The mitochondrial acyl carrier protein (ACP) coordinates mitochondrial fatty acid synthesis with iron sulfur cluster biogenesis
Figures
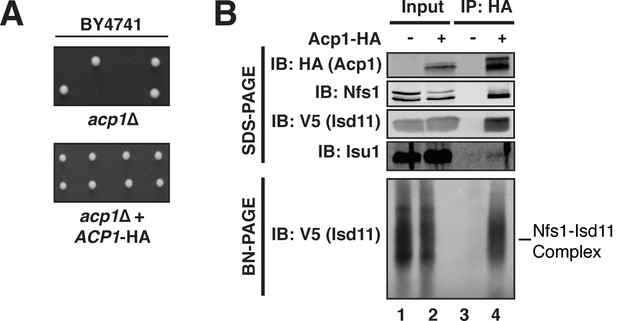
Acp1 is a stable subunit of the ISU complex.
(A) acp1Δ heterozygous diploids were dissected with and without a plasmid expressing Acp1-HA (BY4741) and spores were grown on YPAD medium for 2 days. Sporulation of the heterozygous deletion strain failed to generate haploid ACP1 deletion strains unless a vector borne ACP1 gene was present. (B) Purified mitochondria from cells either expressing Acp1-HA or not were solubilized by digitonin (input) and then subjected to anti-HA immunoprecipitation. The resulting eluates and input samples were subjected to SDS-PAGE and BN-PAGE and immunoblot.
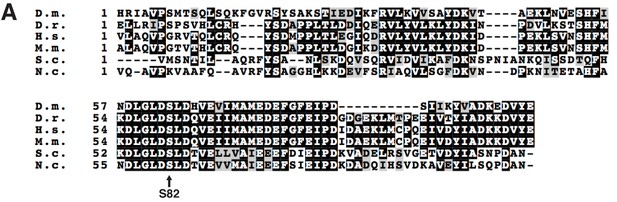
Protein sequence alignment of ACP from eukaryotes.
(A) Protein sequence alignment of the ACP proteins from the indicated species (Clustal Omega). D.m. – Drosophila melanogaster, D.r. – Danio rerio, H.s. – Homo sapiens, M.m. – Mus musculus, S.c. – Saccharomyces cerrevisiae, N.c. – Neuropsora crassa. The invariant Ser residue to which 4-PP is conjugated is indicated.
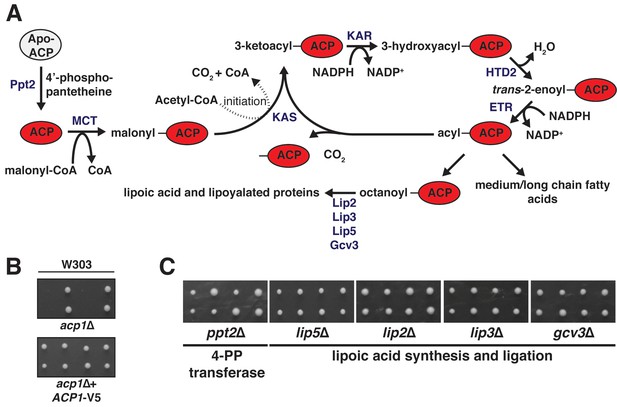
Eukarotic mitochondrial fatty acid biosynthesis (FASII) pathway.
(A) Eukaryotic cells have maintained two distinct fatty acid synthesis pathways—the canonical cytoplasmic FASI and the mitochondrial FASII, which is homologous to the prokaryotic fatty acid synthesis pathway. FASII, which is dependent on ACP, is comprised of a set of monofunctional enzymes that catalyze the various steps in fatty acid synthesis. In order to facilitate mitochondrial fatty acid synthesis, apo-ACP must first be converted to holo-ACP by a 4’-phosphopanthetheine transferase (Ppt2), which catalyzes the covalent attachment of the 4-phosphopantetheine prosthetic group (4-PP) to an absolutely conserved Ser residue on ACP. The 4-PP contains a terminal thiol that serves as the attachment site to enable Acp1 to scaffold de novo fatty acid synthesis. The enzymes of FASII use malonyl-coA to initiate the fatty acid chain and acetyl-coA for acyl chain elongation. The canonical product of FASII is octanoate, which is the precursor for lipoic acid, an important mitochondrial cofactor. Following ACP-dependent octanoate synthesis, Lip2, Lip3, Lip5, and Gcv3 support the synthesis of lipoic acid and ligation to its target proteins including the E3 component of pyruvate dehydrogenase and α-ketoglutarate dehydrogenase. Ppt2 – 4’phophopantetheine transferase; MCT – malonyl-coA transferase; KAS – ketoacyl synthetase; KAR – ketoacyl reductase; HTD2 – hydroxyacyl-thioester reductase type 2; ETR – enoyl-thioester reductase; Lip2, Lip3, Lip5, Gcv3 – lipoic acid biosynthesis and ligation. (B) acp1Δ heterozygous diploids in the W303 strain background were dissected with and without a plasmid expressing Acp1-V5 and spores were grown on YPAD medium for 2 days. (C) ppt2Δ, lip5Δ, lip2Δ, lip3Δ, and gcv3Δ heterozygous diploids were dissected and spores were grown on YPAD medium for 2 days.

