Activity of the C. elegans egg-laying behavior circuit is controlled by competing activation and feedback inhibition
Figures
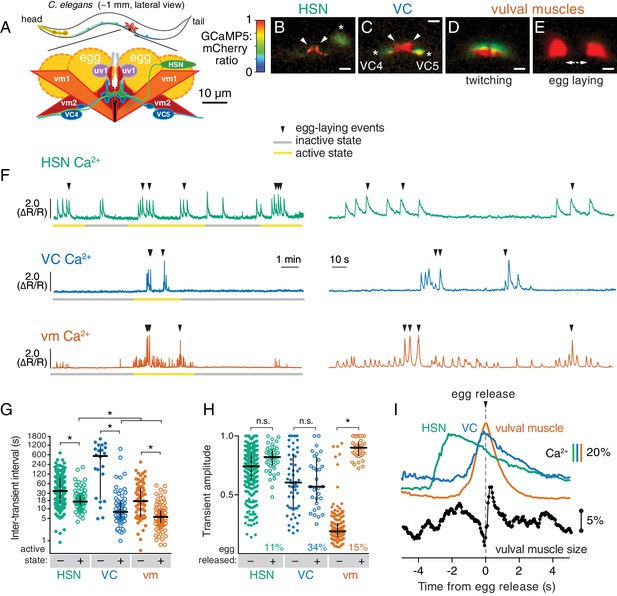
Cell-specific reporters of activity in the C.elegans egg-laying behavior circuit.
(A) Schematic of the circuit. HSN (green) and VC (blue) motor neurons synapse onto the vm2 muscle postsynaptic termini (center of schematic). The uv1 neuroendocrine cells (pink) extend processes (grey) along the vulval slit and vm2 postsynaptic terminus. (B–E) Individual video frames of the GCaMP5:mCherry fluorescence ratio showing active state Ca2+ transients in HSNs (B), VCs (C), and vulval muscles during twitching (D) and egg-laying behaviors (E). Arrowheads, HSN and VC presynaptic termini; asterisks, cell bodies; scale bar, 10 µm. (F) 30 min recordings of HSN, VC, and vulval muscle activity (left panel), showing distinct active (yellow) and inactive (grey) egg-laying behavior states, with expanded timescale of one active state at right. Arrowheads show egg-laying events. (G) Scatter plots and median HSN, VC, and vulval muscle (vm) inter-transient intervals during egg-laying inactive (–, filled circles) and active (+, open circles) states. Asterisks indicate significant differences (p<0.0001). (H) Relationship between Ca2+ transient amplitude and egg release. Scatter plots and medians of normalized amplitude with (+; open circles) and without (–; closed circles) egg release. Also shown is the percent of total transients that accompanied egg release. (I) Timing of HSN, VC, and vulval muscle Ca2+ transients and egg release. Shown at top is a curve of the median of Ca2+ from HSN (green), VC (blue), and vulval muscles (orange) from normalized ∆R/R traces (with the peak Ca2+ set to 100%) synchronized to the moment of egg release (0 s, arrowhead and dotted line). Bars indicate 20% change in median GCaMP5/mCherry ratio. The timing of the HSN Ca2+ peak is significantly different from that of the VCs and vulval muscles (p<0.0001). Shown at bottom is a trace of median vulval muscle size. Bar shows a 5% change in median object size based on mCherry fluorescence.
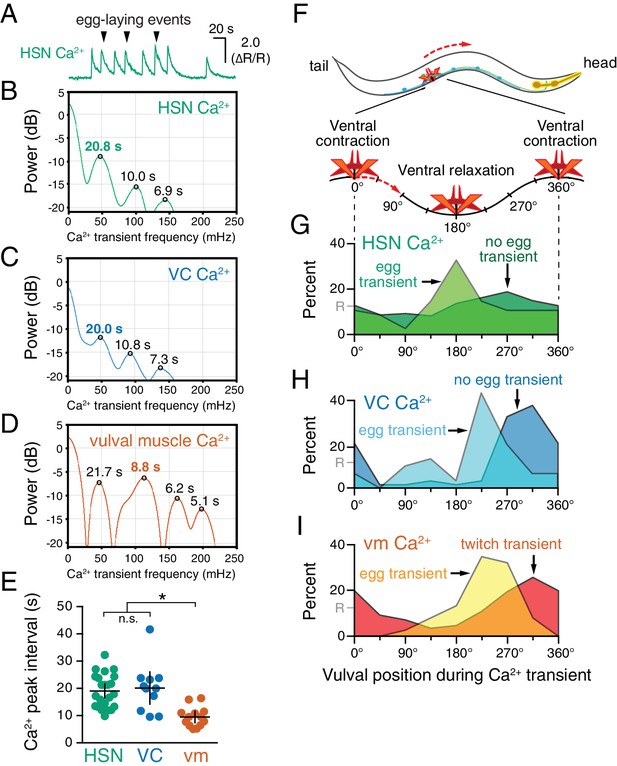
HSN, VC, and vulval muscle activity is rhythmic and phased with animal locomotion.
