Fatty acid analogue N-arachidonoyl taurine restores function of IKs channels with diverse long QT mutations
Abstract
About 300 loss-of-function mutations in the IKs channel have been identified in patients with Long QT syndrome and cardiac arrhythmia. How specific mutations cause arrhythmia is largely unknown and there are no approved IKs channel activators for treatment of these arrhythmias. We find that several Long QT syndrome-associated IKs channel mutations shift channel voltage dependence and accelerate channel closing. Voltage-clamp fluorometry experiments and kinetic modeling suggest that similar mutation-induced alterations in IKs channel currents may be caused by different molecular mechanisms. Finally, we find that the fatty acid analogue N-arachidonoyl taurine restores channel gating of many different mutant channels, even though the mutations are in different domains of the IKs channel and affect the channel by different molecular mechanisms. N-arachidonoyl taurine is therefore an interesting prototype compound that may inspire development of future IKs channel activators to treat Long QT syndrome caused by diverse IKs channel mutations.
https://doi.org/10.7554/eLife.20272.001eLife digest
Every heartbeat relies on an electric wave that travels through the heart. This wave must reach different parts of the heart in a specific sequence to ensure that the heart muscle cells contract in a coordinated manner. Such coordinated contractions enable the heart to pump enough blood around the body. By allowing specific ions to flow into or out of the heart muscle cell, proteins called ion channels in the cell membrane generate the electric wave, keep it going and stop it. One such protein called the IKs channel controls the flow of potassium ions, and in doing so stops the electric wave in heart muscle cells.
About 300 different mutations in the IKs channel have been shown to cause abnormal heart rhythms in individuals with a disorder called long QT syndrome. People with this condition may suddenly black out because their heart develops prolonged electric waves that prevent blood from being pumped properly.
To investigate how mutations in the IKs channel produce heart rhythm abnormalities, Liin et al. genetically engineered the egg cells of African clawed frogs to have one of eight mutant forms of the human IKs channel. Studying these channels revealed that the mutations reduce how well the channels work in a wide variety of ways. However, treating the cells with a particular fatty acid helped to normalize how each of the mutant channels worked. Therefore, variants of the fatty acid could potentially form a useful treatment for people with heart rhythm problems caused by mutations in the IKs channel.
More studies are needed to confirm whether the fatty acid is as effective at combating the effects of the mutations in whole hearts and animals. As ion channels related to the IKs channel are found in many types of cells, it is also important to investigate whether treatment with the fatty acid could cause any side effects that affect other organs.
https://doi.org/10.7554/eLife.20272.002Introduction
Long QT syndrome (LQTS) is a condition of the heart which in most cases is caused by a mutation in cardiac ion channels (Hedley et al., 2009; Morita et al., 2008). In LQTS, the action potential of the heart is prolonged, which is observed as a prolonged QT interval in the electrocardiogram. LQTS patients have an increased risk of developing ventricular tachyarrhythmias called torsades de pointes when exposed to triggers such as adrenergic stress (Morita et al., 2008; Cerrone et al., 2012). These arrhythmias can cause palpitation, syncope or sudden death due to ventricular fibrillation. To improve the clinical outcome of LQTS patients, it is therefore critical to prevent these LQTS-induced life-threatening arrhythmias.
Most mutations causing LQTS are located in the KCNQ1 gene (Hedley et al., 2009). KCNQ1 codes for the potassium channel KV7.1, which in the heart co-assembles with the beta-subunit KCNE1 to form the slowly-activating, voltage-dependent potassium channel IKs (Barhanin et al., 1996; Sanguinetti et al., 1996). The IKs channel provides one of the important delayed rectifier outward potassium currents that repolarizes the cardiomyocyte and terminates the cardiac action potential (Nerbonne and Kass, 2005). Reduced IKs function therefore tends to delay cardiomyocyte repolarization, thereby causing prolonged cardiac action potential durations and a prolonged QT interval. The cardiac IKs channel consists of four KV7.1 subunits and two to four KCNE1 subunits (Nakajo et al., 2010; Plant et al., 2014; Murray et al., 2016). Throughout this work, we will refer to the IKs channel as KV7.1+KCNE1. KV7.1 has six transmembrane segments named S1-S6 (Liin et al., 2015) (Figure 1a). S1-S4 of each KV7.1 subunit forms a voltage-sensing domain where S4 is the voltage sensor with three positive gating charges. S5 and S6 from all four KV7.1 subunits form the pore domain with a putative gate in S6 that needs to move to open the ion-conducting pore of the channel. KCNE1 has a single-transmembrane segment (Figure 1a) and is proposed to be localized in the otherwise lipid-filled space between two voltage-sensing domains of neighbouring KV7.1 subunits (Nakajo and Kubo, 2015). Upon cardiomyocyte depolarization, the voltage sensor of KV7.1 moves outward in relation to the membrane. It has been proposed that this movement of the voltage sensor is transferred to the pore domain via the S4-S5 linker and induces channel opening by moving the S6 gate (Liin et al., 2015).
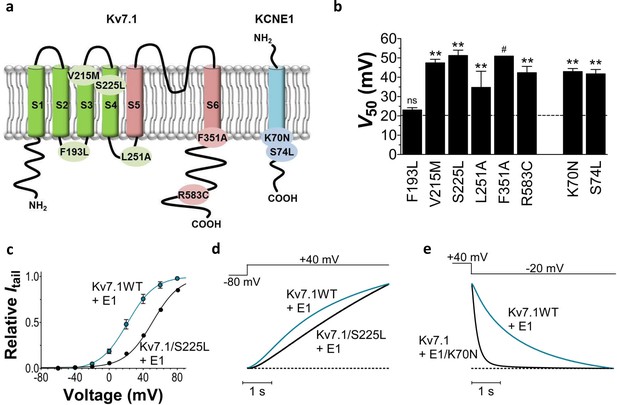
Biophysical properties of LQTS and LQTS-like KV7.1+KCNE1 channel mutants expressed in Xenopus oocytes.
(a) Topology of KV7.1 and KCNE1, and position of tested LQTS and LQTS-like mutants. (b) G(V) midpoints (V50) from the Boltzmann fits for mutants co-expressed with KCNE1. n = 5–11. Data as mean ± SEM. The statistics represent one-way ANOVA with Dunnett’s Multiple Comparison Test to compare the mutants to wild-type KV7.1+KCNE1; **p<0.01; ns is p≥0.05. # denotes lowest estimate. Dashed line denotes wild-type V50. (c) Representative example of KV7.1/S225L+KCNE1 G(V) (black line and symbols) compared to wild-type KV7.1+KCNE1 (blue line and symbols, mean ± SEM, n = 5). (d–e) Representative example of KV7.1/S225L+KCNE1 opening kinetics and KV7.1+KCNE1/K70N closing kinetics (black lines) compared to wild-type KV7.1+KCNE1 (blue lines).
Altogether, about 300 mutations in KCNQ1 and KCNE1 have been identified in patients suffering from LQTS (Hedley et al., 2009) (http://www.fsm.it/cardmoc/). These mutations are distributed throughout the channel sequence and are therefore likely to cause channel dysfunction by different mechanisms, which are, however, largely unknown. Potential mechanisms for KV7.1+KCNE1 channel loss of function by a mutation could, for example, be interference with voltage sensor movement, gate opening, or membrane expression. LQTS is today treated with drugs that prevent the triggering of arrhythmic activity, such as beta-blockers, or with arrhythmia-terminating implantable cardioverter defibrillator (Hedley et al., 2009). A different treatment strategy for LQTS caused by loss-of-function mutations in the KV7.1+KCNE1 channel would be to pharmacologically augment the KV7.1+KCNE1 channel function of these LQTS mutants, thereby shortening the prolonged QT interval and lower the risk of arrhythmia development. However, there is currently no clinically approved KV7.1+KCNE1 channel activator.
