Macrophage PPARγ inhibits Gpr132 to mediate the anti-tumor effects of rosiglitazone
Figures
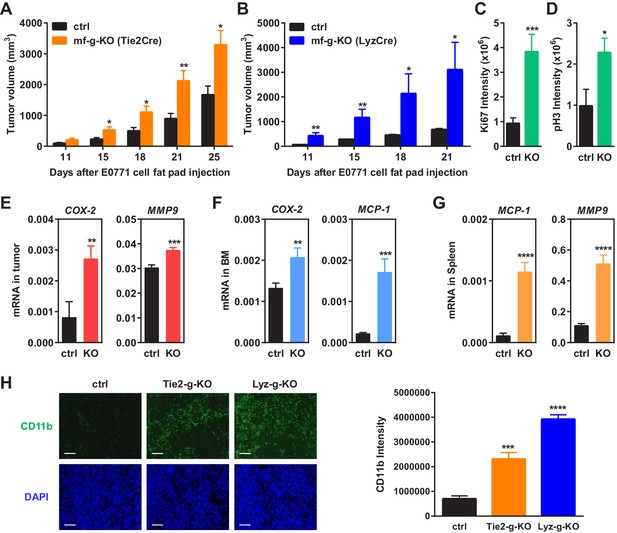
Macrophage PPARγ deletion enhances mammary tumor growth in vivo.
(A) Tie2Cre-induced mf-g-KO mice (n = 26) showed enhanced tumor growth compared to control mice (n = 16) as indicated by earlier onset and larger tumor volume. EO771 mouse mammary tumor cells were injected into the mammary fat pad of 6–8 weeks old female mice. (B) LyzCre-induced mf-g-KO mice (n = 6) showed augmented tumor growth compared to control mice (n = 6) as indicated by earlier onset and larger tumor volume. (C–D) Quantification of cell proliferation markers Ki67 (C) and phosphor histone H3 (PH3) (D) in tumor sections showed increased cell proliferation in mf-g-KO mice (n = 4). (E–G) RT-QPCR analyses showed an increased expression of pro-inflammatory genes in tumor tissues (E), bone marrow (BM) (F) and spleen (G) from mf-g-KO mice (n = 3). (H) Immunofluorescence staining of tumor sections for macrophage marker CD11b showed an enhanced macrophage recruitment in the tumors from both Tie2Cre- and LyzCre-induced mf-g-KO mice compared with control mice (n = 4). All tumors were collected 21 days after cancer cell injection. Error bars, SD; *p<0.05; **p<0.01; ***p<0.005; ****p<0.001; n.s. non-significant.
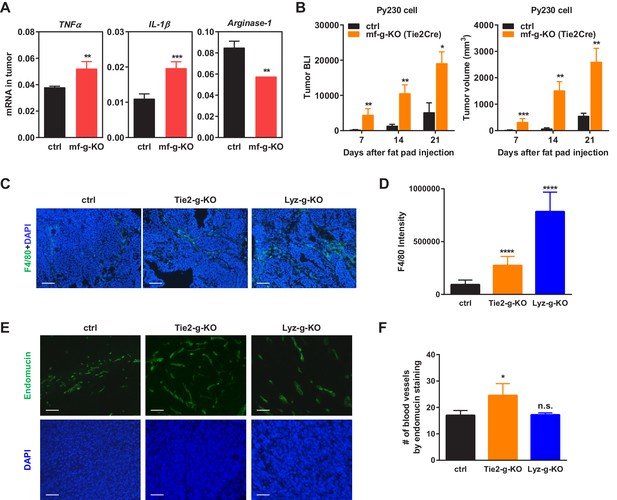
Additional analyses of tumors.