(A) Active-state segments, such as the one shown here, were extracted from Ca2+ recordings, and analyzed for rhythmicity by power spectrum analysis. Underlying rhythm frequencies and peak inter-transient intervals were thus extracted for HSN (B), VC (C), and vulval muscle (D) traces, and the peak of maximum rhythm for each active state is indicated in bold. (E) The average vulval muscle Ca2+ peak interval (~10 s) was significantly different than the ~20 s rhythm observed in HSN (p<0.0003) and VC (p<0.0006) Ca2+ recordings. HSN and VC rhythms were not significantly different from each other (p>0.9999; one-way ANOVA). (F) The position of the vulva within a sinusoidal locomotor body bend at the moment a Ca2+ transient peaked was used to assign a body bend ‘phase’, in units of degrees, with 180° representing ventral relaxation and 0/360° representing ventral contraction. Plots show the percent of Ca2+ transients observed in each of eight 45° bins for HSN (G), VC (H), and vulval muscle (I), with data for transients accompanied by an egg-laying event (‘egg transient’) plotted in different colors from data for transients not accompanied by an egg-laying event (‘no egg transient’). These plots show data pooled from recordings of 8–11 animals, and Figure 2—figure supplement 1 shows plots for each animal separately. The phasing of VC and vulval muscle transients that did not lead to egg laying is significantly different from an equal number of randomly distributed events (R, at 12.5%; p<0.0001; Kruskal-Wallis test).
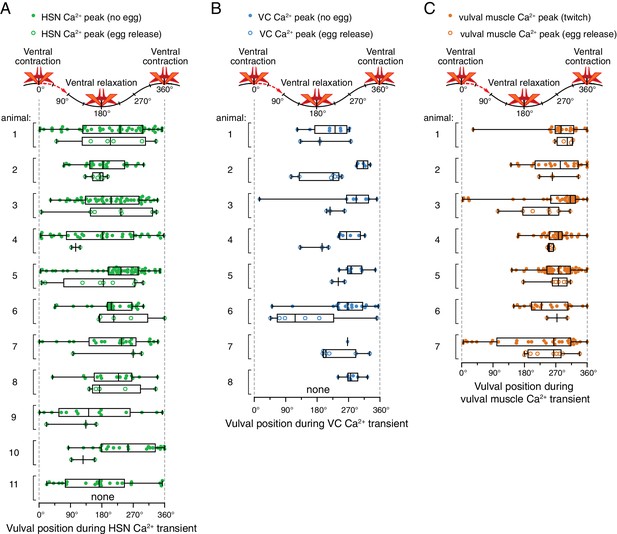
Relative timing of HSN, VC, and vulval muscle Ca2+ transients during locomotion body bends.
The position of the vulva within a sinusoidal locomotor body bend was used to assign a body bend ‘phase’, in units of degrees, for each Ca2+ transient analyzed in Figure 1 as described in the Materials and methods. The timing of each HSN (A), VC (B), or vulval muscle (C) Ca2+ transient peak with egg release (open circles) or without egg release (closed circles) was determined relative to the position of the vulva during the body bends of locomotion. Bar and whisker plots indicate the median and quartiles for each transient phase.
