Loss of MeCP2 disrupts cell autonomous and autocrine BDNF signaling in mouse glutamatergic neurons
Figures
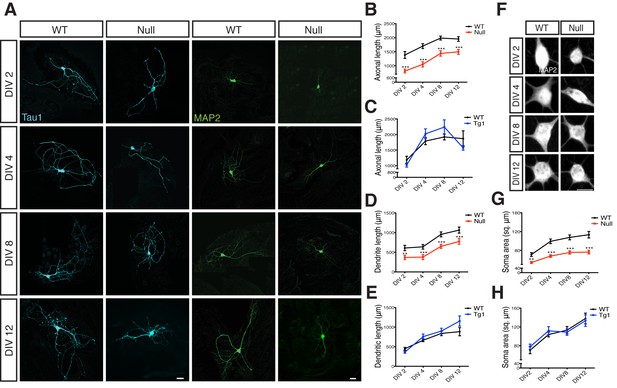
Loss of MeCP2 alters neurite outgrowth and neuronal soma size.
(A) Co-immunostaining of glutamatergic hippocampal neurons from WT and Mecp2Null/y mice at DIV 2, 4, 8 and 12 for Tau1 (cyan) and MAP2 (green). Scale bars represent 20 µm. (B–E) Mean axonal length and dendritic length measured at different time points for Mecp2Null/y (B,D) and Mecp2Tg1 neurons (C,E). (F) Somatic labeling of glutamatergic hippocampal neurons from WT and Mecp2Null/y mice at DIV 2, 4, 8 and 12 with MAP2. Scale bar represents 20 µm. (G,H) Mean soma area measured at different time points for Mecp2Null/y (G) and Mecp2Tg1 neurons (H). Data shown as mean ± SEM. **p<0.01; ***p<0.001.
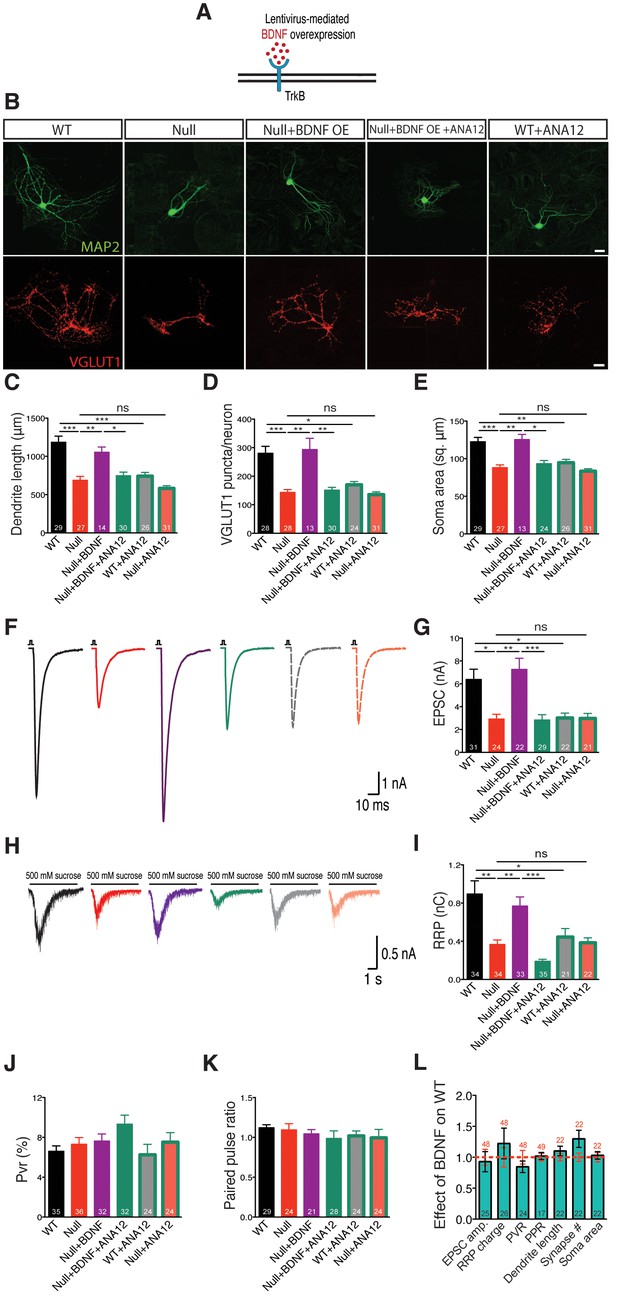
BDNF overexpression in Mecp2Null/y autaptic neurons normalizes cell morphology and restores synaptic output.