In this study, we investigate the biophysical properties and potential mechanism of action of LQTS-associated KV7.1+KCNE1 channel mutations and test the ability of the fatty acid analogue N-arachidonoyl taurine (N-AT) to restore the function of these mutants.
We selected eight mutations of residues mutated in patients with LQTS located in different segments of the KV7.1+KCNE1 channel and that were previously shown to form active channels (Bianchi et al., 2000; Yamaguchi et al., 2003; Eldstrom et al., 2010; Henrion et al., 2009; Yang et al., 2013; Yang et al., 2002; Harmer et al., 2010; Splawski et al., 1997). We measure the movement of the S4 voltage sensor in selected mutants using voltage clamp fluorometry to further our understanding of the molecular mechanisms underlying the defects caused by the diverse mutations. We find that the eight LQTS-associated mutations affect the voltage dependence and/or closing kinetics, in some cases by different molecular mechanisms. Moreover, we find that N-AT restores much of the channel activity in these eight LQTS-associated KV7.1+KCNE1 mutants. This suggests that N-AT may function as a general activator of KV7.1+KCNE1 channels with diverse mutational defects.
Results
LQTS mutants show altered biophysical properties
We first study the biophysical properties of six point mutations in KV7.1 (F193L, V215M, S225L, L251P, F351S, R583C), and two in KCNE1 (K70N, S74L) identified in patients with LQTS (Yamaguchi et al., 2003; Yang et al., 2002; Splawski et al., 1997; Priori et al., 1999; Napolitano et al., 2005; Lai et al., 2005) (Figure 1a). As L251P and F351S did not produce functional channels (Napolitano et al., 2005; Deschenes et al., 2003) (Figure 1—figure supplement 1), we engineered the milder L251A and F351A mutants instead. L251A and F351A will be referred to as 'LQTS-like mutants'. When expressed alone in Xenopus oocytes, all investigated KV7.1 mutants, except F193L and V215M, display a shifted conductance versus voltage curve (G(V)) compared to the wild-type KV7.1 channel (Figure 1—figure supplement 2; Supplementary file 1). S225L, L251A and F351A shift the G(V) towards positive voltages compared to wild-type KV7.1. In contrast, R583C shifts the half-maximal activation, V50, ~10 mV towards negative voltages compared to wild-type KV7.1. This apparent negative shift is likely caused by the pronounced inactivation of the R583C mutant (Figure 1—figure supplement 3a), which is seen to a considerable smaller extent in the other KV7.1 mutants and wild-type KV7.1 (inset in Figure 1—figure supplement 3a). When a fraction of the channels are released from inactivation, by introducing a brief hyperpolarizing pulse between the test pulse and the tail pulse, R583C has a V50 fairly comparable to wild-type KV7.1 (Figure 1—figure supplement 3b).
When the KV7.1 mutants are co-expressed with KCNE1, all KV7.1 and KCNE1 mutants except KV7.1/F193L+KCNE1 have a G(V) that is shifted towards positive voltages compared to the wild-type KV7.1+KCNE1 channel (Figure 1b). KV7.1/F351A causes the most dramatic change by shifting V50 more than +30 mV. We are therefore only able to record the foot of the G(V) curve of KV7.1/F351A+KCNE1, and a shift in V50 of +30 mV is a lower estimate of the change in V50 (ΔV50). One of the other mutants with dramatically shifted G(V) is KV7.1/S225L+KCNE1. V50 for KV7.1/S225L+KCNE1 is shifted almost +30 mV compared to wild-type KV7.1+KCNE1 (Figure 1c; Supplementary file 1). S225L also slows down KV7.1+KCNE1 channel opening kinetics (p<0.01; Figure 1d; Supplementary file 1). All mutations, except for L251A, accelerate channel closing kinetics compared to wild-type KV7.1+KCNE1 (Supplementary file 1). K70N has the most dramatic effect on KV7.1+KCNE1 channel closing by accelerating the closing kinetics by approximately a factor of 5 (Figure 1e; Supplementary file 1). When comparing the amplitude of K+ currents generated by these mutants with the current amplitude of the wild-type KV7.1+KCNE1 channel in the same batch of oocytes, we note that all mutants generate smaller currents than wild-type over a large voltage range (Figure 1—figure supplement 4). Although defective trafficking may contribute to these reduced currents in Xenopus oocytes, the current amplitudes for most mutants matches fairly well with the predicted current amplitude from channels with G(V) curves shifted towards positive voltages as observed for these mutants (Figure 1—figure supplement 4a), suggesting that the reduced current amplitudes in Xenopus oocytes are mainly a result of gating defects (and not trafficking defects).
To summarize, all mutations change channel function by altering the voltage dependence of opening and/or the kinetics of opening and/or closing. Reduced function of the KV7.1+KCNE1 channel induced by these LQTS and LQTS-like mutations may largely be explained by the right-shifted G(V) and the faster closing kinetics caused by these mutations. F193L does not alter the G(V), but speeds up KV7.1+KCNE1 channel closing by a factor of 2 (Supplementary file 1). These results are consistent with previous reported findings for some of these mutants (Bianchi et al., 2000; Yamaguchi et al., 2003; Eldstrom et al., 2010; Henrion et al., 2009; Yang et al., 2013; Yang et al., 2002; Harmer et al., 2010).
Heterozygous expression reduces LQTS mutant severity
Patients with LQTS mutations can be either homozygous or heterozygous for the mutation. To mimic heterozygous expression, we co-inject the mutated KV7.1 subunit and KCNE1 subunit together with the wild-type KV7.1 subunit (or wild-type KCNE1 subunit for KCNE1 mutants) (cartoon in Figure 2). We refer to this as heterozygous expression. Figure 2a–b compares the homozygous expression (KV7.1wt+KCNE1mut or KV7.1mut+KCNE1wt) with heterozygous expression (KV7.1wt+KV7.1mut+KCNE1wt or KV7.1wt+KCNE1wt+KCNE1mut) for KV7.1/S225L (Figure 2a) and KCNE1/K70N (Figure 2b). Both of these examples show that heterozygous expression generates channels with more wild-type like opening or closing kinetics and G(V) compared to homozygous expression of the mutant subunit. A milder biophysical phenotype upon heterozygous expression is generally seen for the LQTS and LQTS-like mutants in terms of G(V), current amplitude, and/or closing kinetics (Figure 2c–d, Figure 1—figure supplement 4, Supplementary file 2). This milder phenotype indicates that the wild-type subunit can partly restore KV7.1+KCNE1 function. Alternatively, for mutants with a G(V) that is very shifted to positive voltages (e.g. F351A), it may be that channel complexes that contain the mutated subunits are largely out of the physiological voltage range and therefore do not contribute substantially to the recorded current. Also, for mutants with low membrane expression (e.g. possibly F193L [Yamaguchi et al., 2003]), it may be that channels containing the wild-type subunit are favoured so that in most KV7.1+KCNE1 channel complexes the majority (or all) of the subunits will be wild-type subunits.
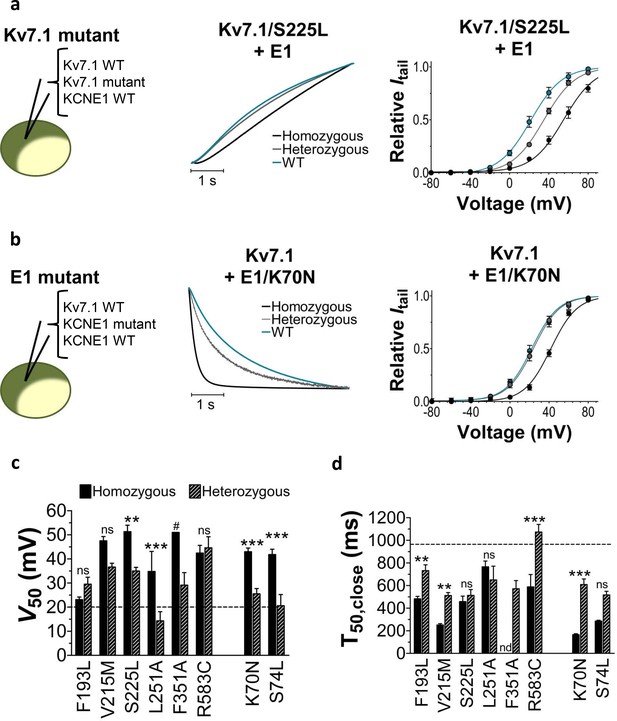
Comparison of homozygous and heterozygous expression of LQTS and LQTS-like mutants.