(A) Tumors from PPARγ-deficient mice exhibited higher pro-inflammatory M1-like markers (TNFα and IL1β) but lower M2-like markers (Arginase 1). (B) Tumor growth from another C57BL/6-compatible mouse breast cancer cell line Py230 was also increased in mf-g-KO mice compared with control mice. Luciferase-labelled Py230 cells were injected into mammary fat pad, and tumor growth was quantified by bioluminescence imaging (BLI) (left) and tumor volume (right) (n = 3). Error bars, SE. (C–F) Immunofluorescence staining of tumor sections. (C–D) Macrophage recruitment was increased in both Tie2Cre-PPARγ-KO and LyzCre-PPARγ-KO mice compared to WT control mice. Tumor sections were stained for a macrophage marker F4/80 (n = 4). (E–F) Angiogenesis was increased in Tie2Cre-PPARγ-KO mice but not in LyzCre-PPARγ-KO mice. Tumor sections were stained for an endothelial marker endomucin (n = 4). All tumors were collected 21 days after cancer cell injection. (C,E) Representative images of staining; scale bars, 25 μm. (D,F) Quantification of F4/80 intensity (D) and number of blood vessels (F). Error bars, SD.
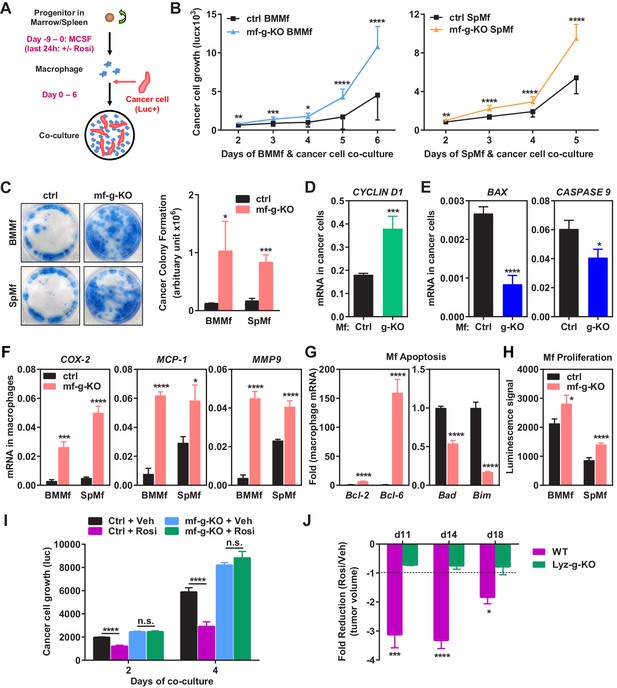
Macrophage PPARγ deletion exacerbates breast cancer cell proliferation and attenuates the anti-tumor effect of rosiglitazone.
(A) A diagram of mouse macrophage and human breast cancer cell co-culture. Progenitors in bone marrow or spleen were differentiated into macrophages with M-CSF for nine days before the seeding of luciferase-labelled 1833 human breast cancer cells to the cultures. For rosiglitazone (Rosi) pre-treatment, macrophages were treated with 1 μM Rosi or vehicle control for the last 24 hr of macrophage differentiation; after medium was removed and cells were washed, cancer cells were added to the macrophage cultures in fresh medium without Rosi or vehicle. (B) Cancer cell proliferation was increased when co-cultured with PPARγ-deficient macrophages derived from bone marrow (left) or spleen (right) of mf-g-KO mice compared with WT control macrophages (n = 3). Cancer cell growth was quantified by luciferase signal for 2–6 days. (C) PPARγ-deficient macrophages promoted tumor cell colony formation in the co-cultures (n = 3). Tumor cells were cultured for 11–12 days for the colonies to form. Left, representative images of crystal violet staining. Right, quantification of colony formation. (D–E) Co-culture with PPARγ-deficient macrophages resulted in higher expression of proliferation markers (D) and lower expression of apoptosis markers (E) in breast cancer cells (n = 3). Human gene expression in cancer cells was quantified by RT-QPCR and human-specific primers. (F) PPARγ-deficient macrophages exhibited a higher expression of pro-inflammatory genes (n = 3). BMMf, bone marrow macrophage; SpMf, spleen macrophage. (G) PPARγ-deficient macrophages displayed higher levels of anti-apoptotic genes (left) and lower levels of pro-apoptotic genes (right) (n = 3). (H) PPARγ-deficient macrophages showed increased proliferation (n = 3). The number of metabolically active cells was determined by ATP content using the CellTiter-Glo Assay. (I) Co-culture with Rosi pre-treated macrophages inhibited breast cancer cell growth compared with vehicle (Veh) pre-treated macrophages in a macrophage-PPARγ-dependent manner (n = 3). (J) The ability of Rosi to suppress tumor growth in vivo was significantly attenuated in mf-g-KO mice (n = 6). Four days after EO771 cell mammary fat pad injection, mf-g-KO mice or control mice were treated with Veh or Rosi (10 mg/kg) every two days before tumor volume measurement. Error bars, SD; *p<0.05; **p<0.01; ***p<0.005; ****p<0.001; n.s. non-significant.