Acp1 is a stable subunit of the ISU complex.
(A) Isd11-V5 and Acp1- HA were expressed from their endogenous loci and Isd11-V5 was immunoprecipitated from digitonin-solubilized mitochondria followed by the indicated immunoblots. (B) Nfs1-V5 and Acp1- HA were expressed from their endogenous loci and Nfs1-V5 was immunoprecipitated from digitonin-solubilized mitochondria followed by the indicated immunoblots.
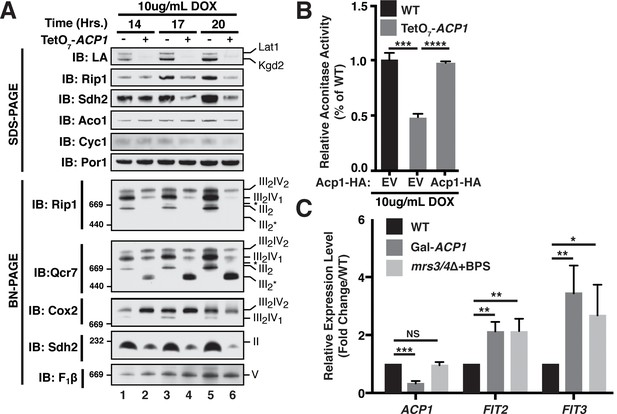
Acp1 is required for FeS biogenesis.
(A) Isolated mitochondria from the indicated strains were resolved by SDS-PAGE (upper panel) or solubilized in 1% digitonin and resolved by BN-PAGE (lower panel). The time course indicates the time following addition of 10 μg/mL doxycycline to the cultures, which suppresses expression from the TetO7-ACP1 allele. The indicated proteins and protein complexes were assessed by immunoblot. LA indicates lipoic acid-conjugated Lat1 (PDH complex subunit; upper band) and Kgd2 (α-ketoglutarate dehydrogenase complex subunit; lower band). (B) Aconitase activity was measured in whole cell lysates from the indicated strains containing the indicated plasmids 18 hr post-addition of 10 μg/mL doxycycline (± SEM; N = 3 biological replicates. ***p<0.0005, ****p<0.00005). (C) qPCR was used to measure the expression of ACP1, FIT2, and FIT3 in the indicated strains. The Gal-ACP1 strain was harvested at 28 hr post-transfer to raffinose medium to suppress ACP1 expression. The mrs3/4∆ strain lacks both Mrs3 and Mrs4 mitochondrial iron transporters. BPS (80 μM) is a Fe(II) chelator that causes depletion of bioavailable iron. FIT2 and FIT3 are components of the iron regulon that is induced upon loss of cytosolic FeS (± SEM; N = 3 biological replicates. *p<0.05, **p<0.005, ***p<0.0005).
-
Figure 2—source data 1
Source data for Figure 2.
This file contains raw source data used to make the graphs presented in Figure 2B,C Figure 2—figure supplement 2B and C. PRISM software was used to graph all quantitative data and perform statistical analyses. p values for pairwise comparisons were determined using a Student’s t test.
