Local chromosome context is a major determinant of crossover pathway biochemistry during budding yeast meiosis
Figures
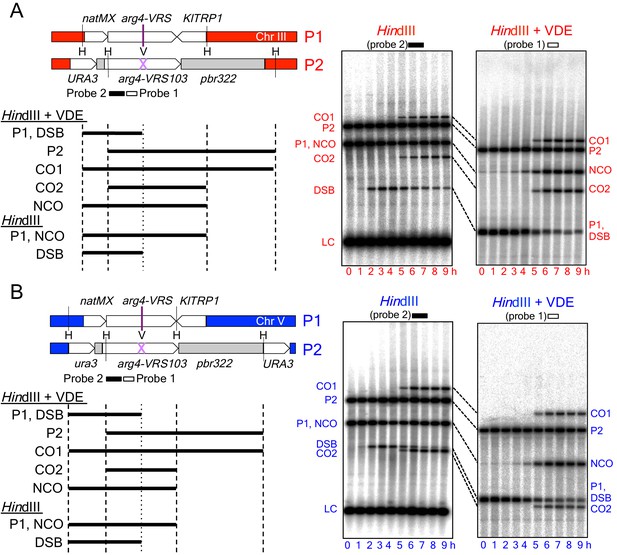
Inserts used to monitor VDE-initiated meiotic recombination.
The HIS4 and URA3 loci are denoted throughout this paper in red and blue, respectively, and are in Red1/Hop1 enriched and depleted regions, respectively (see Figure 4A and Figure 4—figure supplement 1, below). (A) Left—map of VDE-reporter inserts at HIS4, showing digests used to detect recombination intermediates and products. One parent (P1) contains ARG4 sequences with a VDE-recognition site (arg4-VRS), flanked by an nourseothricin-resistance module [natMX, (Goldstein and McCusker, 1999)] and the Kluyveromyces lactis TRP1 gene [KlTRP1, (Stark and Milner, 1989)]; the other parent (P2) contains ARG4 sequences with a mutant, uncuttable VRS site [arg4-VRS103, (Nogami et al., 2002) flanked by URA3 and pBR322 sequences. Digestion with HindIII (H) and VDE (V) allows detection of crossovers (CO1 and CO2) and noncrossovers (NCO); digestion with HindIII alone allows detection of crossovers and DSBs. P2, CO1 and CO2 fragments are drawn only once, as they are the same size in HindIII digests as in HindIII + VDE digests. Right—representative Southern blots. HindIII-alone digests are probed with a fragment (probe 2) that hybridizes to the insert loci and to the native ARG4 locus on chromosome VIII; this latter signal serves as a loading control (LC). Times after induction of meiosis that each sample was taken are indicated below each lane. (B) map of VDE-reporter inserts at URA3 and representative Southern blots; details as in (A). Strain, insert and probe details are given in Materials and methods and Supplementary file 1.
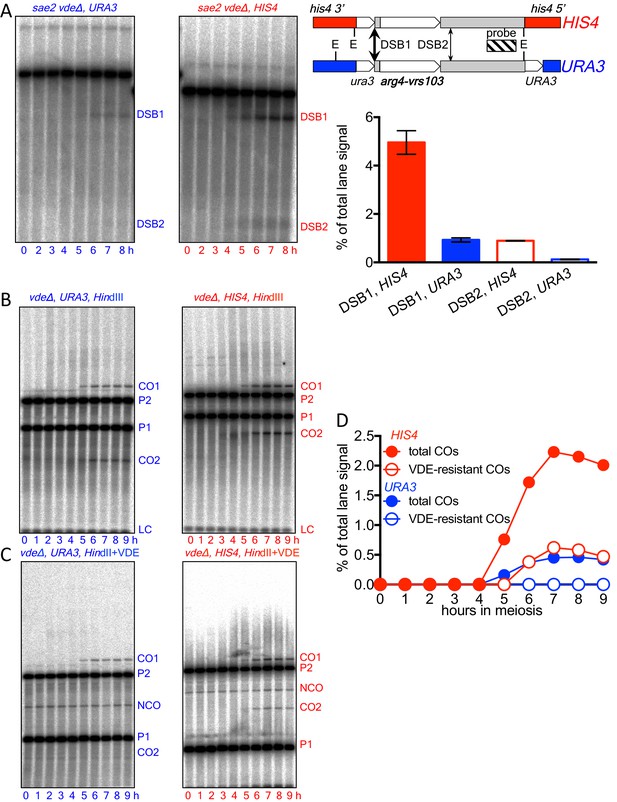
Spo11-initiated events at the two insert loci.