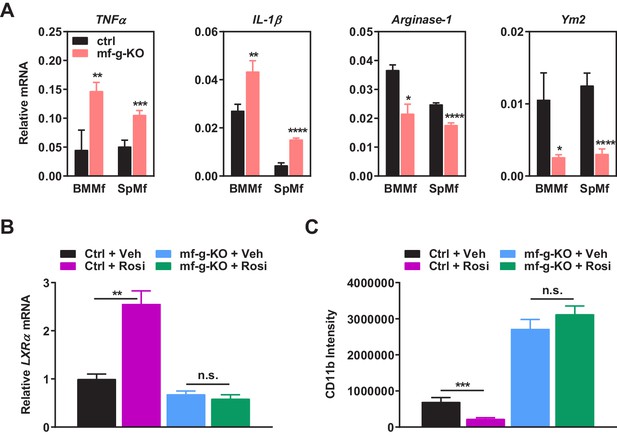
Additional analyses of co-cultures and rosiglitazone treatment.
(A) PPARγ-deficient macrophages exhibited increased expression of pro-inflammatory M1-like markers (TNFα and IL1β) but decreased expression of M2-like markers (Arginase 1 and Ym2) in macrophage-cancer cell co-cultures (n = 3). BMMf, bone marrow macrophage; SpMf, spleen macrophage. (B) As a positive control, rosiglitazone treatment (1 μM, 24 hr) increased the expression of previously reported PPARγ target gene LXRα in WT macrophages but not g-KO macrophages (n = 3). Error bars, SD. (C) Rosiglitazone treatment reduced the abundance of tumor associated macrophages in WT control mice but not in mf-g-KO (LyzCre) mice (n = 6). Error bars, SD.
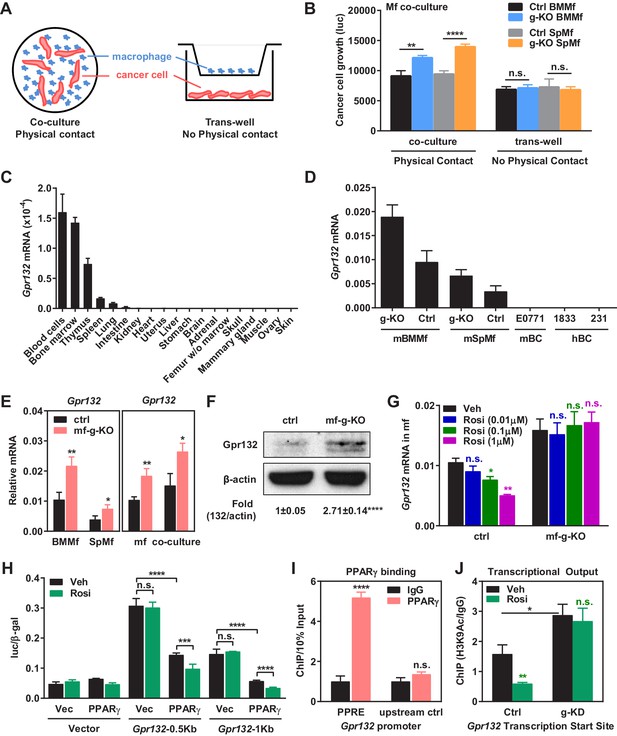
PPARγ represses Gpr132 transcription in macrophages.
