YAP is essential for mechanical force production and epithelial cell proliferation during lung branching morphogenesis
Figures
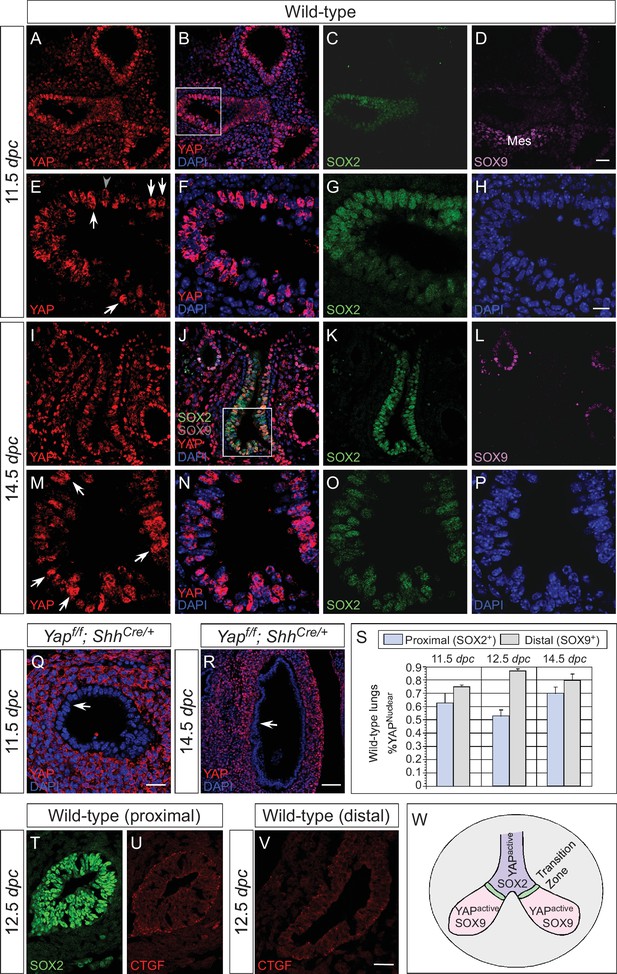
Nuclear YAP is active throughout the mouse lung epithelium during development.
(A–R) Immunostaining of lung sections collected from wild-type and Yapf/f; ShhCre/+ mice at 11.5 and 14.5 days post coitus (dpc). The proximal airway is marked by SOX2 expression, while the distal airway is distinguished by SOX9 expression. Note that the strong SOX9 signal in the lower left corner of (D) is derived from the mesenchyme (mes). Nuclear YAP could be frequently found in both SOX2+ and SOX9+ domains and was not restricted to the ‘transition zone’, a small SOX2+ domain abutting the SOX9+ compartment. The boxed regions in (B) and (J) were analyzed at a higher magnification as shown in (E–H) and (M–P), respectively. Representative epithelial cells with nuclear YAP (white arrows) or cytoplasmic YAP (grey arrowhead) are indicated in (E) and (M). Note that SOX2 and SOX9 staining is nuclear. YAP immunoreactivity was completely absent in the epithelium (arrow) but retained wild-type levels in the mesenchyme of Yapf/f; ShhCre/+ mice (Q,R), demonstrating the specificity of YAP antibodies used in this study. (S) Quantification of lung epithelial cells with nuclear YAP in both the proximal and distal airways. A high percentage of cells exhibited nuclear YAP expression along the entire lung epithelium. A small fraction of epithelial cells with nuclear YAP also had cytoplasmic YAP. n = 8 for 11.5 dpc; n = 10 for 12.5 dpc; n = 10 for 14.5 dpc. (T–V) Immunostaining of lung sections collected from wild-type mice at 12.5 dpc. Expression of CTGF, a YAP target, was detected in both the proximal and distal airways. CTGF signal was barely detectable in Yapf/f; ShhCre/+ lungs (not shown). (W) Schematic diagram that illustrates the distribution of active nuclear YAP throughout the entire lung epithelium. Scale bar = 25 μm for A–D, I–L; 10 μm for E–H, M–P; 25 μm for Q; 75 μm for R; 25 μm for T–V.