(a–b) Representative example of kinetics (middle panel) and G(V) (right panel) for homozygous expression and heterozygous expression of S225L (a) and K70N (b). Currents in response to steps from –80 mV to +40 mV (a, middle pane) and from +40 mV to –20 mV (b, middle panel). Homozygous expression (black), heterozygous expression (gray), and KV7.1+KCNE1 wild-type (blue). n = 7–13. (c–d) Summary of V50 (c) and T50 for closing (d) for homozygous and heterozygous expression. Data as mean ± SEM. n = 5–13. The statistics represent one-way ANOVA with pair-wise Bonferroni’s Test to compare homozygous and heterozygous expression; **p<0.01; ***p<0.001; ns is p≥0.05. # denotes lowest estimate. Not determined (nd). The statistics was not calculated for F351A. Dashed lines denote corresponding values for wild-type KV7.1+KCNE1.
Different mutants display different fluorescence versus voltage profiles
Although most of the mutations shift channel voltage dependence and affect channel closing kinetics, the underlying mechanism of mutation-induced changes in KV7.1+KCNE1 channel function is most likely different for different mutations. For instance, mutations located in S5 and S6 (e.g. F351A) may mainly affect gate movement, while mutations in S1–S4 (e.g. S225L) are more likely to affect voltage sensor movement. To explore whether different mutations interfere with different gating transitions, we use voltage clamp fluorometry, in which the movement of the voltage sensor in KV7.1 can be tracked by the fluorescence change from the fluorescent probe Alexa-488-maleimide attached to G219C in the S3-S4 loop (referred to as G219C*) (Barro-Soria et al., 2014; Osteen et al., 2010; Osteen et al., 2012). Voltage sensor movement (measured by fluorescence) and gate movement (measured by ionic currents) are then monitored under two-electrode voltage clamp. The KV7.1/G219C* construct by itself or co-expressed with KCNE1 gives voltage-dependent fluorescence changes (Figure 3a). As previously reported, the fluorescence versus voltage (F(V)) curve of KV7.1/G219C* correlates well with the G(V) curve (Figure 3a, left panel), while the F(V) curve of KV7.1/G219C*+KCNE1 is divided into two components (Figure 3a, right panel) (Barro-Soria et al., 2014; Osteen et al., 2010; Osteen et al., 2012). For KV7.1/G219C*+KCNE1, the first fluorescence component (F1) has been suggested to represent the main voltage sensor movement and the second fluorescence component (F2) to be correlated with gate opening (Barro-Soria et al., 2014). We introduce G219C into KV7.1/S225L and KV7.1/F351A. The G(V) curves of both KV7.1/G219C*/S225L and KV7.1/G219C*/F351A are shifted towards more positive voltages compared to the wild-type channel, but the F(V) curves are differentially affected by the two mutations (Figure 3b–c, left panels). For KV7.1/G219C*/S225L, the F(V) curve is shifted to a similar extent as the G(V) curve, while for KV7.1/G219C*/F351A, the F(V) curve is shifted to a considerably smaller extent (Osteen et al., 2010). When these mutants are co-expressed with KCNE1, we observe different effects on the voltage dependence of the two fluorescent components F1 and F2 induced by the mutations. The S225L mutation primarily shifts F1 towards positive voltages so that F1 and F2 of KV7.1/G219C*/S225L+KCNE1 are hardly distinguishable in the F(V) curve (Figure 3b, right panel). In contrast, the F351A mutation primarily shifts F2 towards positive voltages so that F1 and F2 are clearly separated (Figure 3c, right panel). Thus, S225L and F351A seem to shift the G(V) curve of KV7.1+KCNE1 towards positive voltages by interfering with different gating transitions.
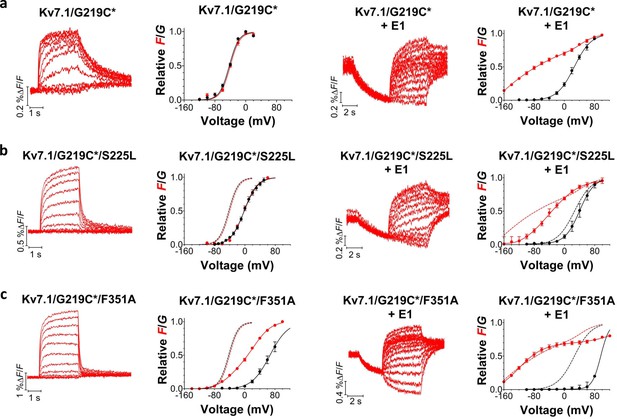
Voltage-clamp fluorometry recordings of wild-type and mutated KV7.1+KCNE1 channels.
(a-c) Representative fluorescence traces and mean F(V)/G(V) curves for KV7.1/G219C* (a), S225L (b), and F351A (c). Left panels without KCNE1 and right panels with KCNE1. The holding voltage is –80 mV, the pre-pulse –120 mV for 2 s (left panels) and –160 mV for 5 s (right panels), and test voltages between –140 and +80 mV for 3 s (left panels) and between –160 and +80 mV for 5 s (right panels) in 20 mV increments. The tail voltage is –80 mV (left panels) and −40 mV (right panels). For KV7.1/G219C*/F351A+KCNE1, the pre-pulse is –120 mV for 3 s, and test voltages ranging between –160 and +100 mV. The bottom of the fit of the KV7.1/G219C*/S225L+KCNE1 F(V) curve (which saturates fairly well at negative voltages) is set to 0 in the normalized F(V) curves in the right panels. The F1 amplitude of KV7.1/G219C*/F351A+KCNE1 is normalized to the F1 amplitude of wild-type. Data as mean ± SEM. n = 4–14. The dashed lines in (b) and (c) denote F(V) (red) and G(V) (black) for wild-type (from a).
Kinetic modeling recapitulates experimental findings
To further explore the different effects of S225L and F351A in the voltage-clamp fluorometry experiments, we use two kinetic models previously developed to reproduce the currents and fluorescence from KV7.1/G219C* (Osteen et al., 2012) and KV7.1/G219C*+KCNE1 channels (Barro-Soria et al., 2014), respectively. The KV7.1/G219C* model is an allosteric model with 10 states (Figure 3—figure supplement 1a), where the horizontal transition is the main S4 movement (which generates the main fluorescence component F1) and the vertical transition is channel opening accompanied by an additional smaller S4 movement (that generates a smaller additional fluorescence component F2) (Osteen et al., 2012; Zaydman et al., 2014). The KV7.1/G219C* model allows for channel opening after only a subset of four S4s are activated, which thereby generates F(V) and G(V) that are close in the voltage dependence (reference (Osteen et al., 2012); and Figure 3—figure supplement 2a). The KV7.1/G219C*+KCNE1 model has 6 states (Figure 3—figure supplement 1b), where the horizontal transition is the main S4 movement (which generates the main fluorescence component F1) and the vertical transition is channel opening accompanied by an additional smaller S4 movement (that generates a smaller additional fluorescence component F2) (Osteen et al., 2012; Zaydman et al., 2014). The KV7.1/G219C*+KCNE1 model only allows for channel opening after all four S4s are activated, which thereby generates F(V) and G(V) that are separated in voltage dependence (reference [Barro-Soria et al., 2014]; and Figure 3—figure supplement 2a).