(A) Experimental scheme of lentivirus-mediated BDNF overexpression in Mecp2Null/yneurons, showing neuronal membrane (black), BDNF (red) and TrkB receptor (blue). (B) Representative images of neuronal morphology under the following conditions (from left to right): WT, Null, Null + BDNF, Null + BDNF + ANA12, WT + ANA12. Dendritic outgrowth (top) and glutamatergic synapses (bottom) indicated by MAP2 (green) and VGLUT1 (red) labeling. Scale bars represent 20 µm. (C–E) Bar graphs show mean dendrite length (C), glutamatergic synapse number (D) and neuronal soma area (E). (F) Representative traces of evoked EPSCs recorded from autapses under the following conditions: WT, Null, Null + BDNF, Null + BDNF + ANA12, WT + ANA12, Null + ANA12. (G) Bar graph shows mean evoked EPSC amplitude. (H) Representative traces of average postsynaptic response to 5 s application of 500 mM sucrose. Experimental groups same as (F). (J–K) Bar graphs show mean RRP size (I), vesicular release probability Pvr (J) and paired-pulse ratio with 25 ms inter-stimulus interval (K). (L) Bar graph shows EPSC amplitude, RRP size and Pvr, PPR and dendrite length, glutamatergic synapse number and soma area, normalized to WT (dashed red line). Number of neurons (n) shown in the bars for all graphs. Data shown as mean ± SEM. *p<0.05; **p<0.01; ***p<0.001; ns: not significant.
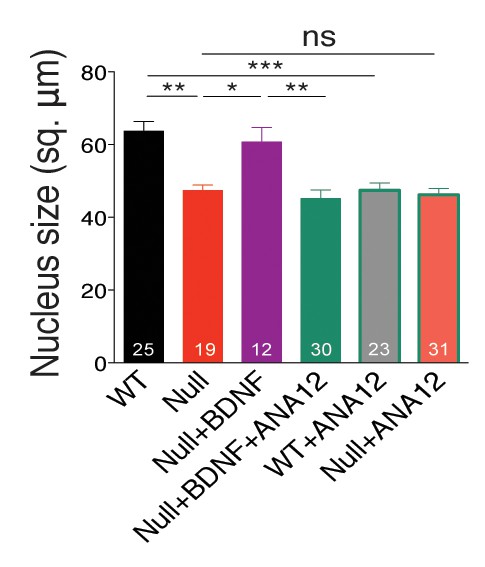
BDNF overexpression in Mecp2Null/y autaptic neurons normalizes nuclei size.
Bar graph shows mean neuronal nuclei size. Number of neurons (n) shown in the bars for all graphs. Data shown as mean ± SEM. *p<0.05; **p<0.01; ***p<0.001; ns: not significant.
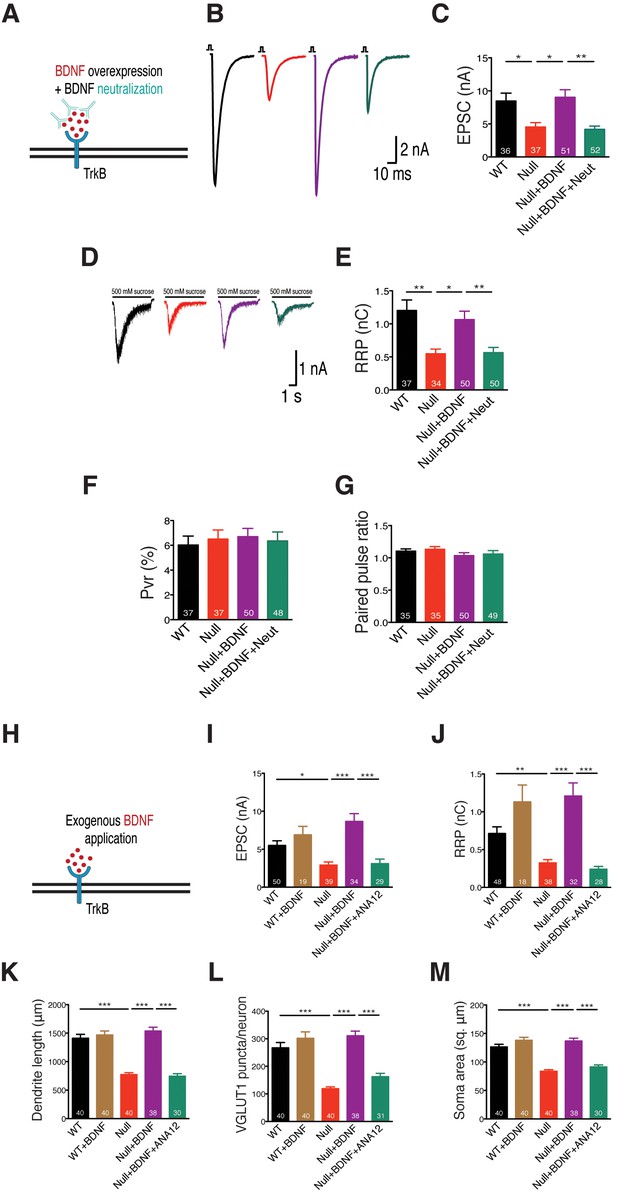
BDNF neutralization fails to rescue physiological phenotypes while exogenous application of BDNF restores physiological and morphological phenotypes, reaffirming specificity of BDNF-TrkB interaction in Mecp2Null/y glutamatergic neurons.