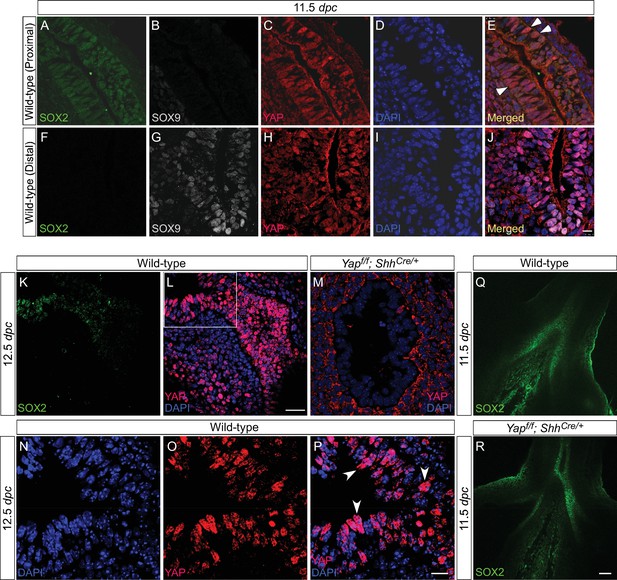
Active nuclear YAP is distributed throughout the mouse lung epithelium during development.
(A–P) Immunostaining of lung sections collected from wild-type mice at 11.5 and 12.5 days post coitus (dpc). The boxed region in (L) indicates areas shown in (N–P). The proximal airway is marked by SOX2 expression, while the distal airway is distinguished by SOX9 expression. Nuclear YAP can be frequently found in both SOX2+ and SOX9+ domains and is not restricted to the junction (the ‘transition zone’) between SOX2+ and SOX9+ domains. Representative cells with nuclear YAP (arrowhead) are indicated in (E,P). YAP immunoreactivity is completely absent in the epithelium (but present in the mesenchyme) of Yapf/f; ShhCre/+ mice (M), demonstrating the specificity of YAP antibodies used in this study. Immunofluorescence and immunohistochemistry yielded the same results (data not shown for immunohistochemistry). (Q–R) Whole-mount immunostaining of wild-type and Yap mutant lungs at 11.5 dpc. Distinct domains of SOX2 were discerned in the absence of YAP. Scale bar = 10 μm for A–J; 25 μm for K, L; 10 μm for N–P; 50 μm for Q, R.
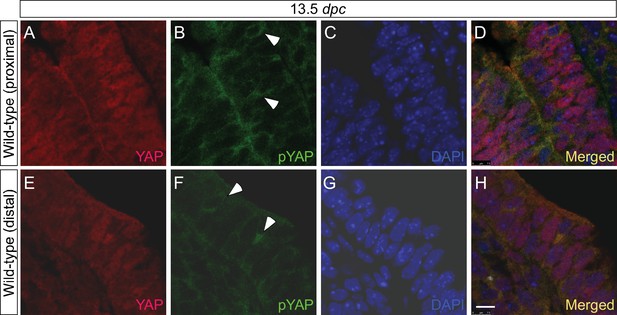
YAP and phospho-YAP are detected in both the proximal and distal airways during lung development.
(A–H) Immunostaining of lung sections collected from wild-type mice at 13.5 days post coitus (dpc). The proximal airway is marked by SOX2 expression, while the distal airway is distinguished by SOX9 expression (not shown). Nuclear YAP can be frequently found in both SOX2+ and SOX9+ domains. Similarly, phospho-YAP at S112 (pYAP) could be detected in both the proximal and distal airways. pYAP levels were, in general, higher in the proximal than distal epithelium but pYAP levels varied significantly from cell to cell in both the proximal and distal airways. Representative cells with higher levels of pYAP (arrowhead) are indicated in (B,F). In many cells, low levels of pYAP were associated with the presence of nuclear YAP. This is consistent with a model in which pYAP is sequestered by 14-3-3 proteins in the cytoplasm and degraded but also indicate a dynamic shuttling and distribution of YAP along the entire airway epithelium. Similar results were obtained for lungs collected at 12.5 dpc. Scale bar = 7.5 μm for A–H.