(A–B) Physical contact is required for the pro-tumor effects of PPARγ-deficient macrophages. (A) A schematic diagram of the co-culture vs. trans-well systems. (B) Tumor cell proliferation was enhanced by co-culture with PPARγ-deficient macrophages but not by their conditioned medium delivered via trans-well (n = 3). (C) Gpr132 was predominantly expressed in the hematopoietic cell types and tissues (n = 3). (D) Gpr132 was expressed in macrophages but largely absent in breast cancer cells. mBMMf, mouse bone marrow macrophage; mSpmf, mouse spleen macrophage; mBC, mouse breast cancer cells; hBC, human breast cancer cells. (E) Gpr132 mRNA levels were significantly higher in PPARγ-deficient macrophages compared with control macrophages, either in macrophage cultures alone or in macrophages co-cultured with human breast cancer cells (n = 3). (F) Gpr132 protein expression was significantly higher in PPARγ-deficient macrophages (n = 3). (G) PPARγ activation by rosiglitazone reduced Gpr132 mRNA in WT macrophages but not in PPARγ-deficient macrophages (n = 3). (H) Transcriptional output from both 0.5 Kb and 1 Kb Gpr132 promoters was reduced by the co-transfection of PPARγ and further diminished by rosiglitazone (n = 3). HEK293 cells were transfected with PPARγ and its heterodimer partner retinoic X receptor α (RXRα), together with a luciferase reporter driven by 0.5 Kb or 1 Kb Gpr132 promoter, and compared with vector-transfected controls. Next day, cells were treated with rosiglitazone or vehicle control for 24 hr before harvest and reporter analyses. (I) ChIP assay of PPARγ binding to the endogenous Gpr132 promoter in macrophages. A PPRE region in the Gpr132 promoter was pulled down with anti-PPARγ antibody or an IgG control antibody in RAW264.7 mouse macrophages and detected by QPCR (n = 3). An upstream Gpr132 promoter region served as a negative control. (J) ChIP assay of H3K9Ac active transcription histone mark at the Gpr132 transcription start site showed that rosiglitazone represses the transcriptional activity from a Gpr132 promoter in a PPARγ-dependent manner (n = 3). Control or PPARγ knockdown (KD) RAW264.7 macrophages were treated with 1 μM rosi or vehicle control for 4 hr before harvest. Error bars, SD; *p<0.05; **p<0.01; ***p<0.005; ****p<0.001; n.s. non-significant.
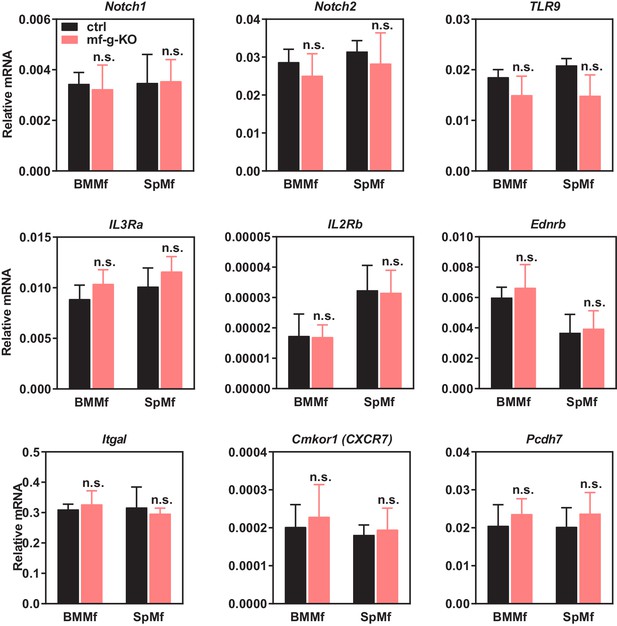
Expression of other candidate genes was unaltered in PPARγ-deficient macrophages.
Candidate genes that encode membrane proteins and are potentially regulated by macrophage PPARγ were selected from published microarray databases. Their expression in bone marrow macrophages (BMMf) or spleen macrophages (SpMf) derived from mf-g-KO mice or littermate control mice were quantified by RT-QPCR (n = 3). LGR5 and CCR4 expression was also examined, but the expression was too low to detect. Error bars, SD.