(A) Spo11-catalyzed DSBs are more frequent at HIS4 that at URA3. Left—Southern blots of EcoRI digests of DNA from vde∆ strains, probed with pBR322 sequences, showing Spo11-DSBs in the Parent 2 insert (see Figure 1) in resection/repair-deficient sae2∆ mutant strains. Right—location of DSBs and probe and DSB frequencies (average of 7 and 8 hr samples from a single experiment; error bars represent range). Spo11-DSBs in the Parent 1 inserts at HIS4 and URA3 were at different locations within the insert, but displayed similar ratios between the two loci (data not shown). (B) Southern blots of HindIII digests of DNA from vde∆ strains, to detect total Spo11-initiated crossovers. (C) Southern blots of HindIII-VDE double digests of the same samples, to determine the background contribution of Spo11-initiated COs in subsequent experiments measuring VDE-initiated COs, which will be VDE-resistant due to conversion of the VRS site to VRS103. Probes were as shown in Figure 1. (D) Quantification of data in panels B (total COs; filled circles) and C (VDE-resistant COs; open circles). Data are from a single experiment.
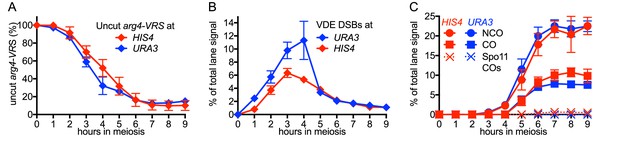
VDE-initiated recombination occurs at similar levels at the two insert loci.
(A) Cumulative DSB levels are similar at the two insert loci. The fraction of uncut VRS-containing chromosomes (Parent 1) was determined by subtracting the amount of the NCO band in HindIII + VDE digests from the amount of the Parent 1 + NCO band in HindIII digests. (B) Non-cumulative VDE-DSB frequencies, measured as fraction of total lane signal, excluding loading controls, in HindIII digests. (C) Crossover (average of CO1 and CO2) and noncrossover frequencies, measured in HindIII-VDE digests. Solid lines—recombinants from cells expressing VDE; dashed lines—Spo11-initiated crossovers from vde- strains, measured in HindIII-VDE digests and thus corresponding to VDE-resistant products (see also Figure 1—figure supplement 1C). Values are the average of two independent experiments; error bars represent range. Representative Southern blots are shown in Figure 1 and Figure 1—figure supplement 1C.
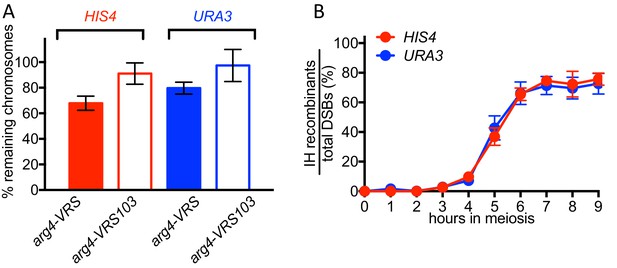
70–80% of VDE-DSBs are repaired.
(A) Fraction of inserts remaining, calculated using HindIII digests (see Figure 1). For the arg4-VRS103 insert, the ratio (Parent 2 + CO2)/ (0.5 x LC) was calculated at 9 hr, and was then normalized to the 0 hr value. For the arg4-VRS insert, a similar calculation was made: (Parent 1 + NCO + CO1)/(0.5 x LC) (B) Relative recovery of interhomolog recombination products, calculated using HindIII-VDE double digests (see Figure 1). The sum of CO (average of CO1 and CO2) and NCO frequencies was divided by the frequency of total DSBs, as calculated in Figure 2A. Data are the average of two independent experiments; error bars represent range.