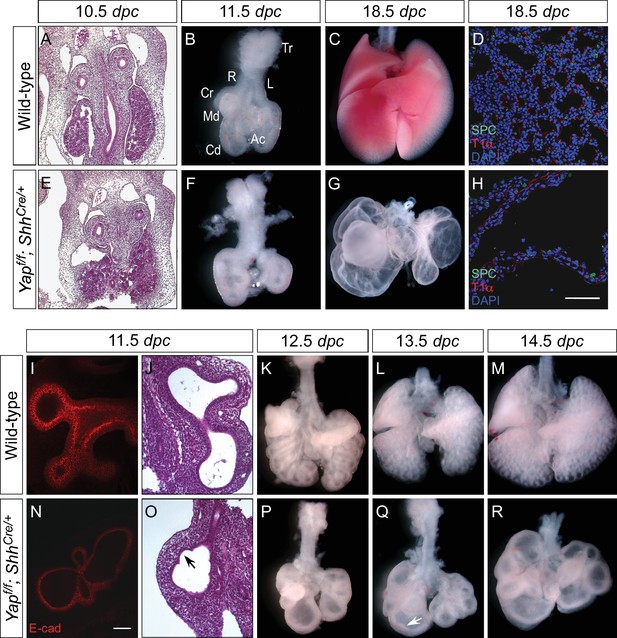
Loss of epithelial Yap leads to lung cysts.
(A,E) Hematoxylin and eosin-stained sections of wild-type and Yapf/f; ShhCre/+ embryos at 10.5 dpc. No apparent difference in the morphology of lung buds was observed between wild-type and Yap mutants. (B,C,F,G) Ventral view of dissected lungs from wild-type and Yapf/f; ShhCre/+ mice at 11.5 and 18.5 dpc. Five buds were produced in both control and Yap-deficient lungs at 11.5 dpc; defective lung branching along the entire lung epithelium could already be detected at this stage in Yap mutants. As lung development proceeded, failure to execute a stereotyped program of branching in the absence of Yap resulted in lungs consisting only of multiple cysts at 18.5 dpc. R, right; L, left; Tr, trachea; Cr, cranial; Md. middle; Cd, caudal; Ac, accessory. (D,H) Immunostaining of lung sections collected from wild-type and Yapf/f; ShhCre/+ mice at 18.5 dpc. Cell types in the proximal airway of Yapf/f; ShhCre/+ mice failed to be specified. For instance, expression of markers for Clara [club] cells (CC10+), ciliated cells (acetylated-tubulin [Ac-tub]+) and pulmonary neuroendocrine cells (CGRP+) were barely detectable (not shown). Reduction in the expression of distal lung cell markers, such as SPC (type II cells) and T1α (type I cells), in the cysts of Yapf/f; ShhCre/+ lungs was also noted. (I,N) Whole-mount immunostaining of wild-type and Yapf/f; ShhCre/+ lungs at 11.5 dpc by two-photon microscopy. Lung epithelium was identified by E-cadherin (E-cad). (J,O) Hematoxylin and eosin-stained sections of wild-type and Yapf/f; ShhCre/+ embryos at 11.5 dpc. The arrow points to ‘evagination’ of epithelial cells in Yap-deficient lung buds. (K–M, P–Q) Ventral view of dissected lungs from wild-type and Yapf/f; ShhCre/+ embryos at the developmental stages indicated. Epithelial ‘evagination’ (arrow in Q) could be seen in Yap-deficient lung buds but they failed to produce new buds subsequently. All views are ventral. Scale bar = 200 μm for C,G; 50 μm for D,H; 100 μm for I,N.