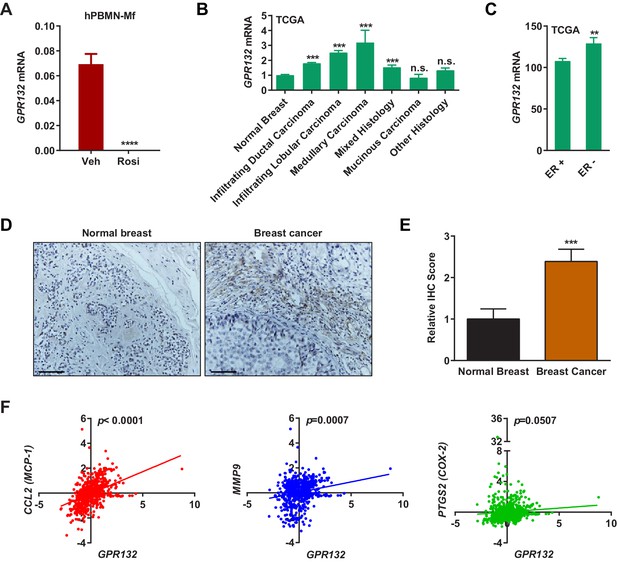
Gpr132 is repressed by PPARγ in human macrophages and correlates with human breast cancer.
(A) Human Gpr132 expression in hPBMN-derived macrophages was blunted by rosiglitazone treatment (n = 3). Macrophages were treated with 1 μM rosiglitazone or vehicle for 4 hr. (B) TCGA BRCA data analysis showed that compared with normal breast samples, breast cancer lesions displayed higher Gpr132 expression. Normal Breast (n = 111); Infiltrating Ductal Carcinoma (n = 750); Infiltrating Lobular Carcinoma (n = 168); Medullary Carcinoma (n = 5); Mixed Histology (n = 29); Mucinous Carcinoma (n = 14); Other Histology (n = 44). Error bars, SE. (C) TCGA BRCA data analysis showed that compared with ER+ breast cancers (n = 746), ER- breast cancers (n = 221) exhibited higher Gpr132 expression. Error bars, SE. (D–E) Immunohistochemistry (IHC) of human tissue microarrays showed higher Gpr132 expression in breast cancer tissues (n = 16) compared with normal breast tissues (n = 13). Tissues were stained with anti-Gpr132 (brown) and hematoxylin (blue). (D) Representative images. Scale bars, 200 μm. (E) Quantification of relative IHC scores. Error bars, SE. (F) Linear regression analyses of TCGA BRCA data showed that Gpr132 expression was positively correlated with the expression of CCL2 (MCP-1), MMP9 and PTGS2 (COX-2) in breast cancer lesions (n = 805). Error bars, SD (A) or SE (B,C,E); *p<0.05; **p<0.01; ***p<0.005; ****p<0.001; n.s. non-significant.
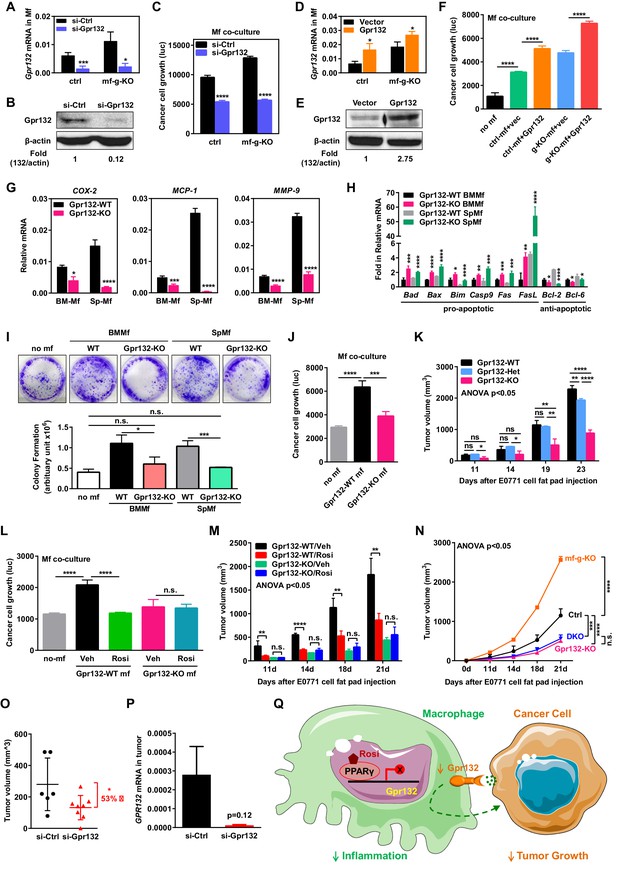
Macrophage Gpr132 promotes tumor growth and mediates the anti-tumor effect of rosiglitazone.