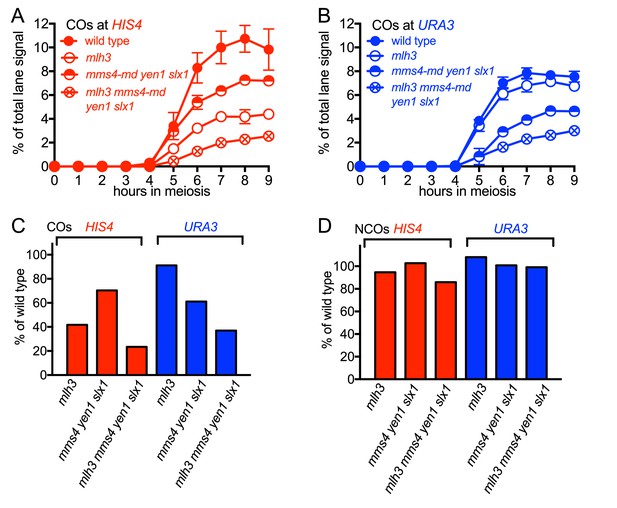
Different resolvase-dependence of crossover formation at the two insert loci.
(A) Crossover frequencies (average of CO1 and CO2) measured as in Figure 2C from HIS4 insert-containing mutants lacking MutLγ (mlh3), structure-selective nucleases (mms4-md yen1 slx1) or both resolvase activities (mlh3 mms4-md yen1 slx1). (B) Crossover frequencies in URA3 insert-containing strains, measured as in panel A. Values are the average of two independent experiments; error bars represent range. (C) Final crossover levels (average of 8 and 9 hr values for two independent experiments), expressed as percent of wild type. Note that, in mlh3 mutants, crossovers in HIS4 inserts are reduced by nearly 60%, while crossovers in URA3 inserts are reduced by less than 10%. (D) Final noncrossover levels, calculated as in C, expressed as percent of wild type. Representative Southern blots are in Figure 3—figure supplement 2.
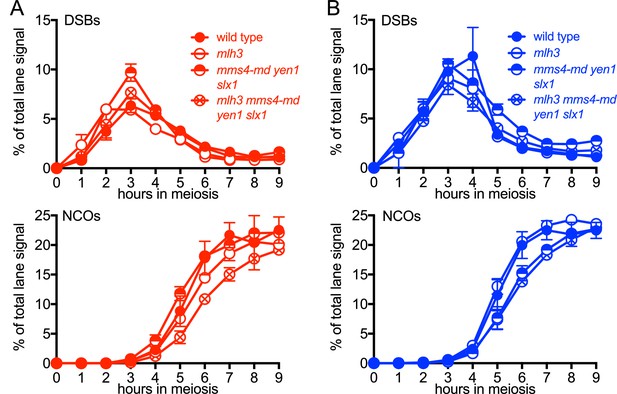
VDE-DSB and NCO frequencies in resolvase mutants.
(A) VDE-DSB frequencies (top), measured as in Figure 2B, and NCO frequencies (bottom), measured as in Figure 2C, from HIS4 insert-containing strains. (B) As panel A, with strains containing inserts at URA3. Data are the average of two independent experiments; error bars represent range. Representative Southern blots are in Figure 3—figure supplement 2.