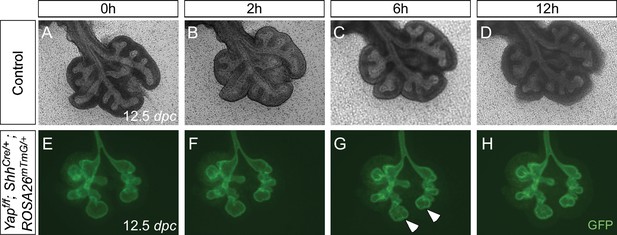
Aborted branching in the absence of epithelial Yap in the lung.
(A–H) Time-lapse microscopy of dissected of lungs from control and Yapf/f; ShhCre/+; ROSA26mTmG/+ mice at 12.5 days post coitus (dpc). Lungs were grown on nuclepore membranes for live imaging. At this stage, limited lung branching in Yap-deficient mutants would soon stop. While ‘evaginations’ (arrowheads in G,H) from existing lung buds were noted in the absence of YAP, no new lung buds were generated. Branching occurred in control lungs, but the rate of lung development was slowed down in ex vivo lung explants.
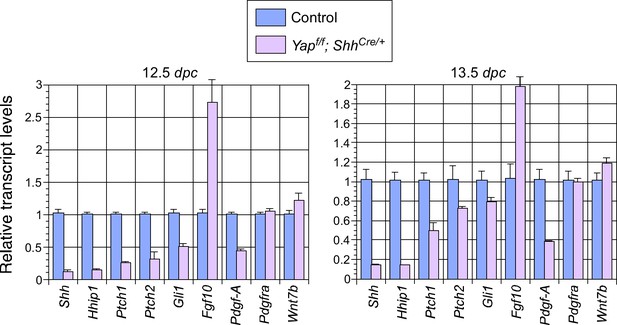
Changes in major signaling pathways in the absence of YAP.
qPCR analysis of lungs from control and Yapf/f; ShhCre/+ lungs at 12.5 and 13.5 days post coitus (dpc). The expression levels of Shh were reduced while Fgf10 was upregulated in Yap-deficient lungs. This is consistent with a negative regulatory relationship between Shh and Fgf10. How YAP influences the expression of Shh and Fgf10 and interacts with major signaling pathways requires further investigation. Note that the use of ShhCre has contributed to the reduction in Shh expression levels although it is unlikely to be solely responsible for the low levels of Shh in the absence of Yap.
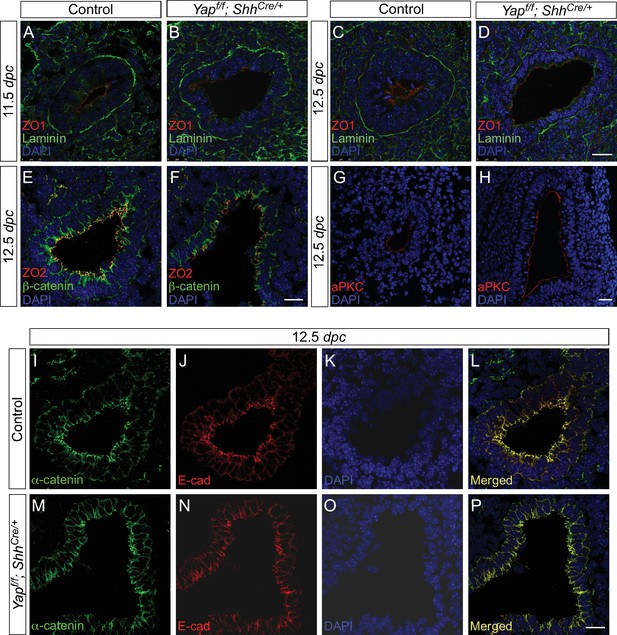
Cell junctions and cell polarity are not disrupted due to the loss of epithelial Yap in the lung.