Using these models, we can reproduce the main features of the fluorescence and currents from KV7.1/G219C*/S225L and KV7.1/G219C*/S225L+KCNE1 by only shifting the main voltage sensor movement by +50 mV in both models (Figure 3—figure supplement 2b), as if the S225L mutation mainly affects the main S4 movement. In the KV7.1 model, shifting the main voltage sensor movement by +50 mV shifts both the G(V) and F(V) curves by +35–40 mV, similar to the effect induced by the S225L mutation in the experimental data. In the KV7.1+KCNE1 model, shifting the main voltage sensor movement by +50 mV results in that the F1 and F2 components overlap in voltage, such that it is hard to distinguish the two components, and that the G(V) is shifted by +10 mV. Both effects are similar to the effects induced by the S225L mutation in the experimental data (cf. Figure 3b).
We can reproduce the main features of the fluorescence and currents from KV7.1/G219C*/F351A and KV7.1/G219C*/F351A+KCNE1 by only shifting the voltage dependence of the opening transition by +140 mV in both models (Figure 3—figure supplement 2c), as if the F351A mutation mainly affects the opening transition. In the KV7.1 model, shifting the opening transition by +140 mV shifts the G(V) by +100 mV whereas the F(V) is shifted less and has a shallower slope, similar to the effects induced by the F351A mutation in the experimental data. In the KV7.1+KCNE1 model, shifting the opening transition by +140 mV results in that the F1 and F2 components are further separated in voltage and that the G(V) is shifted by +100 mV. Both effects are similar to the effects induced by the F351A mutation in the experimental data (cf. Figure 3c).
In summary, our voltage-clamp fluorometry experiments together with kinetic modeling are compatible with a model in which the S225L mutation primarily interferes with the main S4 movement, whereas the F351A mutation interferes with later gating transitions associated with pore opening. One note of caution is that the interpretation of the mutational effects is dependent on the models used for the wild-type channels. Other models for KV7.1 and KV7.1+KCNE1 channels have been proposed (Zaydman et al., 2014; Ruscic et al., 2013), but these have not been as extensively tested or developed as our models. Although other alternative mechanisms for the effects of these mutations are possible, the different impacts of S225L and F351A on the fluorescence versus voltage relationships suggest that these mutations introduce distinct molecular defects.
N-AT enhances the activity of all tested LQTS and LQTS-like mutants
We previously observed that the effect of regular polyunsaturated fatty acids, such as docosahexaenoic acid, on KV7.1 is impaired by co-expression with the KCNE1 subunit (Liin et al., 2015). In contrast, we found that the PUFA analogue N-arachidonoyl taurine (N-AT, structure in Figure 4) retained its ability to activate the KV7.1 channel also in the presence of KCNE1. N-AT activated the wild-type KV7.1+KCNE1 by shifting the G(V) roughly –30 mV (Liin et al., 2015) (Figure 4—figure supplement 1). The magnitude of this N-AT-induced shift is comparable to, but in the opposite direction, to the G(V) shifts observed for several of the LQTS and LQTS-like mutants. We therefore here test the ability of N-AT to enhance the function of the eight KV7.1+KCNE1 mutant channels. Figure 4a–b shows representative effects of 7–70 µM N-AT on KV7.1/S225L+KCNE1. 70 µM N-AT increases current amplitude by a factor of 16 at +20 mV (Figure 4a) and shifts the G(V) curve by about –50 mV (Figure 4b, Supplementary file 3). Steady state of N-AT effects is reached within a few minutes (Figure 4—figure supplement 2). We note a small instantaneous ‘leak’ component in the 70 µM N-AT trace of KV7.1/S225L+KCNE1 (Figure 4a). This leak component in KV7.1/S225L+KCNE1 is observed also in the absence of N-AT, but at more positive voltages (Figure 4—figure supplement 3). We do not observe this leak component in wild-type KV7.1+KCNE1 upon application of N-AT (Figure 4—figure supplement 1a), which suggests that this phenomenon is associated with the S225L mutation. The human ventricular action potential has a duration of about 300–400 ms and a systolic voltage range of about 0 to +40 mV (O'Hara et al., 2011; Piacentino et al., 2003). To test the behaviour of the S225L mutation during shorter stimulating pulses, we apply repetitive 300 ms pulses to +40 mV at a frequency of 1 Hz and at 28°C (37°C was not tolerated by the oocytes). In response to this protocol, the KV7.1/S225L+KCNE1 channel barely opens and thus generates only minor currents (Figure 4c). In contrast, we observe large KV7.1/S225L+KCNE1 currents upon application of 70 µM N-AT (Figure 4c). N-AT also restores the gradual increase in current amplitude during repetitive pulsing seen experimentally (inset in Figure 4c) and in computer simulations (Silva and Rudy, 2005) for the wild-type KV7.1+KCNE1 channel.
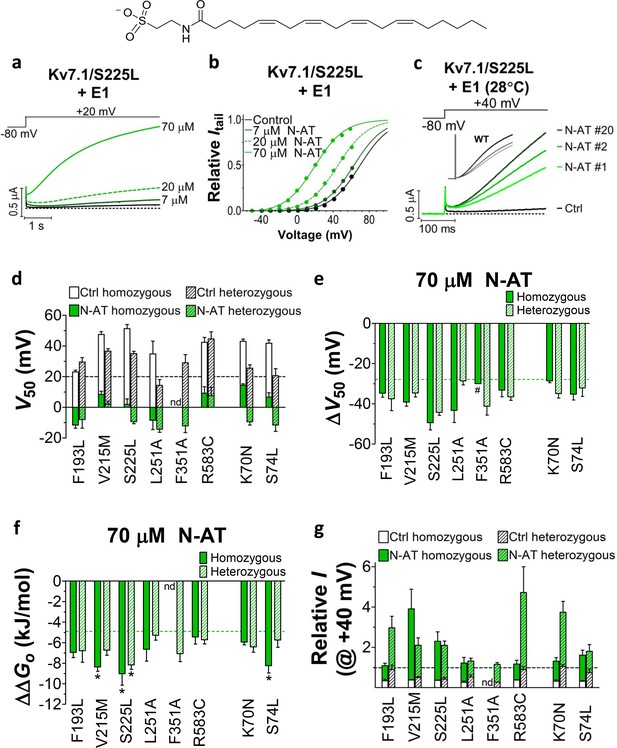
Effect of N-AT on LQTS and LQTS-like mutants.
All these experiments are done in the presence of KCNE1. Structure of N-AT is shown. (a–b) Representative effect of 7–70 µM N-AT on current amplitude (a) and G(V) (b) of KV7.1/S225L+KCNE1. Dashed line in (a) denotes 0 µA. (c) Representative currents generated by KV7.1/S225L+KCNE1 during pulsing at 1 Hz and +28°C in control solution (black) and after the cell had been bathed continuously in 70 µM N-AT (light to dark green, # denotes sweep order). Inset: corresponding currents from wild-type KV7.1+KCNE1 scaled similarly as KV7.1/S225L+KCNE1. Light grey trace denotes sweep #1, grey trace denotes sweep #2, and dark grey trace denotes sweep #20. (d) Summary of V50 for LQTS and LQTS-like mutants before and after 70 µM N-AT application. Dashed line denotes V50 for wild-type KV7.1+KCNE1. (e–f) Summary of ΔV50 (e) and ΔΔGo (f) for LQTS and LQTS-like mutants induced by 70 µM N-AT. # denotes an approximation. Dashed lines denote corresponding ΔV50 and ΔΔGo induced by 70 µM N-AT for wild-type KV7.1+KCNE1. The statistics in (f) represent one-way ANOVA with Dunnett’s Multiple Comparison Test to compare the N-AT-induced change in ΔΔGo of mutants to N-AT-induced change in ΔΔGo of wild-type KV7.1+KCNE1; *p≤0.05. Only significant differences shown in (f), other comparisons have p>0.05. (g) Estimate of the ability of 70 µM N-AT to restore LQTS and LQTS-like mutant current amplitude at +40 mV. The mean N-AT induced increase in current amplitude for each mutant (from Figure 4—figure supplement 4b) is multiplied with the control amplitude for each mutant (from Figure 1—figure supplement 4d). Not determined (nd). Data as mean ± SEM. n = 5–12. Dashed line denotes relative wild-type KV7.1+KCNE1 current amplitude in control solution (i.e. without N-AT).