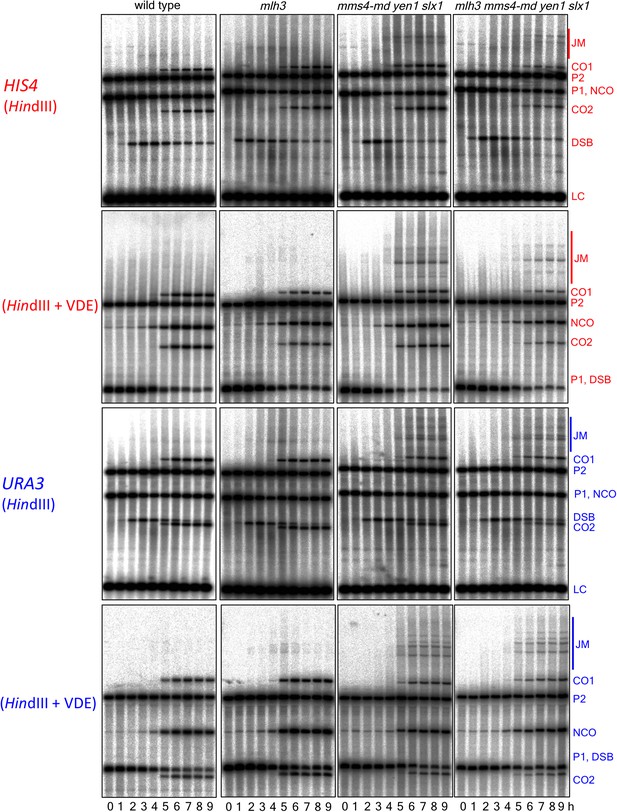
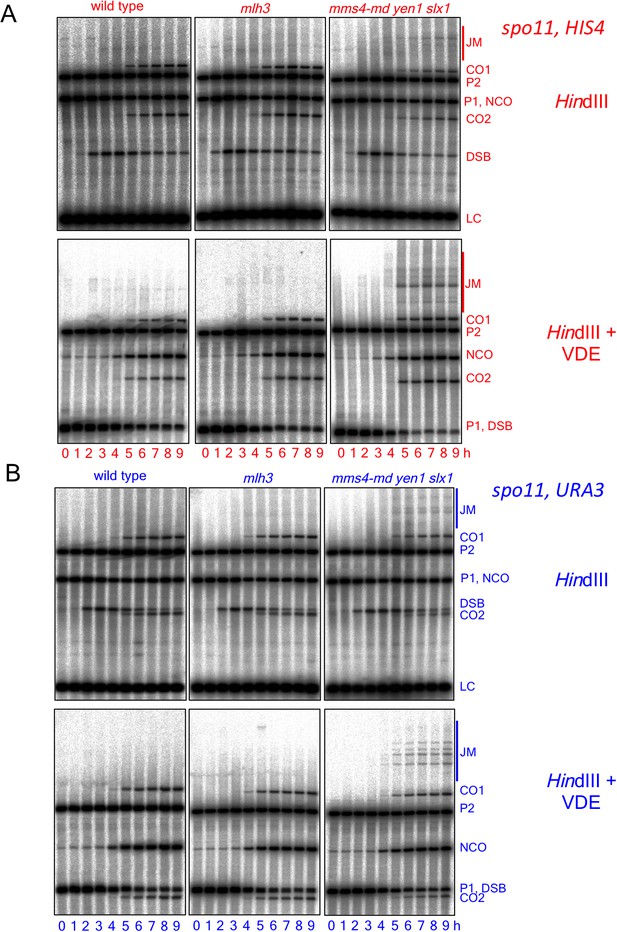
Southern blots of HindIII and HindIII-VDE digests of DNA from HIS4 insert-containing strains (top) and from URA3 insert-contaning strains (bottom).
Probes and gel labels are as in Figure 1; JM—joint molecule recombination intermediates.
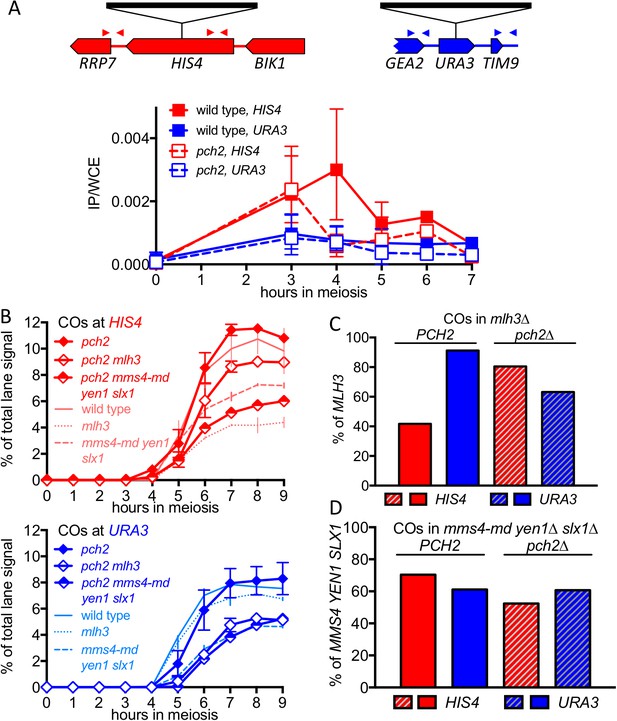
pch2∆ mutants display altered Hop1 occupancy and crossover MutLγ-dependence.