(A) Experimental scheme of lentivirus-mediated BDNF overexpression and BDNF neutralization in Mecp2Null/yneurons, showing neuronal membrane (black), BDNF (red), TrkB receptor (blue) and BDNF neutralizing antibody (cyan). (B) Representative traces of evoked EPSCs recorded from autapses under the following conditions: WT, Null, Null + BDNF, Null + BDNF + neutralization. (C) Bar graph shows mean evoked EPSC amplitude. (D) Representative traces of average current response to 5 s application of 500 mM sucrose. Experimental groups same as (B). (E–G) Bar graphs show mean RRP size (E), Pvr (F) and 25 ms ISI – PPR (G). (H) Experimental scheme depicting exogenous application of BDNF in Mecp2Null/y neurons, showing neuronal membrane (black), BDNF (red) and TrkB receptor (blue). (I–M) Bar graphs show mean evoked EPSC amplitude (I), RRP size (J), dendrite length (K), glutamatergic synapse number (L) and neuronal soma area (M). Number of neurons (n) shown in the bars for all graphs. Data shown as mean ± SEM. *p<0.05; **p<0.01; ***p<0.001; ns: not significant.
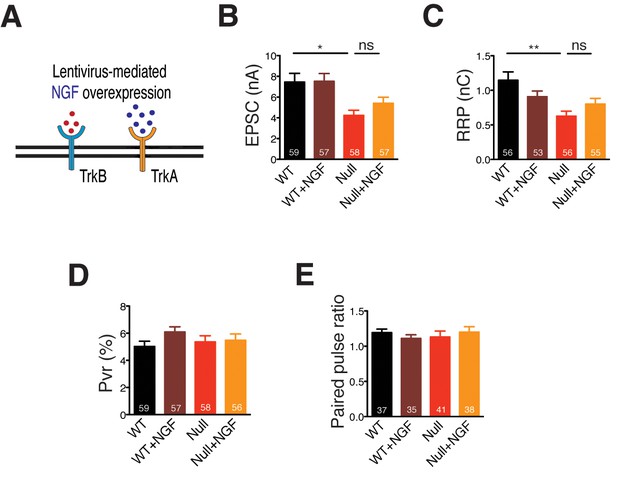
NGF overexpression in Mecp2Null/y neurons does not rescue glutamatergic synaptic output.
(A) Experimental scheme of lentivirus-mediated NGF overexpression in Mecp2Null/y neurons, showing neuronal membrane (black), BDNF (red), TrkB receptor (blue), NGF (dark blue) and TrkA receptor (yellow). (B–E) Bar graphs show mean evoked EPSC amplitude (B), RRP size (C), Pvr (D) and 25 ms ISI – PPR (E). Number of neurons (n) shown in the bars for all graphs. Data shown as mean ± SEM. *p<0.05; **p<0.01; ns: not significant.
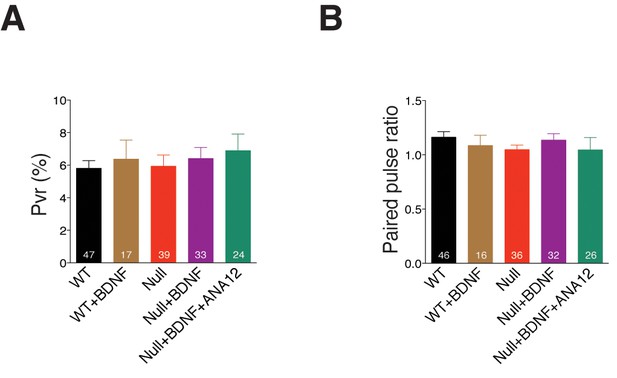
Exogenous application of BDNF does not alter release efficiency or short-term plasticity in Mecp2Null/y hippocampal neurons.