(A–P) Immunostaining of lung sections from control and Yapf/f; ShhCre/+ lungs at 11.5 and 12.5 days post coitus (dpc). No apparent difference in the distribution of apical (aPKC), subapical (ZO1 and ZO2) and basal (Laminin) markers was found between control and Yap mutant lungs. Moreover, the distribution of markers for tight junctions (e.g. ZO1 and ZO2) and adherens junctions (e.g. E-cadherin, β-catenin and α-catenin) was similar between control and Yap-deficient lungs. This suggests that cell junction and cell polarity remain intact in the absence of Yap. Scale bar = 25 μm for A–D, G–H; 100 μm for E–F, I–P.
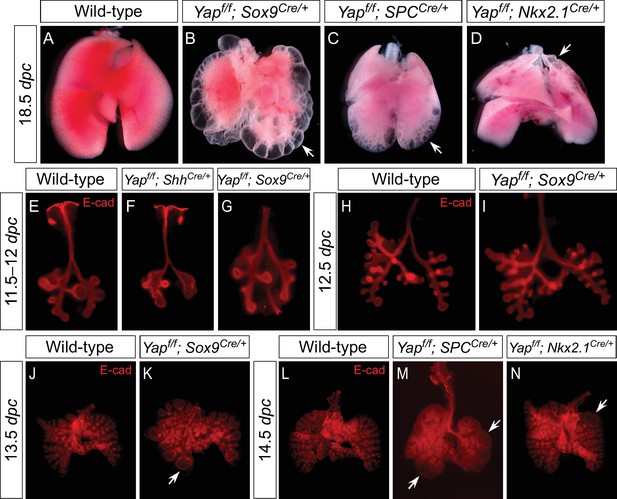
Regional loss of epithelial Yap leads to localized lung cysts.
(A–D) Ventral view of dissected lungs from wild-type, Yapf/f; Sox9Cre/+, Yapf/f; spcCre/+ and Yapf/f; Nkx2.1Cre/+ mice at 18.5 dpc. Lung cysts in Yapf/f; Sox9Cre/+ and Yapf/f; spcCre/+ mice were largely confined to the distal airway (arrows in B,C), while lung cysts were found in the upper lobes (arrow in D) of Yapf/f; Nkx2.1Cre/+ mice. The location of lung cyst formation is correlated with the sites of strong Cre activity and Yap removal. This suggests that loss of Yap at a given region leads to localized lung cysts. (E–N) Whole-mount immunostaining of dissected lungs from wild-type, Yapf/f; ShhCre/+, Yapf/f; Sox9Cre/+, Yapf/f; spcCre/+ and Yapf/f; Nkx2.1Cre/+ mice at the stages indicated. Lung epithelium was visualized by E-cadherin (E-cad). While defective branching was apparent in Yapf/f; ShhCre/+ lungs at 11.5 dpc, branching defects and cyst formation in Yapf/f; Sox9Cre/+ lungs did not appear until 13.5 dpc. Cyst formation in Yapf/f; Sox9Cre/+ lungs was confined to the distal airways (arrow in K). Similarly, cyst formation in Yapf/f; spcCre/+ lungs was detected primarily in the distal airways (arrows in M). By contrast, cyst formation was found in the upper lobes (arrow in N) of Yapf/f; Nkx2.1Cre/+ lungs. This suggests that loss of Yap at a given region leads to localized lung cysts. All views are ventral.