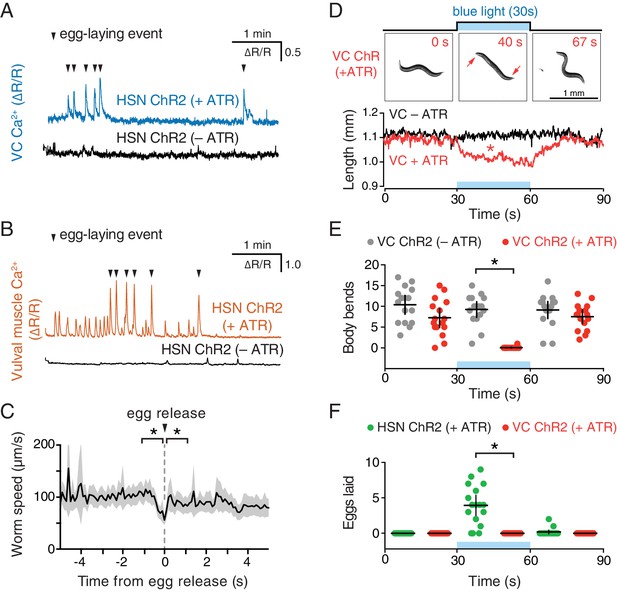
Optogenetic activation of HSN neurons initiates the egg-laying active state, and optogenetic activation of VC neurons slows locomotion.
(A) Animals expressing Channelrhodopsin-2 (ChR2) in the HSNs and GCaMP5/mCherry in the VC neurons were grown in the presence (+ATR; top, blue) or absence (–ATR; bottom, black) of all-trans retinal. 489 nm laser light was used to simultaneously stimulate ChR2 activity and excite GCaMP5 fluorescence during the entire recording. Arrowheads indicate egg-laying events. (B) The same experiment as (A) except that GCaMP5/mCherry were expressed in the vulval muscles rather than VCs. (C) Brief reduction of animal speed during optogenetically-induced egg laying. The egg-laying active state in animals expressing ChR2 in the HSN neurons was induced with 30 s exposure to blue light. Average speed (black trace) about each egg-laying event (0 s) was calculated from the worm centroid position, and the grey area shows the 95% confidence interval. Average speed at the moment of egg laying release (0 s) is reduced compared to 1 s before or after (p<0.001). See also Video 4. (D) Activation of the VC neurons slows locomotion but fails to induce egg laying. Animals expressing ChR2 in the VCs were grown in the presence (+ATR; red) or absence (–ATR; black/grey) of all-trans retinal, and worm length (upper photos and graph) was determined. Micrographs are still images from a representative animal at indicated time points (see Video 5). Inset arrows indicate regions of the head and tail that remain mobile after VC activation. Scatter plots of body bends (E) and egg laying (F) from the same recordings shown in D. There was a significant change in worm length and body bends, but not egg laying, during blue light in animals grown on ATR (red; p<0.0001). Positive control animals expressing ChR2 in the HSNs (green) showed a significant increase in egg laying during blue light (p<0.0001).
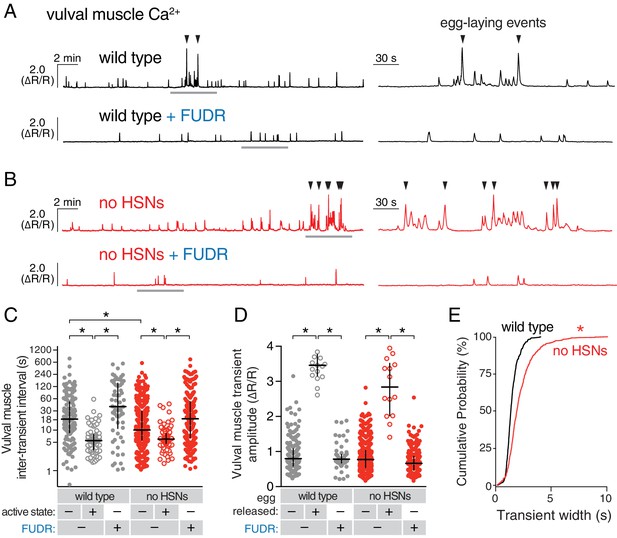
Active states require egg production but not the HSNs.