Further testing of N-AT show that 70 µM N-AT shifts the G(V) curve of all tested mutants by 30–50 mV towards more negative voltages (Figure 4d–e, Supplementary file 3). The G(V) curve of wild-type KV7.1+KCNE1 is shifted by –27.0 ± 2.5 mV (Liin et al., 2015). Thus, 70 µM N-AT completely corrects the positive G(V) shifts induced by the mutations so that in the presence of N-AT the G(V) is similar to or shifted negative compared to the G(V) of the wild-type KV7.1+KCNE1 channel (Figure 4d, F351A homozygous expression was not included in this analysis because of the very shifted G(V) curve of this mutant). The G(V) of mutants is shifted about equally by N-AT for homozygous and heterozygous expression (Figure 4e). The slope of the G(V) curve varies slightly (10.4 to 16.3) among the mutants (Supplementary file 3). To correct for this difference in slope and to better compare the functional effect of N-AT-induced G(V) shifts on the different mutants, we also calculate the change in Gibbs free energy for channel opening (ΔΔGo) that 70 µM N-AT induces. 70 µM N-AT reduces the energy required to open the channel by 5.3–9.0 kJ/mol depending on mutant (4.9 ± 0.7 kJ/mol (n = 5) for wild-type) (Figure 4f). To estimate the functional effect of N-AT on the KV7.1+KCNE1 current amplitude of each mutant, we calculate the ratio of the current amplitude at the end of the 5 s test pulse before and after application of N-AT at +20 and +40 mV. The 5 s voltage pulse to +20 mV (or + 40 mV) at room temperature was chosen to make the KV7.1+KCNE1 channel activate to a similar extent as during a ventricular action potential (300–400 ms) at body temperature (note that KV7.1+KCNE1 channels have a relatively high Q10 of around 5–7.5 [Busch and Lang, 1993; Seebohm et al., 2001]). 70 µM N-AT increases the current amplitude of all mutants at these voltages (Figure 4—figure supplement 4a–b, Supplementary file 3). As expected, current amplitude is most increased for those mutants that have the most shifted G(V) curve towards more positive voltages (e.g. V215M and S225L). This is because these mutants are still at the foot of their G(V) curve at +20 and +40 mV and a N-AT-induced shift towards more negative voltages results in a relatively larger increase in the current amplitude. By multiplying these relative N-AT-induced increases in current amplitude with the relative current amplitude of each mutant (compared to wild-type KV7.1+KCNE1 channels, from Figure 1—figure supplement 4c–d), we observe that 70 µM N-AT compensates fairly well (or overcompensates) for the mutation-induced reduction in current amplitude (Figure 4g, Figure 4—figure supplement 4c). Moreover, for all mutant and wild-type KV7.1+KCNE1 channels, 70 µM N-AT speeds up the opening kinetics at +40 mV by a factor of 1.3–2.5 (Supplementary file 3). 70 µM N-AT also slows down the closing kinetics for most mutants and wild-type KV7.1+KCNE1 (Supplementary file 3). For F351A heterozygous expression and R583C homozygous expression, 70 µM N-AT restores the closing kinetics so that the closing kinetics is not statistically different (p>0.05) from wild-type KV7.1+KCNE1 closing kinetics (737 ± 62 ms and 833 ± 74 ms, respectively compared to 967 ± 47 ms for wild-type). In the presence of KCNE1, channels made with F193L heterozygous expression, L251A homozygous expression, and R583C heterozygous expression have wild-type like closing kinetics already before application of N-AT.
N-AT affects both S4 movement and gate opening in mutants
We next use voltage clamp fluorometry on KV7.1/G219C*/S225L+KCNE1 and KV7.1/G219C*/F351A+KCNE1 to explore the mechanism by which N-AT enhances the activity of two mechanistically different mutants. Surprisingly, N-AT caused a dramatic decrease in the fluorescence from Alexa488-labeled KV7.1/G219C*+KCNE1 channels (Figure 5—figure supplement 1a). In contrast, N-AT did not decrease the fluorescence from Alexa488-labeled KV7.1/G219C* channels nor did high concentrations of taurine decrease the fluorescence from unbound Alexa488 (even up to concentrations of 0.5 M taurine; Figure 5—figure supplement 1b), suggesting that N-AT is not a collisional quencher of Alexa488. The mechanism of the N-AT-induced decrease of fluorescence from Alexa488-labeled KV7.1/G219C*+KCNE1 channels is not clear, but could be due to N-AT inducing a conformational change in KCNE1 or KV7.1 that brings a quenching residue close to Alexa488.
Due to the dramatic decrease in the fluorescence signal from Alexa488-labeled KV7.1/G219C*+KCNE1 channels, we have to normalize the F(V) curves obtained in N-AT to the amplitude of the F(V) in control solutions. With this normalization, voltage clamp fluorometry experiments on KV7.1/G219C*/S225L+KCNE1 indicate that N-AT shifts both the voltage dependence of the first part (which represents F1) and the second part (which represents F2) of the F(V) curve towards more negative voltages (Figure 5—figure supplement 1c). However, due to the not completely saturating F(V) for KV7.1/G219C*/F351A+KCNE1, we are unable to reliably normalize the F(V) curves in the presence of N-AT to the control F(V) curves. We instead explore the effect of N-AT on the kinetics of the two fluorescence components: F1, which is seen as a fast fluorescence change at negative voltages, and F2, which is seen as a slow fluorescence change on top of the F1 component at positive voltages (Barro-Soria et al., 2014). F1 correlates with the measured gating currents in KV7.1+KCNE1 channels (and the initial delay in the KV7.1+KCNE1 ionic currents), whereas F2 correlates with the opening of KV7.1+KCNE1 channels (Barro-Soria et al., 2014). For both mutants, 70 µM N-AT speeds up F1 kinetics (Figure 5a,d, measured at –40 mV where virtually no channels open and the fluorescence is mainly composed of F1). Numeric values for N-AT effects on channel kinetics are summarized in Figure 5f. Moreover, N-AT accelerates the channel opening kinetics (Figure 5b,e) and both the F1 and F2 fluorescence components at +80 mV for KV7.1/G219C*/S225L+KCNE1 (Figure 5f). The change in the F2 component is probably larger than what the fits of a double-exponential function suggest, because the slow part of the fluorescence, mainly F2, overlay nicely on the currents in both the presence and absence of 70 µM N-AT (Figure 5c, upper panel). As a control, we show that the fluorescence in N-AT does not, however, overlay the currents in control solutions and vice versa (Figure 5c, middle and lower panel). For KV7.1/G219C*/F351A+KCNE1, the G(V) curve and the F2 component are so shifted towards depolarizing voltages that we cannot reliably quantify the F2 component in our fluorescence traces. 70 µM N-AT does, however, speed up KV7.1/G219C*/F351A+KCNE1 current kinetics (Figure 5e), which suggests that N-AT also speeds up F2 in KV7.1/G219C*/F351A+KCNE1. Altogether, these results suggest that N-AT accelerates both conformational changes during the main gating charge movement and channel opening.