(A–B) Gpr132 knockdown decreased Gpr132 mRNA (A) and protein (B) in macrophages (n = 3). (C) In co-cultures, Gpr132 knockdown in macrophages reduced cancer cell growth (n = 3). (D–E) Gpr132 over-expression increased both mRNA (D) and protein (E) in macrophages (n = 3). (F) In co-cultures, Gpr132 over-expression in macrophages enhanced cancer cell growth (n = 3). Cancer cell alone without macrophages (no mf) served as a negative control. (G) Gpr132-KO macrophages exhibited lower expression of pro-inflammatory genes compared with WT controls (n = 3). (H) Gpr132-KO macrophages displayed higher levels of pro-apoptotic genes and lower levels of anti-apoptotic genes (n = 3). (I–J) In vitro co-cultures showed that Gpr132 deletion in macrophages significantly reduced the ability of macrophages to promote cancer cell colony formation (I) and proliferation (J) (n = 3). (K) In vivo mammary fat pad tumor grafts showed that tumor growth was significantly diminished in Gpr132-KO mice compared with WT or Gpr132-Het mice (n = 6). (L) In in vitro co-cultures, Rosi pre-treated WT macrophages but not Rosi pre-treated Gpr132-KO macrophages were able to inhibit cancer cell growth (n = 3). (M) The ability of Rosi to suppress tumor growth in vivo was abolished in Gpr132-KO mice (n = 6). Four days after EO771 cell mammary fat pad injection, Gpr132-KO or WT mice were treated with Veh or Rosi (10 mg/kg) every two days. (N) The ability of macrophage PPARγ deletion to exacerbate tumor growth in vivo was abolished in Gpr132-KO mice (n = 4). DKO, mf-g/Gpr132 double KO. (O–P) Pharmacological Gpr132 inhibition impeded mammary tumor growth. Female mice (six-week-old) were treated with si-Gpr132 (n = 8) or si-Ctrl (n = 6) for 18 days via intravenous injection at 10 μg/mouse twice/week, three days before and 15 days after EO771 cell mammary fat pad injection. (O) Tumor volume was significantly decreased by si-Gpr132 treatment. (P) Gpr132 expression in tumors was effectively depleted. Error bars, SD; *p<0.05; **p<0.01; ***p<0.005; ****p<0.001; n.s. non-significant. (Q) A simplified working model for how macrophage PPARγ inhibits inflammation and tumor growth by repressing the transcription of macrophage Gpr132, a novel pro-inflammatory and pro-tumor membrane receptor. Upon sensing and activation by tumor signals, macrophage Gpr132 may modulate macrophage intracellular signaling and downstream targets, which in turn promotes cancer cell proliferation (indicated by the dashed line). Macrophage PPARγ deficiency increases Gpr132 level to afford better tumor sensing by macrophages, thereby promoting tumor growth. Pharmacological Gpr132 inhibition via either PPARγ agonist or Gpr132 blockade attenuates breast cancer progression. Moreover, both macrophage PPARγ and Gpr132 are key mediators of the anti-tumor effects of the clinically used TZD drug rosiglitazone.
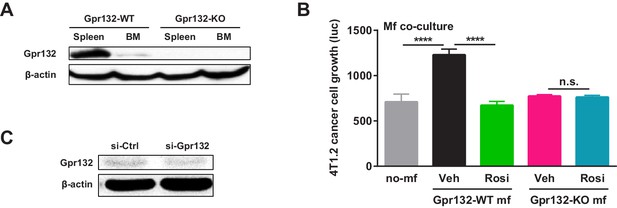
Additional analysis of Gpr132.
(A) Western blot of Gpr132 protein in spleen and bone marrow (BM) from WT and Gpr132-KO mice. (B) Gpr132-null macrophages were refractory to the anti-tumor effects of rosiglitazone pre-treatment. Rosiglitazone pre-treatment of WT macrophages, but not Gpr132-KO macrophages, inhibited the growth of luciferase-labeled 4T1.2 mouse mammary tumor cells in the co-cultures (n = 3). Error bars, SD. (C) Western blot of tumors from mice treated with si-Gpr132 or si-Ctrl showed that si-Gpr132 conferred ~62% knockdown.