(A) Vulval muscle activity in wild-type animals either untreated or after sterilization with floxuridine (FUDR). Left panel shows 30 min recordings with the grey underlined regions expanded at right. (B) Vulval muscle activity in egl-1(n986dm) mutants lacking HSNs. (C–D) Scatter plots of vulval muscle inter-transient intervals (C) and amplitudes (D). Line indicates median, error bars indicate quartiles, and asterisks indicate significant differences (p<0.01). (E) Vulval muscle transients are wider in animals lacking HSNs. Cumulative distribution plots of Ca2+ transient peak widths; asterisk indicates p<0.0001 (Mann-Whitney test).
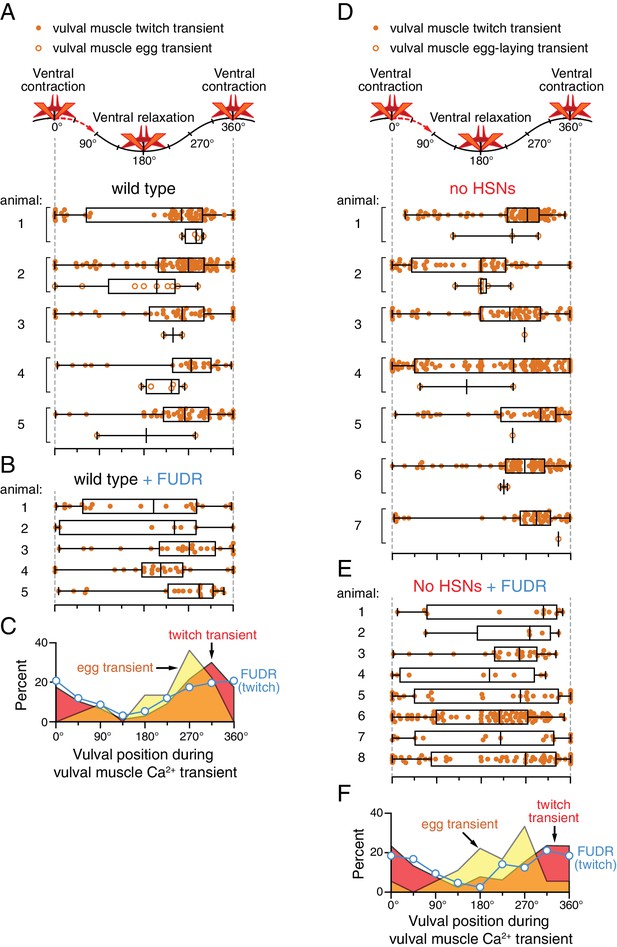
Persistent phasing of rhythmic vulval muscle activity in animals lacking HSNs and after sterilization.
Phasing of vulval muscle Ca2+ transients during twitching (closed circles) and egg laying (open circles) in five untreated wild-type animals (A) and five animals sterilized with FUDR (B). Bar and whisker plots indicate the median and quartiles for each transient phase. (C) Histograms of pooled data showing phasing of vulval muscle twitches (red) and egg-laying transients (yellow) in untreated wild-type animals and twitches after FUDR treatment (blue). Phasing of vulval muscle Ca2+ transients during twitching (closed circles) and egg laying (open circles) in seven untreated egl-1(n986dm) mutant animals lacking HSNs (D) and eight egl-1(n986dm) animals sterilized with FUDR (E). Bar and whisker plots indicate the median and quartiles for each transient phase. (F) Histogram of pooled data showing phasing of vulval muscle twitches (red) and egg-laying transients (yellow) in untreated egl-1(n986dm) animals and twitches after FUDR treatment (blue).
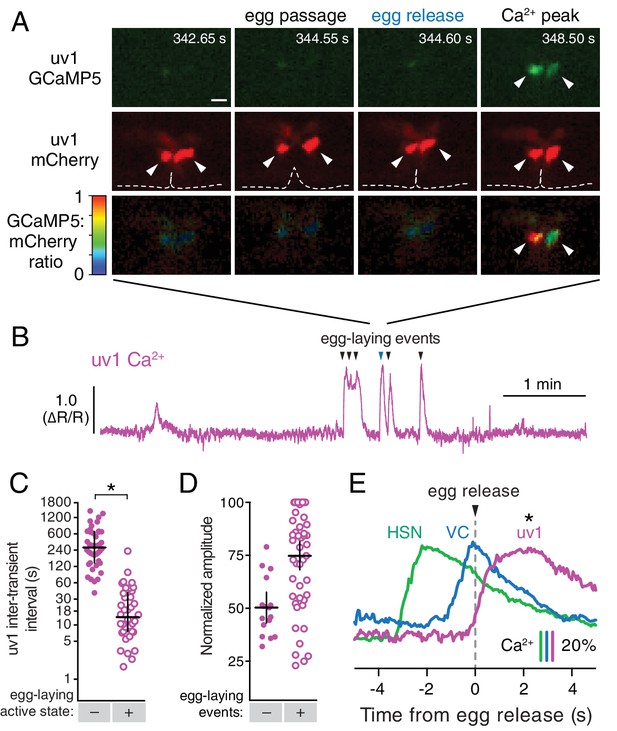
The tyraminergic uv1 neuroendocrine cells are mechanically deformed and activated by egg laying.
