p=0.04, F(2,18) = 3.73). For the 2 groups of trained animals, there was a significant difference in the time to stop 24 hr after the training (comparison of 2 and 3 – p=0.027; t = 2.50, df = 13; two-tailed t-test). There was no significant difference between the time to stop in animals treated with DRB, and in control animals that had not been trained (comparison of 1 and 3 p=0.49; t = 0.71, df = 12; two-tailed t-test). Thus, the DRB blocked memory that would have been formed after training with anisomycin. (C) A diagram showing that after the brief training with 10 µM anisomycin, long-term memory depends on transcription and translation, The DRB blocks memory because it blocks transcription, and a 30 µM dose of anisomycin, which has a longer-lasting effect, blocks the transcription-dependent translation.
-
Figure 6—source data 1
Learning during the inactive phase is transcription dependent.
- https://doi.org/10.7554/eLife.17769.014
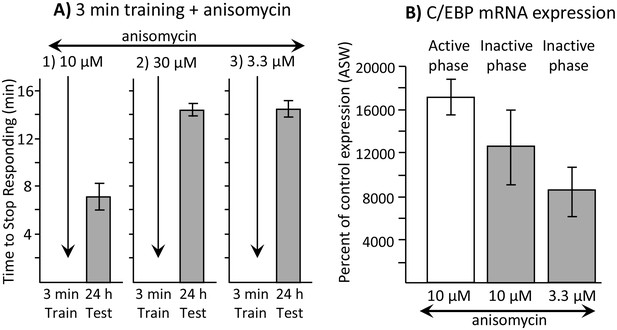
Dissociation between memory and C/EBP expression.
Data are from A. californica. (A) Neither 30 µM (N = 6) nor 3.3 µM (N = 7) anisomycin injected 10 min before a brief training during the inactive phase produce 24 hr memory. The times to stop responding 24 hr after treatment with 30 µM anisomycin and 3.3 µM anisomycin are significantly longer than the time to stop 24 hr after 10 µM (N = 5) anisomycin (for 30 µM: p=0.005, t = 5.98, df = 9; for 3.3 µM: p=0.0002, t = 6.42, df = 10; two tailed t-tests with Bonferroni corrections). (B) C/EBP expression in the buccal ganglia 2 hr after injecting 10 µM anisomycin during the active (N = 5) and inactive (N = 6) phases increases, and after injecting 3.3 µM (N = 6) anisomycin during the inactive phase. Data are normalized to C/EBP expression in animals that were injected with ASW (not shown). Normalization was to the ASW treatment at the same time as the anisomycin treatment (N = 6 during the active phase, N = 5 during the inactive phase). There were no significant differences in C/EBP expression after ASW treatment between active and inactive phases. Expression after ASW treatment was set at 100%. All treatments with anisomycin produced large (X thousand percent) increases in C/EBP expression, although only 10 µM anisomycin during the inactive phase produces long-term memory when paired with training (For the 3 groups shown: p=0.139; F(2,14) = 2.27.
-
Figure 7—source data 1
Dissociation between memory and C/EBP expression.
- https://doi.org/10.7554/eLife.17769.016
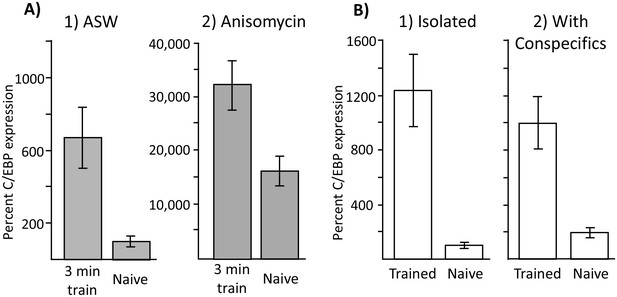
Increased C/EBP expression is a correlate of training, not of memory formation.