- https://doi.org/10.7554/eLife.17828.008
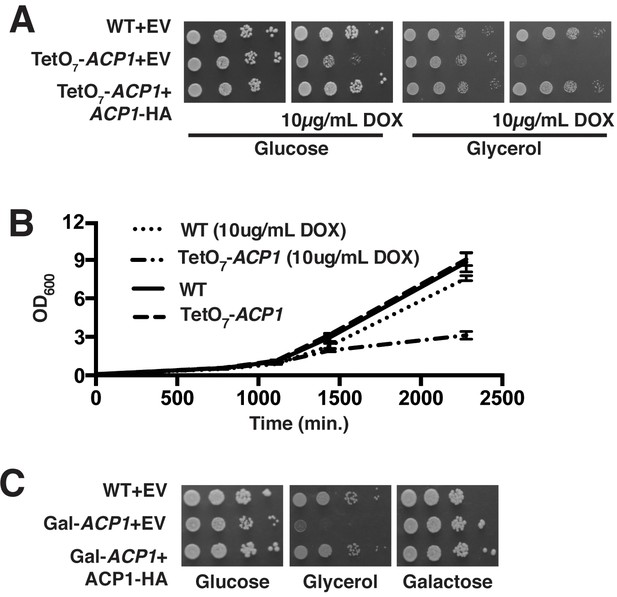
ACP1 expression is required for cell proliferation.
(A) Ten-fold serial dilutions of WT, TetO7-ACP1, and TetO7-ACP1 cells expressing Acp1-HA were plated on synthetic media containing the indicated carbon source with and without 10 μg/mL doxycycline (BY4741 background). (B) Liquid cultures (2% raffinose) with and without 10 μg/mL doxycycline were inoculated to an OD. 025 with either WT or TetO7-ACP1 cells. Growth was monitored by absorbance at 600 nm. (C) Ten-fold serial dilutions of WT, Gal-ACP1, and Gal-ACP1 cells expressing Acp1-HA were serially diluted and plated on synthetic media containing the indicated carbon source. With pre-growth of the Gal-ACP1 strain on glucose medium, subsequent plating of the cells on glucose shows moderate growth impairment. The depletion of Acp1 in TetO7-ACP1 cells is more efficient compared to Gal-ACP1 cells.
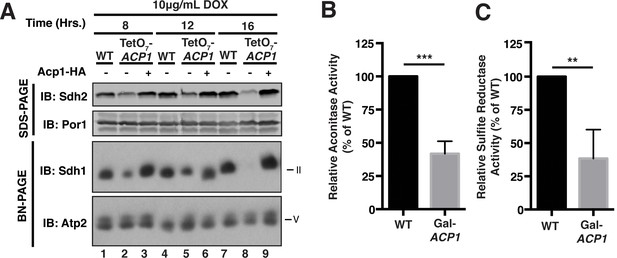
Acp1 is essential for FeS biogenesis.
(A) Isolated mitochondria from WT, TetO7-ACP1, or TetO7-ACP1 cells expressing Acp1-HA were harvested at the indicated times post addition of doxycycline were resolved by SDS-PAGE (upper panels) or solubilized in 1% digitonin and resolved by BN-PAGE (lower panels). (B) Aconitase activity was measured in mitochondrial lysates from WT and Gal-ACP1 cells at 28 hr post-transfer to raffinose-containing medium (± SEM; N = 3 biological replicates. ***p<0.0005). (C) The cytosolic FeS-containing sulfite reductase activity was measured in whole cell lysates from WT and Gal-ACP1 cells at 28 hr post-transfer to raffinose-containing medium (± SEM; N = 3 biological replicates. **p<0.005).
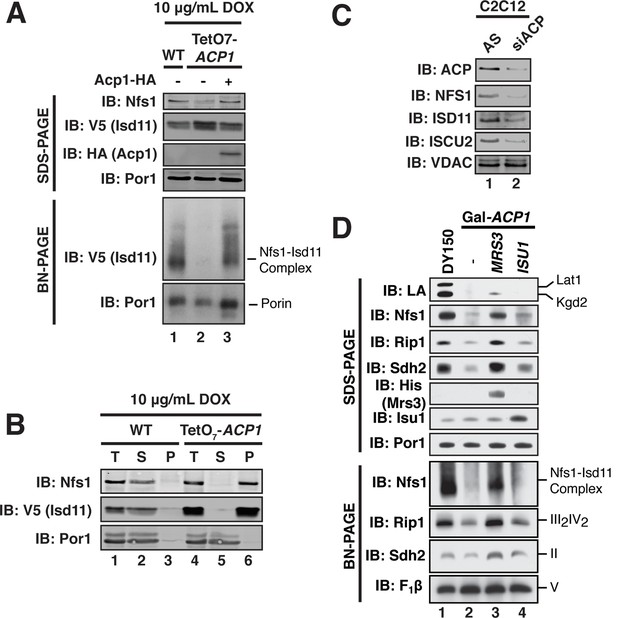
ACP promotes FeS biogenesis by maintaining the stability of the ISU (Nfs1-Isd11) complex.