(A) Fluorescence micrographs of uv1 showing GCaMP5, mCherry, and the GCaMP5/mCherry ratio before egg laying, during egg passage through the vulva, and after egg release. Times of movie frames in seconds are at top, and white scale bar is 10 µm. Arrowheads in mCherry micrographs indicate position of uv1 cells as they are deformed by egg passage, the dotted line indicates position and opening of the vulva. (B) GCaMP5/mCherry ratio (∆R/R) trace of uv1 activity, including an egg-laying active state. Egg-laying events are indicated by arrowheads. (C) Scatter plots of uv1 inter-transient intervals during the inactive (closed circles) and active (open circles) egg-laying behavior states. Line indicates median, error bars indicate quartiles, and asterisks indicate significant differences (p<0.0001). (D) Scatter plots and medians of normalized amplitude with (+; open circles) and without (–; closed circles) an accompanying egg release. Error bars indicate quartiles, and asterisks indicate significant differences (p<0.0001, Mann-Whitney test). (E) uv1 Ca2+ transients follow egg-laying events. Normalized traces of median Ca2+ in HSN (green) and VC (blue) from Figure 1 are compared to uv1 (purple), synchronized to the moment of egg release (0 s, arrowhead and dotted line). Bars indicate 20% change in median GCaMP5/mCherry ratio. Asterisk indicates the uv1 Ca2+ peak is significantly later than the HSN and VC peaks (p<0.0001).
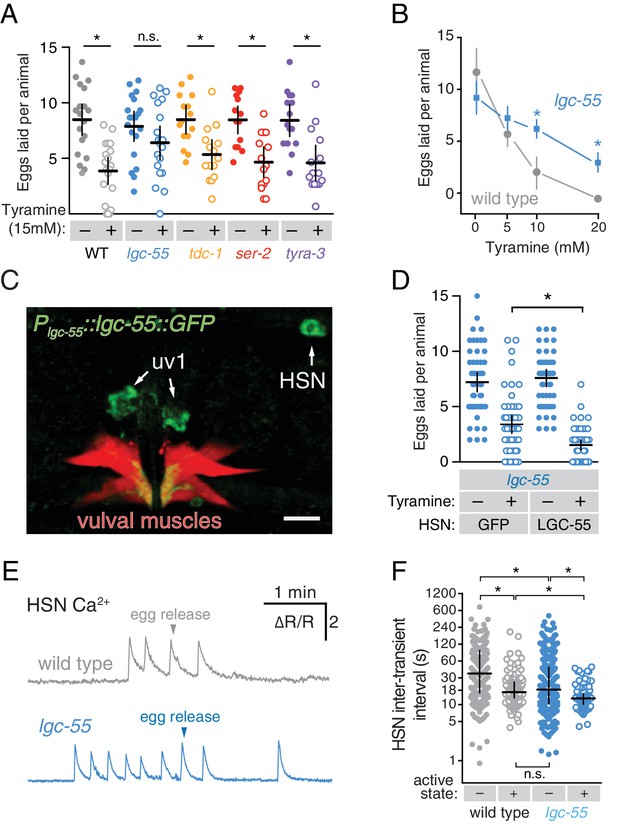
Tyramine inhibits egg laying, in part, through LGC-55 receptors on the HSNs.