(A) Effect of 3 min training during the inactive phase on C/EBP expression. Data are from the buccal ganglia of A. californica. (1) Training 10 min after injection with ASW (Trained: N = 8; Naïve: N = 9) produced a significant increase in C/EBP mRNA expression (p=0.004, t = 3.43, df = 15), even though this treatment did not lead to long-term memory. (2) Training after injection with anisomycin (Trained: N = 8; Naïve: N = 8) also produced a significant increase in C/EBP (p=0.008, t = 3.11, df = 14; both tests are two-tailed unpaired t-tests), over that caused by the injection of anisomycin in naïve controls (see Figure 6) Note that values for all 4 treatments are normalized to the value for naïve animals treated with ASW. As shown above, anisomycin alone caused a large increase in expression. (B) Effects of training in isolation on C/EBP expression. Data are from the buccal ganglia of A. fasciata, in which training during the active phase when animals are maintained in isolation does not produce long-term memory. Note that C/EBP expression in all four groups was normalized to expression in isolated, naïve animals, in which the mean value was set as 100%. (1) Training in A. fasciata maintained in isolation (Trained: N = 10; Naïve: N = 10) produced a significant increase in C/EBP expression (p=0.0009, t = 4.27, df = 18), as did (2) training in animals housed with conspecifics (Trained: N = 6; Naïve: N = 6) (p=0.004, t = 4.13, df = 10). Note that maintenance with conspecifics itself produced a small increase in C/EBP expression (p=0.05, t = 2.52, df = 14; all tests are two-tailed unpaired t-tests with Bonferroni correction).
-
Figure 8—source data 1
Increased C/EBP expression is a correlate of training, not of memory formation.
- https://doi.org/10.7554/eLife.17769.018
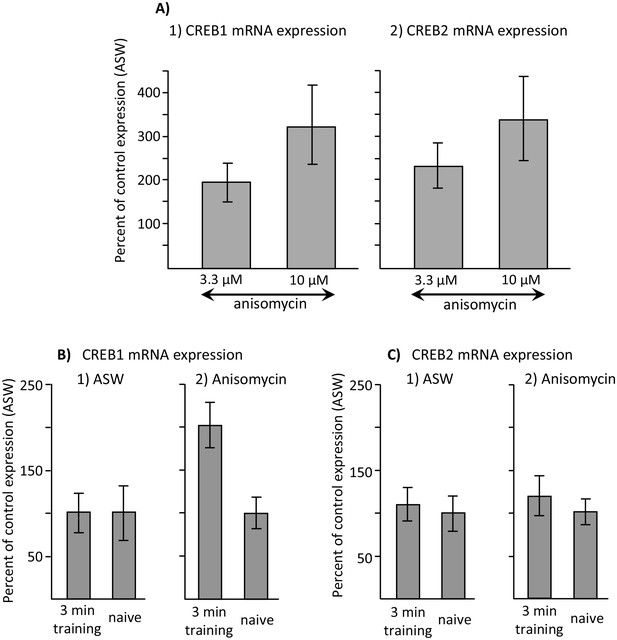
Increased CREB1 expression after training is a correlate of memory.
Data are from the buccal ganglia of A. californica. (A) Increases in CREB1 or CREB2 by anisomycin treatment alone cannot account for memory formation. A preliminary analysis examined whether either CREB1 or CREB2 expression is increased by either 3.3 or 10 µM anisomycin 2 hr after treatment, with respect to expression 2 hr after injection with ASW (not shown). A one-way analyses of variance showed no significant differences in CREB1 expression between ASW (N = 6), 10 µM (N = 8) and 3.3 µM (N = 6) anisomycin (p=0.09, F(2,17) = 2.77) or of CREB2 expression between these three treatments (ASW: N = 6; 10 µM anisomycin: N = 7; and 3.3 µM: N = 5) anisomycin) (p=0.07, F(2,15) = 3.10) 2 hr after the treatments. In addition, there were no significant differences between expression after treatment with 3.3 and 10 µM anisomycin (For CREB1: p=0.29, t = 1.1, df = 12; for CREB2, p=0.42, t = 0.84, df = 10). (B) CREB1, but not CREB2, is a correlate of memory formation. (1) There was a significant increase (p=0.006, t = 3.24, df = 14) in CREB1 expression 2 hr after a 3 min training with 10 µM anisomycin during the inactive phase(N = 8), with respect to naïve animals treated with anisomycin (N = 8). However, there was no significant increase in expression in animals treated with ASW (p=0.97, t = 0.03, df = 15. N = 8 for the trained group, N = 9 for the naïve group). (2) CREB2 expression was unaffected by training after either treatment (For animals treated with ASW: p=0.71, t = 0.37, df = 15, N = 8 trained animals and 9 untrained animals; for animals treated with 10 µM anisomycin: p=0.50, t = 0.69, df = 12, N = 7 trained and 7 untrained animals). Titles and brief legends for the source data files.
-
Figure 9—source data 1
Increased CREB1 expression after training is a correlate of memory.
- https://doi.org/10.7554/eLife.17769.020