(A) Hop1 occupancy at insert loci, determined by chromatin immunoprecipitation and quantitative PCR. Top—cartoon of insert loci, showing the location of primer pairs used. Bottom—relative Hop1 occupancy, expressed as the average ratio of immunoprecipitate/input extract for both primer pairs (see Materials and methods for details). Values are the average of two independent experiments; error bars represent range. (B) VDE-initiated CO frequencies measured as in Figure 2C at HIS4 (top) and URA3 (bottom) in pch2∆ (solid diamonds), pch2∆ mlh3∆ (open diamonds), and pch2∆ mms4-md yen1 slx1 (half-filled diamonds) mutants. Crossovers from wild type (solid line), mlh3∆ (dotted line) and mms4-md yen1 slx1mutants (dashed line) from Figure 3 are shown for comparison. Values are from two independent experiments; error bars represent range. Representative Southern blots are in Figure 4—figure supplement 2. (C) Extent of CO reduction in mlh3∆ mutants, relative to corresponding MLH3 strains. (D) Extent of CO reduction in mms4-md yen1 slx1 (ssn) mutants, relative to corresponding MMS4 YEN1 SLX1 strains. For both (C) and (D), PCH2 genotype is indicated at the top; values are calculated as in Figure 3C.
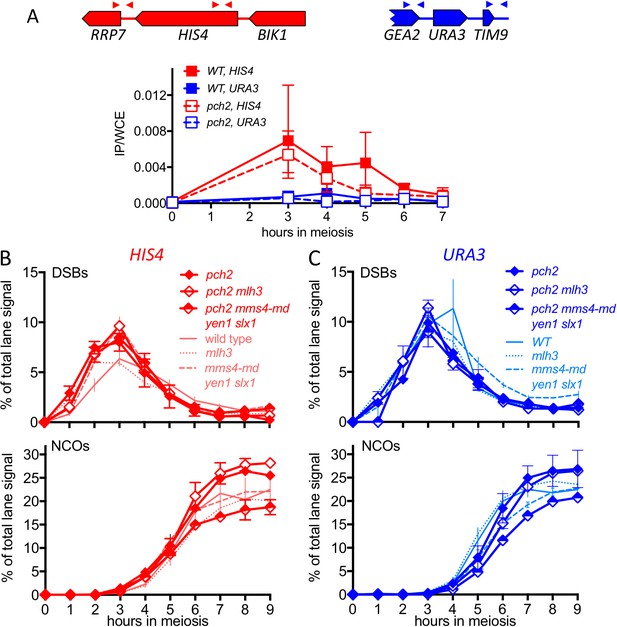
Hop1 occupancy at non-insert loci, DSBs and NCOs in pch2∆ mutants.
(A) Hop1 occupancy at corresponding loci lacking inserts, determined as in Figure 4A. Occupancy at HIS4 is from strains with inserts at URA3, and vice versa. (B) DSBs and NCOs in inserts at HIS4, determined as in Figure 2B and C, respectively. Symbols are as in Figure 4B. (C) DSBs and NCOs in inserts at URA3, details as in panel B. Values are from two independent experiments; error bars represent range. Representative Southern blots are in Figure 4—figure supplement 2.