(A,B) Bar graphs show mean Pvr (A) and 25 ms ISI – PPR (B). Number of neurons (n) shown in the bars for all graphs. Data shown as mean ± SEM.
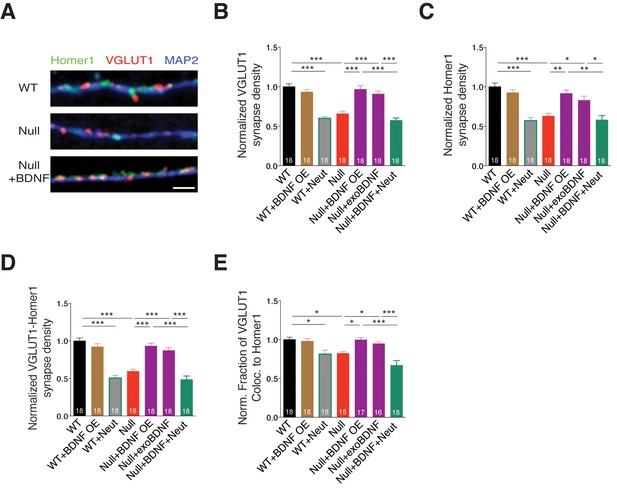
BDNF overexpression in Mecp2Null/y glutamatergic neurons restores synapse density.
(A) Representative images of WT and Mecp2Null/y neurons labeled with MAP2, VGLUT1 and Homer1 under the following conditions (from top to bottom): WT, Null, Null + BDNF. Scale bar represents 3 µm. (B–E) Bar graphs show mean normalized VGLUT1 synapse density (B), Homer1 synapse density (C), colocalized VGLUT1-Homer1 synapse density (D), and normalized fraction of VGLUT1 puncta colocalized to Homer1 (E). Number of neurons (n) shown in the bars for all graphs. Data shown as mean ± SEM. *p<0.05; **p<0.01; ***p<0.001.
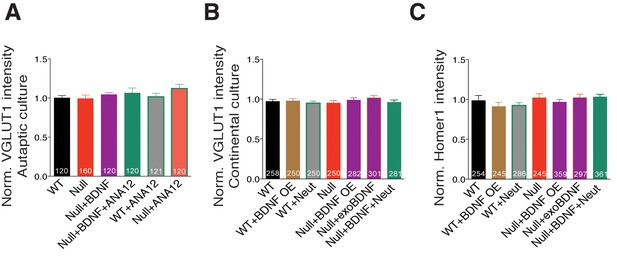
BDNF overexpression in Mecp2Null/y glutamatergic neurons does not alter expression levels of synaptic markers.
(A–C) Bar graphs show mean normalized VGLUT1 fluorescence intensities in autaptic (A) and continental WT and Mecp2Null/y neurons (B), and Homer1 fluorescence intensities (C). Number of synaptic puncta (n) shown in the bars for all graphs. Data shown as mean ± SEM.
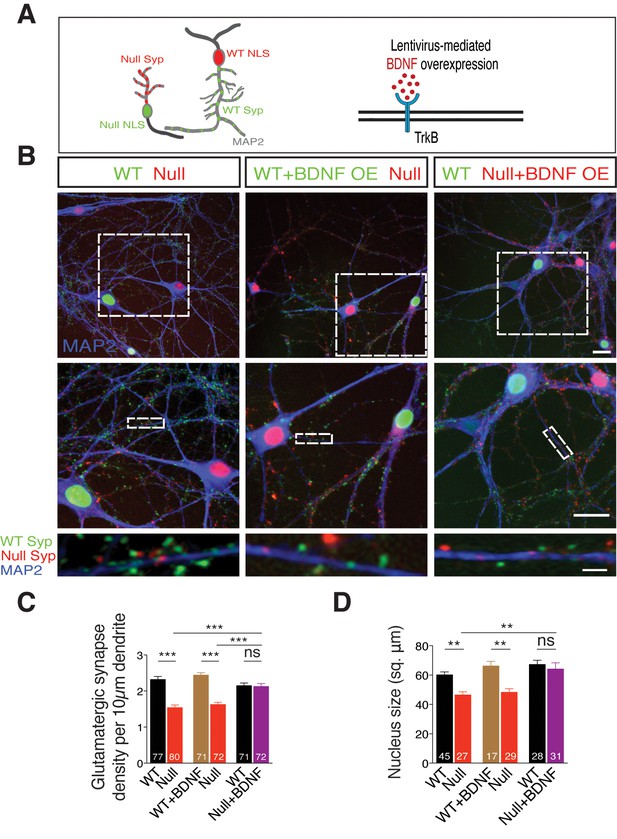
TrkB activation specifically in Mecp2Null/y neurons in an in vitro RTT model normalizes glutamatergic synapse number in a cell autonomous and autocrine manner.