(A) Exogenous tyramine inhibits egg-laying behavior and requires the LGC-55 receptor. Scatter plots and means showing the average number of eggs laid after 30 min by wild type (grey), tdc-1(n3419) tyramine biosynthetic enzyme mutant (blue), and lgc-55(tm2913), ser-2(pk1357), and tyra-3(ok325) tyramine receptor mutant animals (orange, red, and purple, respectively) on plates without (–, closed circles) or with (+, open circles) 15 mM tyramine. Error bars indicate 95% confidence intervals, and asterisks indicate significant responses (p<0.01) while n.s. is not significant (p>0.05). (B) Dose-response curve of tyramine inhibition on egg laying in wild-type and lgc-55 mutant animals. Asterisks indicate significant differences at 10 mM (p<0.0001) and 20 mM tyramine (p=0.0164). (C) LGC-55 is expressed in HSN, uv1, and vulval muscles. Expression of LGC-55 tagged with GFP compared to vulval muscle mCherry was visualized using confocal microscopy; scale bar is 10 µm. GFP is localized to the HSN and uv1 cell bodies (arrows) as well as the ventral tips of the vulval muscles. (D) Re-expression of LGC-55 in HSN restores tyramine inhibition of egg laying. Scatter plots of eggs laid by lgc-55 mutants expressing either GFP or lgc-55 in the HSN from the tph-1 promoter. Asterisk indicates significant differences in egg laying (p=0.0029). (E) Loss of LGC-55 increases HSN activity. Representative Ca2+ ratio traces show HSN activity in wild type (top, grey) and lgc-55 mutant animals (bottom, blue) during the active state. (F) Scatter plots and medians of HSN inter-transient intervals in wild type and lgc-55 mutant animals during the egg-laying inactive (–, closed circles) and active (+, open circles) states. Asterisks indicate statistically significant differences (p<0.0001).
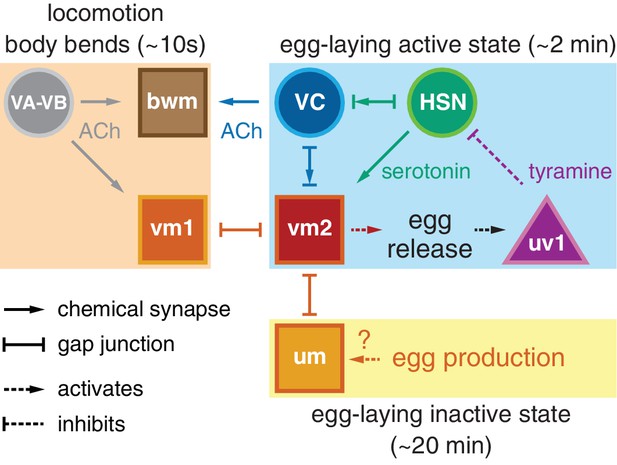
Working model of how circuit connectivity, signaling, and activity contribute to the observed rhythms that accompany the active and inactive egg-laying behavior states.
VA and VB motor neuron synapses release acetylcholine (ACh) that rhythmically excites the body wall muscles (bwm) and vm1 vulval muscles during each locomotion body bend, every ~10 s. Eggs are produced from each gonad arm every 10 min, and we propose the accumulation of 2–3 eggs mechanically excites the uterine muscles (um) facilitating exit from the inactive state. Sustained bursts of HSN command motor neuron activity trigger serotonin release that drives ~2 min active states. HSN also makes synapses and gap junctions with the cholinergic VC motor neurons, and VC makes synapses and gap junctions with the vm2 muscles whose contraction allows egg release. Passage of eggs mechanically activates the uv1 cells triggering release of tyramine and neuropeptides, which inhibit HSN activity to terminate the active state. See text for further details.
Videos
Ratio recording of HSN Ca2+ transients during the egg-laying active state.
High Ca2+ is indicated in red while low calcium is in blue. Large panel is an expanded view showing the freely-moving animal that has been contrast enhanced to make the worm and its laid eggs visible. Inset is cropped to a small area containing the HSN, and stabilized to remove movement. Text labels indicate when Ca2+ transients and egg release occur.
Ratio recording of VC Ca2+ transients during the egg-laying active state.
High Ca2+ is indicated in red while low calcium is in blue. Contrast is enhanced to make the worm visible, although the laid eggs are not easily visible. Text labels indicate when vulval muscle (vm) twitches or egg-laying contractions occur, which result in small or large displacements of the VC4 and VC5 cell bodies, respectively.