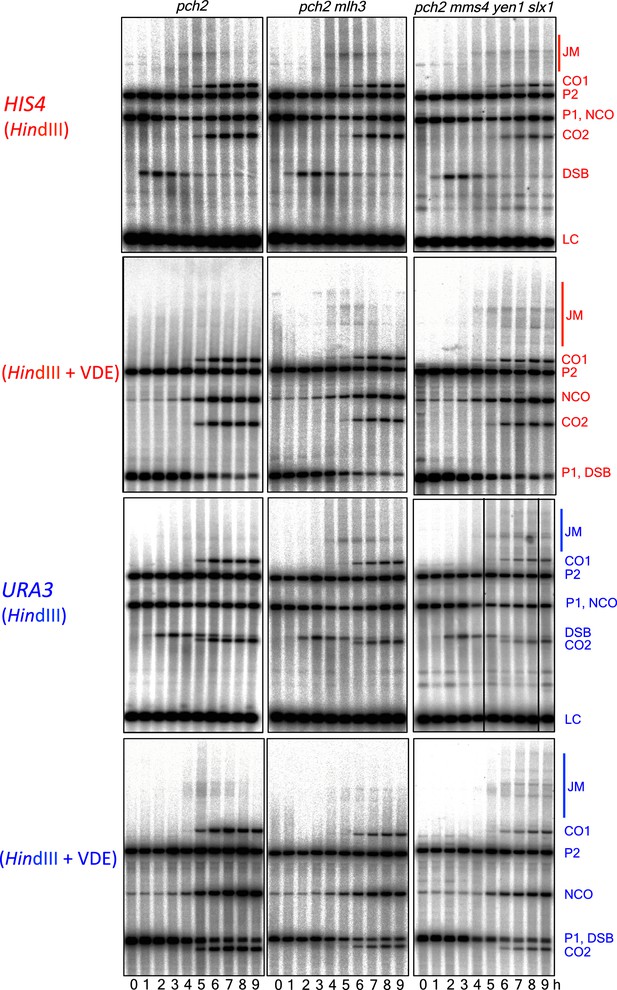
Southern blots of HindIII and HindIII-VDE digests of DNA from HIS4 insert-containing strains (top) and from URA3 insert-contaning strains (bottom).
Gel labels are as in Figure 1; JM—joint molecule recombination intermediates. In the gel with HinDIII digests of samples from a pch2∆ mm4-mn yen1∆ slx1∆ strain with inserts at URA3, the 9 hr sample was originally loaded between the 4 and 5 hr samples; this image was cut and spliced as indicated by vertical lines for presentation purposes.
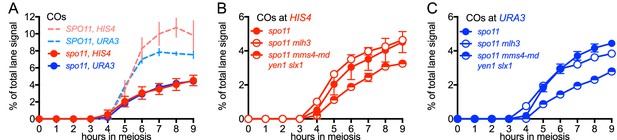
VDE-initiated COs are reduced and are MutLγ-independent in the absence of Spo11 activity.
(A) VDE-initiated crossover frequencies, measured as in Figure 2C in spo11-Y135F strains (dark solid lines) in inserts at HIS4 (red) and at URA3 (blue). Data from the corresponding SPO11 strains (dotted lines, from Figure 2C) are presented for comparison. (B) COs in HIS4 inserts in spo11 strains that are otherwise wild-type (spo11) or lack either Mutlγ or structure-selective nucleases. (C) As in B, but with inserts at URA3. Values are from two independent experiments; error bars represent range. Representative Southern blots are in Figure 5—figure supplement 2.
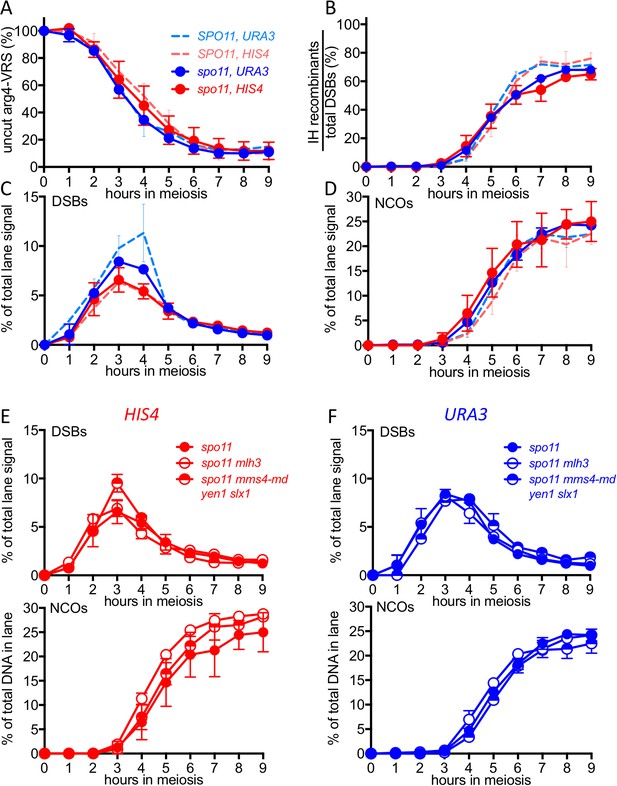
DSBs and recombinant products in spo11 strains.