(A) Purified mitochondria from the indicated strains were either resolved by SDS-PAGE (lower panels) or solubilized in 1% digitonin and resolved by BN-PAGE (upper panels). Cells were grown for 18 hr in the presence of 10 μg/mL doxycycline. The indicated proteins and protein complexes were assessed by immunoblot. (B) Mitochondria purified from the indicated strains 18 hr post-addition of 10 μg/mL doxycycline were solubilized with 1% Triton X-100. Soluble (S) and pellet (P) fractions were separated by centrifugation at 100,000 g. The fractions, along with the total input (T) were resolved by SDS-PAGE and assessed by immunoblot. (C) C2C12 mouse myoblasts were transfected with a pool of siRNA targeting NDUFAB1 (ACP) or a scrambled control. Isolated mitochondria was resolved by SDS-PAGE and assessed by immunoblot. (D) Isolated mitochondria from the WT and Gal-ACP1 strains expressing the indicated gene via a 2 μ plasmid at 28 hr post-transfer to raffinose-containing medium were either resolved by SDS-PAGE (lower panels) or solubilized in 1% digitonin and resolved by BN-PAGE (upper panels). The indicated proteins and protein complexes were assessed by immunoblot.
-
Figure 3—source data 1
Source data for Figure 3.
This file contains raw source data used to make the graphs presented in Figure 3—figure supplement 1C. PRISM software was used to graph all quantitative data and perform statistical analyses. p values for pairwise comparisons were determined using a Student’s t test.
- https://doi.org/10.7554/eLife.17828.012
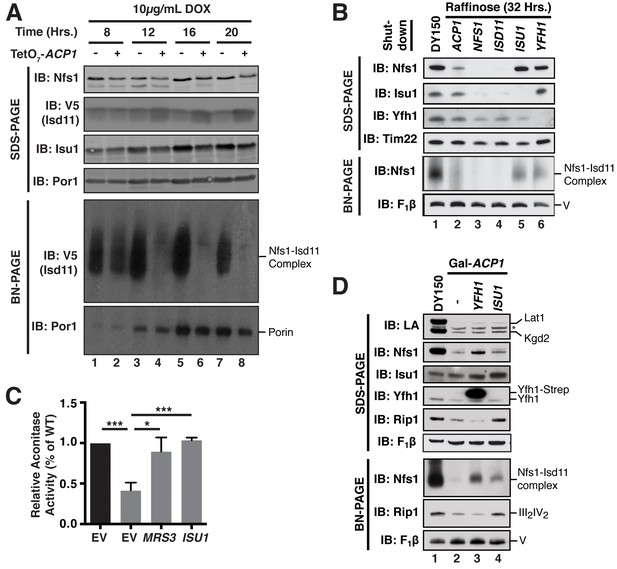
ACP promotes FeS biogenesis by maintaining the stability of the ISU (Nfs1-Isd11) complex.
(A) Isolated mitochondria from WT and TetO7-ACP1 cells harvested at the indicated time points post-addition of doxycycline were resolved by SDS-PAGE (upper panel) or solubilized in 1% digitonin and resolved by BN-PAGE (lower panel). (B) Indicated strains were grown in raffinose for 32 hr to repress expression of the indicated genes. YFH1 was depleted in a Met3-YFH1 strain cultured in the presence of 2.5 mM methionine. Isolated mitochondria from each strain was either resolved by SDS-PAGE (upper panel) or solubilized in 1% digitonin and resolved by BN-PAGE (lower panel). Depletion of ISD11, but not ISU1 subunit destabilizes Nfs1-Isu11 complex on BN-PAGE as ACP1 depletion does, suggesting Acp1 acts through Isd11 to destabilize Nfs1. (C) Aconitase activity was measured in mitochondrial lysates from WT and Gal-ACP1 cells overexpressing MRS3 or ISU1 harvested at 28 hr post-transfer to raffinose-containing medium (± SEM; N = 3 biological replicates. *p<0.05, ***p<0.0005). (D) Isolated mitochondria from the WT and Gal-ACP1 strains expressing the indicated gene via a 2 μ plasmid at 28 hr post-transfer to raffinose-containing medium were either resolved by SDS-PAGE (lower panels) or solubilized in 1% digitonin and resolved by BN-PAGE (upper panels). The indicated proteins and protein complexes were assessed by immunoblot. * indicates non-specific band.