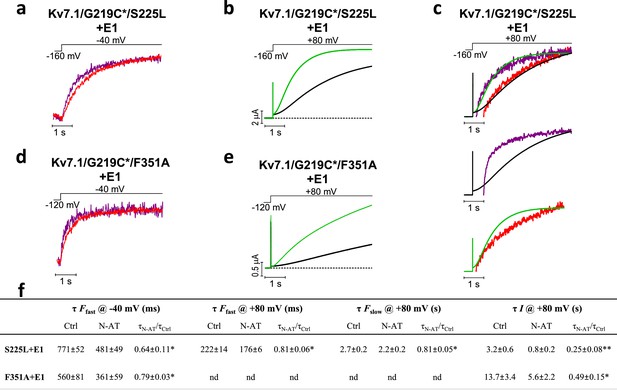
Effect of 70 µM N-AT on S4 movement and gate opening in S225L and F351A mutants.
(a–c) Representative example of the effect of 70 µM N-AT on F1 kinetics (a), current opening kinetics (b), and F2 kinetics (c) in KV7.1/G219C*/S225L+KCNE1. Control fluorescence (red) and current (black). N-AT fluorescence (magenta) and current (green). Top in (c) shows an overlay of the later part of the fluorescence (after most of F1 has occurred) and the later part of the currents (after the initial delay) before and after application of N-AT. Middle and lower (c) show that there is not a great overlap of the fluorescence in the presence of N-AT and the current in control solution (middle) or the fluorescence in control solution and the current in the presence of N-AT (lower). (d–e) Representative example of effect of 70 µM N-AT on F1 kinetics (d) and current opening kinetics (e) in KV7.1/G219C*/F351A+KCNE1. Same colouring as in (a–b). Dashed line in (b) and (e) denotes 0 µA. Fluorescence traces and all traces in (c) have been normalized to better allow temporal comparison. (f) Summary of the effect of 70 µM N-AT on the kinetic parameters of KV7.1/G219C*/S225L+KCNE1 and KV7.1/G219C*/F351A+KCNE1. Kinetics of the fast (F1) and slow (F2) fluorescence components were deduced from a double-exponential function fitted to the fluorescence traces. The kinetics of currents were deduced from a single-exponential function fitted to current traces. Ratios of time constants (τN-AT/τCtrl) were calculated pair-wise (control compared to N-AT) in each oocyte and analysed using two-tailed one sample t-test where ratios were compared with a hypothetical value of 1. Data as mean ± SEM. n = 4 (3for fluorescence kinetics for KV7.1/G219C*/F351A+KCNE1). *p<0.05; **p<0.01. nd = not determined.
Discussion
We show that all studied LQTS and LQTS-like mutations i) shift the G(V) of KV7.1+KCNE1 towards more positive voltages, and/or ii) accelerate KV7.1+KCNE1 closing. This suggests that at least part of the mechanism underlying the reduced ability of these mutants to generate K+ currents is by altering these biophysical properties of the KV7.1+KCNE1 channel. Using voltage clamp fluorometry in combination with kinetic modeling, we further suggest that these altered biophysical properties in mutants may be caused by interference with different gating transitions. Our experimental data and kinetic modeling are consistent with a model in which KV7.1/S225L primarily causes the reduced channel function by altering the main voltage sensor movement, while KV7.1/F351A alters later gating transitions associated with pore opening. The different effects of S225L and F351A on the fluorescence versus voltage relationships in KV7.1/G219C* and KV7.1/G219C*+KCNE1 suggest that these mutations cause channel dysfunction via different molecular mechanisms. Note that we used the LQTS-like F351A mutant, because the LQTS mutant F351S did not generate any currents (Figure 1—figure supplement 1). However, during the review process of this manuscript a new LQTS mutation, F351L, was found (Vyas et al., 2016). The current and fluorescence of this LQTS mutant is very similar to the current and fluorescence of F351A (Figure 3—figure supplement 3), suggesting that our conclusions on the LQTS-like F351A is also relevant for the LQTS mutant F351L.
One of the mutations, F193L, has only minor effects on the biophysical properties of KV7.1+KCNE1. This mutant was previously reported to have reduced current amplitude compared to the wild-type KV7.1+KCNE1 channel and a mild clinical phenotype (Yamaguchi et al., 2003). The F193L mutation may therefore cause loss of function by faster deactivation kinetics and lower current density. Heterozygous expression of mutated subunits and wild-type subunits in equal molar ratios results in general in a milder biophysical phenotype (more close to the wild-type phenotype). This is in line with a milder clinical phenotype generally reported for heterozygous carriers of LQTS mutations compared to individuals with homozygous genotypes (Priori et al., 1998; Jackson et al., 2014; Zhang et al., 2008). Moreover, for different mutations different biophysical effects of the mutations could be dominant or recessive: For S225L and L251A, heterozygous expression in the presence of KCNE1 partially or completely restores wild-type like V50, whereas heterozygous expression does not improve closing kinetics compared to homozygous expression. For KCNE1/K70N and KCNE1/S74L, co-expression with wild-type KCNE1 subunits also restores wild-type like V50, whereas wild-type like closing kinetics is only partially restored. In contrast, for KV7.1/R583C, heterozygous expression restores wild-type like closing kinetics, but not wild-type like V50. However, because of uncertainties regarding the stoichiometry of mutant to wild-type subunits in assembled KV7.1+KCNE1 channels (as mentioned in the Results section), further studies will be required to understand the mechanisms underlying these apparent dominant or recessive effects and to evaluate possible physiological impact of these effects.
Our results show that all tested mutants respond to N-AT. This is in contrast to previously reported KV7 channel activators on disease-causing KV7 mutants, for which mutants show markedly different sensitivity (Seebohm et al., 2003; Xiong et al., 2007; Leitner et al., 2012). 70 µM N-AT shifts the G(V) curve of the wild-type KV7.1+KCNE1 channel and of all LQTS and LQTS-like mutants by approximately (–50)–(–30) mV, accelerates channel opening and slows down channel closing. In the presence of 70 µM N-AT, the V50 of all LQTS and LQTS-like mutants are similar to or more negative than V50 for the wild-type KV7.1+KCNE1 channel. For most mutants, 70 µM N-AT overcompensates for the shift in G(V) and reduction in current amplitude caused by the mutations, indicating that a lower N-AT concentration or a less potent N-AT analogue could be used to restore wild-type like G(V) and current amplitudes. Moreover, KV7.1+KCNE1 opening and closing kinetics are partially or completely restored by N-AT. Also, although the disease aetiology of the F193L mutant is likely mainly reduced channel expression, the N-AT induced augmentation caused by a shift in G(V) and increased currents may at least in part overcome the reduction in currents caused by the reduced channel expression. This general ability of N-AT to, at least partly, compensate for the reduced function of mutants with mutations in different parts of the KV7.1+KCNE1 channel complex and with seemingly different molecular defects, as long as a population of these mutant channels reaches the plasma membrane, suggests that N-AT is an interesting model compound for development of future anti-arrhythmics to treat LQTS caused by diverse KV7.1+KCNE1 mutations.