(A) Left panel: Neuronal pair depicting lentivirus-mediated labeling of nucleus (NLS) and synapses of WT (NLS: red, Synaptophysin: green) and Mecp2Null/y neurons (NLS: green, Synaptophysin: red), co-stained for MAP2 to identify synapses localized on dendrites. Right panel: Experimental scheme of lentivirus-mediated BDNF overexpression in WT or Mecp2Null/y neurons, showing neuronal membrane (black), BDNF (red) and TrkB receptor (blue). (B) Representative images of co-cultured WT and Mecp2Null/y neurons labeled with MAP2 under the following conditions (from left to right): WT/Null; WT+BDNF/Null and WT/Null+BDNF. Bottom panel shows WT (green) and Null (red) Synaptophysin+ synapses localized on a single dendrite. Scale bars on top and middle panel represent 20 µm. Scale bar on bottom panel represents 3 µm. (C,D) Bar graphs show mean glutamatergic synapse density (C) and nucleus size (D) for all co-cultured groups. Number of neurons (n) shown in the bars for all graphs. Data shown as mean ± SEM. **p<0.01; ***p<0.001; ****p<0.0001; ns: not significant.
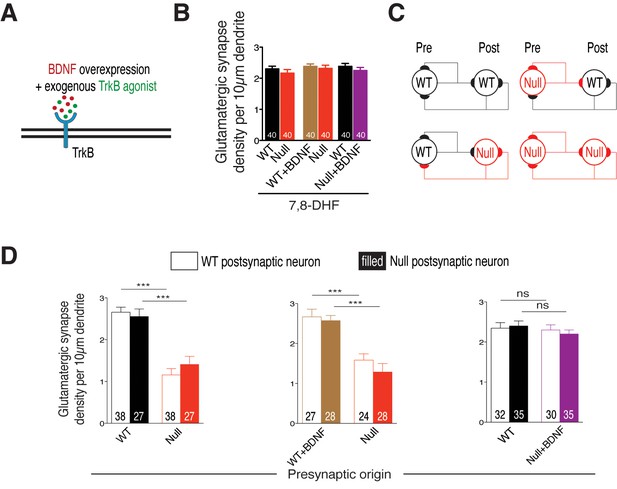
Cell autonomous BDNF-TrkB signaling regulates glutamatergic synapse number by functioning as a presynaptic rate-limiting factor.
(A) Experimental scheme of lentivirus-mediated BDNF overexpression and exogenous application of TrkB agonist in WT or Mecp2Null/y neurons, showing neuronal membrane (black), BDNF (red), TrkB receptor (blue) and TrkB agonist (green). (B) Bar graph shows glutamatergic synapse density for all co-cultured groups upon application of TrkB agonist, 7,8-DHF. (C) Example two-neuron scheme illustrating pre- and postsynaptic neurons (WT or Mecp2Null/y) forming synapses onto itself or onto the partner neuron, in a mixed WT/Null co-culture system. (D) Bar graphs show glutamatergic synapse density measured from a postsynaptic WT (clear bars) and Null (filled bars) neuron for all experimental groups. Number of neurons (n) shown in the bars for all graphs. Data shown as mean ± SEM. ***p<0.001; ns: not significant.
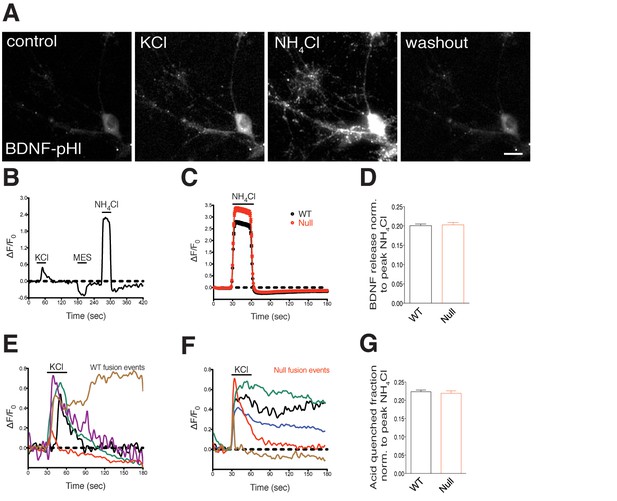
WT and Mecp2Null/y hippocampal neurons reveal transient and persistent fusion events upon stimulation, show equivalent activity-dependent BDNF secretion and membrane-resident BDNF-SpH fraction.