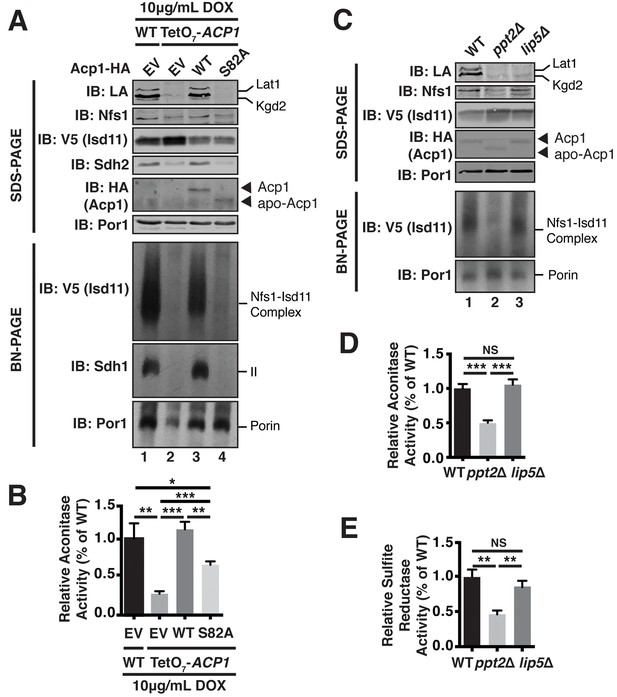
Acp1 requires a 4-PP-conjugated acyl chain to fully stabilize the ISU complex.
(A) Isolated mitochondria from the indicated strains expressing the indicated genes by plasmid were either resolved by SDS-PAGE (upper panels) or solubilized in 1% digitonin and resolved by BN-PAGE (lower panels). The indicated proteins and protein complexes were assessed by immunoblot. Cells were grown for 18 hr in the presence of 10 μg/mL doxycycline. (B) Aconitase activity was measured in whole cell lysates from the indicated strains grown for 18 hr in the presence of 10 μg/mL doxycycline (± SEM; N = 3 biological replicates. *p<0.05, **p<0.005, ***p<0.0005). (C) Isolated mitochondria from the indicated strains were either resolved by SDS-PAGE (upper panels) or solubilized in 1% digitonin and resolved by BN-PAGE (lower panels). The indicated proteins and protein complexes were assessed by immunoblot. (D) Aconitase activity was measured in whole cell lysates from the indicated strains (± SEM; N = 3 biological replicates. ***p<0.0005). (E) Sulfite reductase activity was measured in whole cell lysates from the indicated strains (± SEM; N = 3 biological replicates. **p<0.005).
-
Figure 4—source data 1
Source data for Figure 4.
- https://doi.org/10.7554/eLife.17828.015
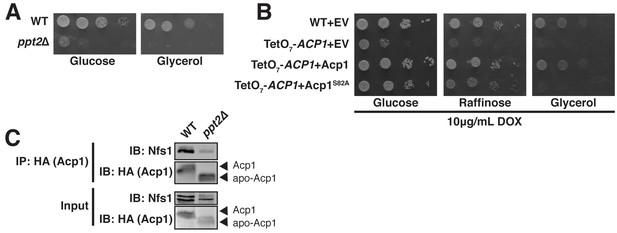
Acp1 requires a 4-PP-conjugated acyl chain to fully stabilize the ISU complex.
(A) Ten-fold serial dilutions of the indicated strains plated on synthetic medium with either glucose or glycerol. (B) Ten-fold serial dilutions of the indicated strains expressing the indicated plasmids were plated on synthetic media containing 10 μg/mL doxycycline and either glucose, raffinose, or glycerol. (C) Purified mitochondria from WT and ppt2Δ cells expressing Acp1-HA were solubilized by digitonin (input) and then subjected to anti-HA immunoprecipitation. The resulting eluates and input samples were subjected to SDS-PAGE.
Additional files
-
Supplementary file 1
Yeast strains used in this study.
This table describes the name, genotype, and source of all yeast strains used in this investigation.
- https://doi.org/10.7554/eLife.17828.017