Ratio recording of vulval muscle Ca2+ transients during the egg-laying active state.
High Ca2+ is indicated in red while low calcium is in blue. Large panel is an expanded view showing the freely-moving animal that has been contrast enhanced to make the worm and its laid eggs visible. Inset is cropped to a small area containing the vulval muscles, and stabilized to remove movement. Text labels indicate when Ca2+ transients and egg release occur.
Activation of the HSNs using Channelrhodopsin-2 induces the egg-laying active state.
Animals were recorded for a total of 90 s with continuous blue light stimulation beginning at 30 s and ending at 60 s, during which five eggs are laid. Recording is sped up three-fold.
Activation of the VCs using Channelrhodopsin-2 induces animal paralysis and shortening of body length.
Animals were recorded for a total of 90 s with continuous blue light stimulation beginning at 30 s and ending at 60 s. Recording is sped up 3-fold.
Ratio recording of uv1 Ca2+ transients during the egg-laying active state.
High Ca2+ is indicated in red while low calcium is in blue. Large panel is an expanded view showing the freely-moving animal. Inset is cropped to a small area containing the uv1s, and stabilized to remove movement. Microscope is focused to image the two uv1 cells on the right side of this animal, while the two additional uv1 cells on the left side are out of focus and not visible. Text labels indicate when egg releases occur, each of which is followed by a uv1 Ca2+ transient.
Tables
Strains used in this study.
Strain | Feature | Genotype | Figures |
|---|---|---|---|
LX1832 | Strain for transgene production | lite-1(ce314) lin-15(n765ts) X | 1–6 |
LX1836 | HSN Channelrhodopsin | wzIs30 IV;lite-1(ce314) lin-15(n765ts) X | 3 |
LX1918 | vulval muscle GCaMP5, mCherry | lite-1(ce314) vsIs164 lin-15(n765ts) X | 1, 2 |
LX1932 | HSN Channelrhodopsin, vulval muscle GCaMP5, mCherry | wzIs30 IV; lite-1(ce314) vsIs164 lin-15(n765ts) X | 3 |
LX1938 | No HSNs, vulval muscle GCaMP5, mCherry | egl-1(n986dm) V; lite-1(ce314) vsIs164 lin-15(n765ts) X | 4 |
LX1986 | uv1 GCaMP5, mCherry | vsIs177; lite-1(ce314) lin-15(n765ts) X | 5 |
LX1960 | VC GCaMP5, mCherry | vsIs172; lite-1(ce314) lin-15(n765ts) X | 1, 2 |
LX1970 | HSN Channelrhodopsin, VC GCaMP5, mCherry | wzIs30 IV; vsIs172; lite-1(ce314) lin-15(n765ts) X | 3 |
LX2004 | HSN GCaMP5, mCherry | lite-1(ce314), vsIs183 lin-15(n765ts) X | 1, 2, 6 |
LX2038 | lgc-55 null mutant HSN GCaMP5, mCherry | lgc-55(tm2913) V lite-1(ce314) vsIs183 lin-15(n765ts) X | 6 |
MIA3 | VC Channelrhodopsin | keyIs3; lite-1(ce314) lin-15(n765ts) X | 3 |
MT13113 | tdc-1 null mutant; no tyramine biosynthesis | tdc-1(n3419) II | 6 |
N2 | Bristol strain | wild type | 6 |
OH313 | ser-2 null mutant | ser-2(pk1357) X | 6 |
VC125 | tyra-3 null mutant | tyra-3(ok325) X | 6 |
QW89 | lgc-55 null mutant | lgc-55(tm2913) V | 6 |
LX2096 | vulval muscle mCherry | vsIs191; lin-15(n765ts) X | |
LX2081 | pan-neuronal TagRFP | unc-119(ed3) I; otIs356 | |
LX2137 | vulval muscle mCherry, lgc-55::gfp | vsIs191; vsEx791 | 6 |
LX1330 | lgc-55 mutant expressing GFP in HSN from tph-1 promoter | lgc-55(tm2913) V; lin-15(n765ts) X vsEx557 | 6 |
LX1329 | lgc-55 mutant expressing LGC-55 in HSN from tph-1 promoter | lgc-55(thm2913) V; lin-15(n765ts) X vsEx558 | 6 |