(A) Cumulative DSB levels, expressed as loss of the VRS-containing insert, calculated as in Figure 2A. (B) Relative recovery of recombination products, calculated as in Figure 2—figure supplement 1B. (C) VDE-DSB frequencies, as in Figure 2B. (D) NCO frequencies, as in Figure 2C. In all four panels, solid lines denote values from spo11 strains; values from wild type (dotted lines, from Figure 2 and Figure 2—figure supplement 1) are presented for comparison. (E) DSB (top) and NCO (bottom) frequencies in spo11-Y135F strains with inserts at HIS4. (F) DSB (top) and NCO (bottom) levels in spo11-Y135F strains with inserts at URA3. For all panels, values are from two independent experiments; error bars represent range. Representative Southern blots are in Figure 5—figure supplement 2.
Southern blots of HindIII and HindIII-VDE digests of DNA from spo11 strains with inserts at HIS4 (top) and at URA3 (bottom).
Gel labels are as in Figure 1; JM—joint molecule recombination intermediates.
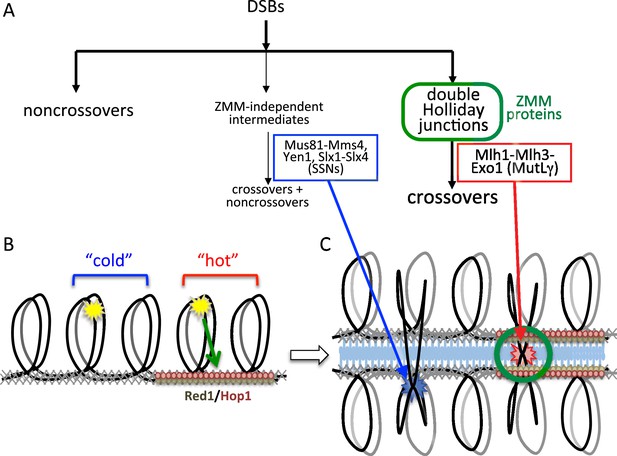
Different resolvase functions in different genome domains.
(A) Early crossover decision model for meiotic recombination (Bishop and Zickler, 2004; Hollingsworth and Brill, 2004) illustrating early noncrossover formation, a major pathway where recombination intermediates form in the context of ZMM proteins and are resolved by MutLγ to form crossovers, and a minor pathway where ZMM-independent intermediates are resolved by SSNs as both crossovers and noncrossovers. (B) Division of the meiotic genome into meiotic axis-protein-enriched 'hot' domains (red) that are enriched for Red1 and Hop1, and 'cold' domains where Red1 and Hop1 are depleted. VDE DSBs (yellow stars) can be directed to form efficiently in either domain, but only VDE DSBs that form in 'hot' domains can be recruited to the meiotic axis. (C) DSBs in 'hot' domains can form joint molecules (red star) in the context of ZMM proteins and the synaptonemal complex, and thus can be resolved by MutLγ-dependent activities. DSBs in 'cold' domains form joint molecules (blue star) outside of this structural context, and are resolved by MutLγ-independent activities.
Additional files
-
Supplementary file 1
Yeast strains.
- https://doi.org/10.7554/eLife.19669.017
-
Supplementary file 2
Primary data for graphs in all figures.
- https://doi.org/10.7554/eLife.19669.018