Defective trafficking of mutant KV11.1 ion channels is a common cause of LQTS type 2. About 80-90% of LQTS type 2-associated hERG mutants are estimated to suffer from defective trafficking (Anderson et al., 2014; Sanguinetti, 2010). The corresponding number for LQTS-associated KV7.1 and KCNE1 mutants is not known. Previous studies identify both trafficking defective and trafficking competent KV7.1 and KCNE1 mutants, e.g. (Anderson et al., 2014; Sanguinetti, 2010). We are mainly interested in understanding the mechanism that underlies abnormal gating of KV7.1 and KCNE1 mutants. To avoid mutants with severe trafficking defects, we therefore selected mutants that have previously been shown to localize abundantly enough to the cell membrane to generate detectable K+ currents. Several of the selected mutants have been shown to traffic well in mammalian systems (KV7.1/V215M and KCNE1/S74L [Eldstrom et al., 2010; Harmer et al., 2010]) or generate clearly detectable currents in mammalian cells (KV7.1/R583C [Yang et al., 2002]). Our Xenopus oocyte experiments that compare mutant current amplitudes with wild-type current amplitudes (Figure 1—figure supplement 4) suggest that the reduced ability of the selected mutants to generate currents in Xenopus oocytes may largely be explained by the shifted G(V) of mutants. Trafficking defects could be disguised in Xenopus oocytes that are cultured at low temperatures that may rescue some trafficking defects (Anderson et al., 2014; Delisle et al., 2004). These current amplitude experiments should therefore be interpreted with caution until trafficking of specific KV7.1 and KCNE1 LQTS mutants in mammalian systems has been explored. Previous studies show that membrane expression of trafficking-defect channel mutants (e.g. for KV11.1 and CFTR) can be pharmacologically rescued using compounds that are suggested to stabilize channel conformation during folding and trafficking (Anderson et al., 2014; Delisle et al., 2004; Sato et al., 1996). However, rescue of membrane expression may only partially compensate for mutation-induced loss of function, if these mutants also suffer from defective gating (Perry et al., 2016). Our proposed N-AT model for pharmacological correction of ‘G(V)’ LQTS mutants could therefore potentially complement pharmacological correction of trafficking-defect LQTS mutants to improve the outcome of patients suffering from LQTS.
We previously suggested that polyunsaturated fatty acids and their analogues (such as N-AT) attract the voltage sensor S4 in KV7.1 by an electrostatic mechanism and thereby shift the G(V) towards more negative voltages and speed up channel opening (Liin et al., 2015). We therefore initially hypothesized that N-AT only would restore the function of those LQTS mutations with altered S4 movement. We were pleasantly surprised when N-AT seems to be able to restore the function of many LQTS and LQTS-like mutants, with diverse mutational defects (such as S225L and F351A). Using voltage clamp fluorometry, we have previously shown that both the main gating charge movement and the gate opening of KV7.1+KCNE1 channels are accompanied by fluorescence signals from fluorophores attached to S4 (Barro-Soria et al., 2014). This suggests that S4 moves both during the main gating charge movement and during the subsequent channel opening in KV7.1+KCNE1 channels (Barro-Soria et al., 2014), which is similar to observations in Shaker KV channels (Börjesson and Elinder, 2011; Pathak et al., 2005; Phillips and Swartz, 2010). Therefore, N-AT could affect both the main gating charge movement and gate opening by acting on the S4 voltage sensor, as has been shown for hanatoxin which targets the voltage-sensing domain in the Shaker KV channel (Milescu et al., 2013). This hypothesis is supported by our voltage-clamp fluorometry experiments using KV7.1/S225L and KV7.1/F351A in which N-AT accelerates the fluorescence components associated with both the main S4 movement (F1) and gate opening (F2), as well as accelerates the kinetics of channel opening. This proposed mechanism would explain why N-AT can restore the function of mutations that mainly target either the main S4 movement or gate opening. However, the dramatic decrease in the fluorescence signal caused by N-AT makes it hard for us to completely determine the effect of N-AT on the F(V) of mutants. Therefore, the complete mechanism of N-AT in the different mutations is not clear.
Future studies are required to assess the clinical utility of PUFA analogues in cardiomyocytes and animal models. We see channel specificity of PUFA analogues as one major challenge and recognize the need to improve PUFA analogue affinity to KV7.1+KCNE1 to reduce required therapeutic concentrations and minimize potential adverse effects. Despite these challenges, our data show that the magnitude of the N-AT-induced voltage shifts are in a similar range as the shifts induced by several LQTS mutations, thereby serving as proof of concept that this PUFA analogue, at least partly, restores channel function in diverse LQTS and LQTS-like mutants.
Materials and methods
Experiments were approved by The Linköping Animal Ethics Committee at Linköping University and The Animal Experiments Inspectorate under the Danish Ministry of Food, Agriculture and Fisheries (University of Copenhagen).
Experiments on Xenopus laevis oocytes
Molecular biology
Request a detailed protocolExpression plasmids human KV7.1 (GenBank Acc.No. NM_000218) in pXOOM and KCNE1 (NM_000219) in pGEM have been previously described (Jespersen et al., 2002; Schmitt et al., 2007). LQTS and LQTS-like point mutations and G219C were introduced into KV7.1 or KCNE1 using site-directed mutagenesis (QuikChange Stratagene, CA). All newly generated constructs were sequenced to ensure integrity (Genewiz, NJ). cRNA was prepared from linearized DNA using the T7 mMessage mMachine transcription kit (Ambion, TX). RNA quality was checked by gel electrophoresis, and RNA concentrations were quantified by UV spectroscopy.
Two-electrode voltage-clamp electrophysiology
Request a detailed protocolXenopus laevis oocytes (from EcoCyte Bioscience, TX, or prepared in house) were isolated and maintained as previously described (Börjesson et al., 2010). 50 nl cRNA (~50 ng KV7.1 for KV7.1-only expression, 25 ng KV7.1 + 8 ng KCNE1 for homozygous expression, or 12.5 ng KV7.1wt + 12.5 ng KV7.1mut + 8 ng KCNE1wt alternatively 25 ng KV7.1wt + 4 ng KCNE1wt + 4 ng KCNE1mut for heterozygous expression) was injected into each oocyte. Currents were measured at room temperature 2–5 days after injection with the two-electrode voltage-clamp technique (CA-1B amplifier, Dagan, MN). For the current amplitude experiments presented in Figure 1—figure supplement 4, the current amplitude of mutants were normalized to the current amplitude of wild-type KV7.1+KCNE1 expressed in the same batch of oocytes and incubated under identical conditions for the same time period. Currents were sampled at 1–3.3 kHz, filtered at 500 Hz, and not leakage corrected. The control solution contained (in mM): 88 NaCl, 1 KCl, 15 HEPES, 0.4 CaCl2, and 0.8 MgCl2 (pH adjusted to 7.4 using NaOH). The holding voltage was generally set to –80 mV. Activation curves were generally elicited by stepping to test voltages between –110 and +60 mV (3–5 s durations and 10 mV increments) followed by a tail voltage of –20 mV. Voltage clamp fluorometry experiments were performed as previously described on oocytes labeled for 30 min with 100 µM Alexa-488-maleimide (Molecular Probes) at 4°C (Barro-Soria et al., 2014; Osteen et al., 2010; Osteen et al., 2012). For voltage clamp fluorometry experiments on KV7.1/G219C*, the holding voltage was –80 mV, the pre-pulse –120 mV for 2 s, and test voltages ranging between –140 and +80 mV for 3 s in 20 mV increments. The tail voltage was –80 mV. For KV7.1/G219C*/KCNE1, the holding voltage was –80 mV, the pre-pulse –160 mV for 5 s, and test voltages ranging between –160 and +80 mV for 5 s in 20 mV increments. The tail voltage was –40 mV. N-arachidonoyl taurine was purchased from Cayman Chemical (MI, USA) and stored, diluted and applied to the oocyte chamber as previously described (Liin et al., 2015). Control solution was added to the bath using a gravity-driven perfusion system.