(A) Representative images of a WT hippocampal neuron expressing BDNF-superecliptic pHluorin perfused with standard extracellular solution, 60 mM KCl, 50 mM NH4Cl and washout with extracellular solution (left to right). Scale bar represents 10 µm. (B) BDNF fluorescence (ΔF/F0) over time reflecting fusion events upon application of KCl, MES and NH4Cl solutions. Black line indicates duration of KCl, MES and NH4Cl application. (C) Average of BDNF fluorescence (ΔF/F0) after NH4Cl application (WT, n = 1521 events, Null, n = 948 events). (D) Bar graph shows activity-dependent BDNF release of WT (1464 events) and Mecp2Null/y neurons (895 events). (E,F) Representative traces of BDNF fluorescence (ΔF/F0) indicating transient and persistent fusion events upon KCl-induced membrane depolarization in WT (E) and Mecp2Null/y neurons (F). (G) Bar graph shows BDNF-SpH fraction in WT and Mecp2Null/y neurons indicating surface accumulation of BDNF upon acid wash (MES, pH 5.5; WT, 1480 events; Mecp2Null/y, 900 events). Data shown as mean ± SEM.
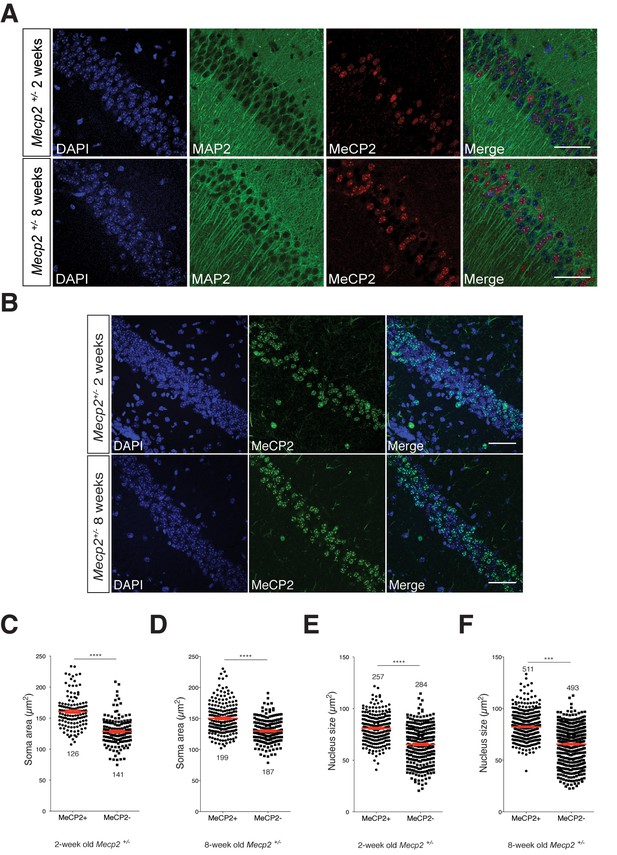
MeCP2-deficient hippocampal CA1 neurons have smaller somata and nuclei in Mecp2+/- heterozygous mice in vivo.
(A) Representative images of hippocampal CA1 from Mecp2+/- mice labeled for DAPI (blue), MAP2 (green) and MeCP2 (red) at two- (top) and eight-weeks of age (bottom). Scale bar represents 50 µm. (B) Representative images of hippocampal CA1 from Mecp2+/- mice labeled for DAPI (blue) and MeCP2 (green) at two- (top) and eight-weeks of age (bottom). Scale bar represents 50 µm. (C–F) Scatter plots show mean soma area and nucleus size of MeCP2+ and MeCP2- neurons at two- (C,E) and eight-weeks of age (D,F). Number of neurons (n) shown in all graphs. Data shown as mean ± SEM. ***p<0.001; ****p<0.0001.
Additional files
-
Supplementary file 1
Detailed statistical data.
- https://doi.org/10.7554/eLife.19374.015