Electrophysiological analysis
Request a detailed protocolTo quantify effects on the G(V), tail currents (measured shortly after initiation of tail voltage) were plotted against the pre-pulse (test) voltage. The following Boltzmann relation was fitted to the data
where is the midpoint (i.e. the voltage at which the conductance is half the maximal conductance estimated from the fit) and s the slope factor (shared slope for control and N-AT curves within the same cell). In figures showing Itail vs voltage, the curves are normalized to the fitted . The same single Boltzmann relation was used to fit the F(V) from voltage clamp fluorometry recordings of KV7.1 without KCNE1 co-expression, where fluorescence at the end of the test pulse was plotted versus the test voltage (Barro-Soria et al., 2014). For voltage-clamp fluorometry recordings of KV7.1 with KCNE1 co-expression (and F351A without KCNE1), a double Boltzmann relation was used (Barro-Soria et al., 2014). For experiments where conductance or fluorescence did not clearly show signs of saturation in the experimental voltage range, these fits should be considered as an approximation. To estimate the effect of N-AT on Gibbs free energy, the following relation was used:
Where z is the gating charge of each channel deduced from the slope of the Boltzmann fits according to , is the N-AT induced shift in the values from the Boltzmann fits, and F is Faraday’s constant (Li-Smerin and Swartz, 2001; Monks et al., 1999; DeCaen et al., 2008). This analysis assumes a two-state model and tends to underestimate the z (Chowdhury and Chanda, 2012). The calculated should therefore be seen as an approximation. For opening and closing kinetics, T50,open was defined as the time it takes to reach 50% of the current in the end of a 3 s (5 s for KCNE1 co-expression) long test pulse to +40 mV. T50,close was defined as the time it takes to reduce the amplitude (= instantaneous tail current – steady state tail current) of the tail current by 50% when stepping to a tail pulse to –20 for 5 s. To analyze the effect of N-AT on fluorescence and current kinetics, single or double exponentials were fitted to the fluorescence or current traces. The ratios of time constants before and after application of N-AT were then calculated.
Modeling
Request a detailed protocolFluorescence and currents from the KV7.1+KCNE1 models were simulated using Berkeley Madonna (Berkeley, CA).
Statistics
Average values are expressed as mean ± SEM. Mutant parameters (e.g. and ) were compared to wild-type parameters using one-way ANOVA with Dunnett’s Multiple Comparison Test. Comparison of homozygous and heterozygous expression was done using one-way ANOVA with pair-wise Bonferroni’s Test. The effects of N-AT on fluorescence and current kinetics were analysed using two-tailed one sample t-test where ratios were compared with a hypothetical value of 1. p<0.05 is considered as statistically significant.
References
-
KCNE1 divides the voltage sensor movement in KCNQ1/KCNE1 channels into two stepsNature Communications 5:3750.https://doi.org/10.1038/ncomms4750
-
Mechanisms of I(Ks) suppression in LQT1 mutantsAmerican Journal of Physiology Heart and Circulatory Physiology 279:H3003–3011.
-
An electrostatic potassium channel opener targeting the final voltage sensor transitionThe Journal of General Physiology 137:563–577.https://doi.org/10.1085/jgp.201110599
-
Electrostatic tuning of cellular excitabilityBiophysical Journal 98:396–403.https://doi.org/10.1016/j.bpj.2009.10.026
-
Estimating the voltage-dependent free energy change of ion channels using the median voltage for activationThe Journal of General Physiology 139:3–17.https://doi.org/10.1085/jgp.201110722
-
Biology of cardiac arrhythmias: ion channel protein traffickingCirculation Research 94:1418–1428.https://doi.org/10.1161/01.RES.0000128561.28701.ea
-
Biophysical characteristics of a new mutation on the KCNQ1 potassium channel (L251P) causing long QT syndromeCanadian Journal of Physiology and Pharmacology 81:129–134.https://doi.org/10.1139/y02-162
-
Mechanistic basis for LQT1 caused by S3 mutations in the KCNQ1 subunit of IKsJournal of General Physiology 135:433–448.https://doi.org/10.1085/jgp.200910351
-
Mechanisms of disease pathogenesis in long QT syndrome type 5AJP: Cell Physiology 298:C263–C273.https://doi.org/10.1152/ajpcell.00308.2009
-
The genetic basis of long QT and short QT syndromes: a mutation updateHuman Mutation 30:1486–1511.https://doi.org/10.1002/humu.21106
-
Long QT syndrome-associated mutations in the voltage sensor of I(Ks) channelsCellular Physiology and Biochemistry 24:11–16.https://doi.org/10.1159/000227828
-
Dual-function vector for protein expression in both mammalian cells and Xenopus laevis oocytesBioTechniques 32:536–538.
-
Restoration of ion channel function in deafness-causing KCNQ4 mutants by synthetic channel openersBritish Journal of Pharmacology 165:2244–2259.https://doi.org/10.1111/j.1476-5381.2011.01697.x
-
The KCNQ1 channel - remarkable flexibility in gating allows for functional versatilityJournal of Physiology 593:2605–2615.https://doi.org/10.1113/jphysiol.2014.287607
-
Opening the shaker K+ channel with hanatoxinJournal of General Physiology 141:203–216.https://doi.org/10.1085/jgp.201210914
-
Helical structure and packing orientation of the S2 segment in the Shaker K+ channelJournal of General Physiology 113:415–423.https://doi.org/10.1085/jgp.113.3.415
-
KCNQ1 channel modulation by KCNE proteins via the voltage-sensing domainJournal of Physiology 593:2617–2625.https://doi.org/10.1113/jphysiol.2014.287672
-
Molecular physiology of cardiac repolarizationPhysiological Reviews 85:1205–1253.https://doi.org/10.1152/physrev.00002.2005
-
The cooperative voltage sensor motion that gates a potassium channelJournal of General Physiology 125:57–69.https://doi.org/10.1085/jgp.200409197
-
Position and motions of the S4 helix during opening of the Shaker potassium channelJournal of General Physiology 136:629–644.https://doi.org/10.1085/jgp.201010517
-
A recessive variant of the Romano-Ward long-QT syndrome?Circulation 97:2420–2425.https://doi.org/10.1161/01.CIR.97.24.2420
-
HERG1 channelopathiesPflügers Archiv - European Journal of Physiology 460:265–276.https://doi.org/10.1007/s00424-009-0758-8
-
Glycerol reverses the misfolding phenotype of the most common cystic fibrosis mutationJournal of Biological Chemistry 271:635–638.https://doi.org/10.1074/jbc.271.2.635
-
The novel C-terminal KCNQ1 mutation M520R alters protein traffickingBiochemical and Biophysical Research Communications 358:304–310.https://doi.org/10.1016/j.bbrc.2007.04.127
-
Dependence of I(Ks) biophysical properties on the expression systemPflüGers Archiv European Journal of Physiology 442:891–895.https://doi.org/10.1007/s004240100608
-
Phenotype guided characterization and molecular analysis of Indian patients with long QT syndromesIndian Pacing and Electrophysiology Journal 16:8–18.https://doi.org/10.1016/j.ipej.2016.03.003
-
An allosteric mechanism for drug block of the human cardiac potassium channel KCNQ1Molecular Pharmacology 83:481–489.https://doi.org/10.1124/mol.112.081513
Article and author information
Author details
Funding
National Institutes of Health (R01GM109762)
- H Peter Larson
American Heart Association (16GRNT30990060)
- H Peter Larson
Svenska Sällskapet för Medicinsk Forskning
- Sara I Liin
Vetenskapsrådet (524-2011-6806)
- Sara I Liin
Northwest Lions Foundation
- Sara I Liin
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Acknowledgements
We thank Frida Starck Härlin (Linköping University) and Briana Watkins (University of Miami) for help with some experiments and Drs. Fredrik Elinder (Linköping University), Laura Bianchi and Feng Qiu (University of Miami), Nicole Schmitt, Mark Skarsfeldt and Federico Denti (University of Copenhagen) for valuable comments.
Ethics
Animal experimentation: Experiments were performed in strict accordance with the recommendations of The Linköping Animal Ethics Committee at Linköping University and The Animal Experiments Inspectorate under the Danish Ministry of Food, Agriculture and Fisheries. Protocols were approved by The Linköping Animal Ethics Committee at Linköping University (#53-13 ) and The Animal Experiments Inspectorate under the Danish Ministry of Food, Agriculture and Fisheries (University of Copenhagen; #2014-15-2934-01061).
Copyright
© 2016, Liin et al.
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 1,430
- views
-
- 329
- downloads
-
- 38
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Citations by DOI
-
- 38
- citations for umbrella DOI https://doi.org/10.7554/eLife.20272