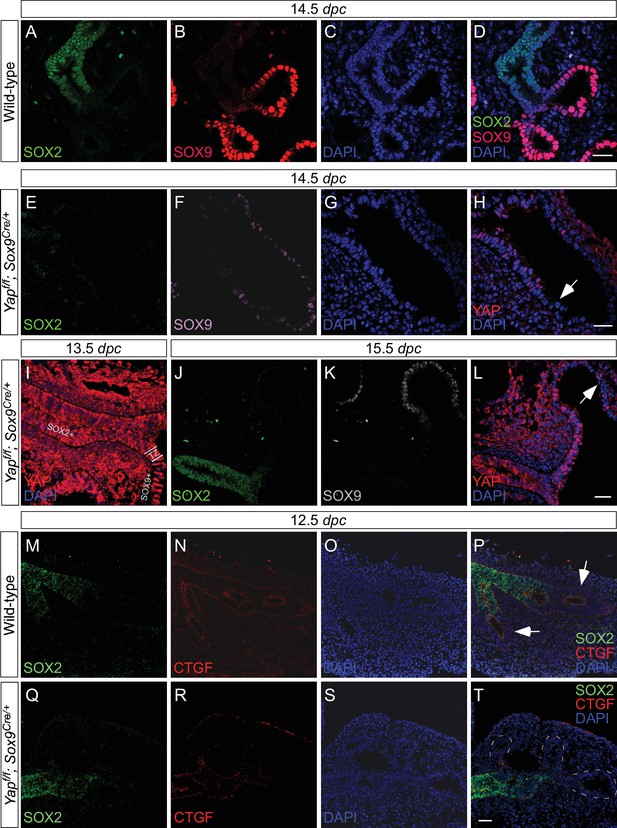
Deletion of Yap in the distal lung epithelium by the Sox9Cre mouse line.
Immunostaining of lung sections collected from wild-type and Yapf/f; Sox9Cre/+ at the stages indicated. (A–D) SOX2 expression marks the proximal airway while the distal airway is distinguished by SOX9 expression. The region where SOX2 and SOX9 expression overlaps is a transition area. (E–L) YAP was lost primarily in distal airways in Yapf/f; Sox9Cre/+ mice, while sporadic loss of YAP can be found in the proximal airway. Loss of YAP was most apparent in the more distal part (arrow in H) of the distal airway, while residual YAP could be found in the more proximal part of the distal airway. (M–T) Removal of YAP in distal airways (dotted lines in T) of Yapf/f; Sox9Cre/+ lungs at 12.5 dpc is associated with loss of CTGF, a direct YAP target. Arrows in P point to CTGF expression in distal airways of wild-type lungs. Together, these results suggest that lung cyst formation in distal airways of Yapf/f; Sox9Cre/+ mice is due to loss of YAP in the distal airway. Scale bar = 25 μm for A–D, E–H; 250 μm for I–L; 100 μm for M–T. dpc, days post coitus.
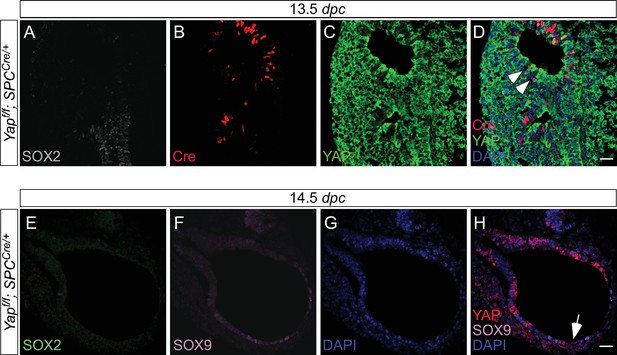
Expression of spcCre is associated with loss of YAP in SOX9+ distal airways.
(A–D) Immunostaining of lung sections collected from Yapf/f; spcCre/+ mice at 13.5 days post coitus (dpc). SOX2 expression marks the proximal airway, while the distal airway is distinguished by SOX9 expression (not shown). High levels of spcCre expression were largely confined to the distal airway, where spcCre expression in a given epithelial cell was correlated with loss of YAP immunoreactivity (e.g. arrowheads in D). (E–H) Immunostaining of lung sections collected from Yapf/f; spcCre/+ mice at 14.5 dpc. Only distal airways are shown. YAP was lost mainly in distal airways in Yapf/f; spcCre/+ mice while sporadic loss of YAP was found in the proximal airway. Loss of YAP was most apparent in the more distal part (arrow in H) of the distal airway, while residual YAP could be found in the more proximal part of the distal airway. Together, these results suggest that lung cyst formation in distal airways of Yapf/f; spcCre/+ mice is due to loss of YAP in the distal airway. Scale bar = 25 μm for A–D; E–H.
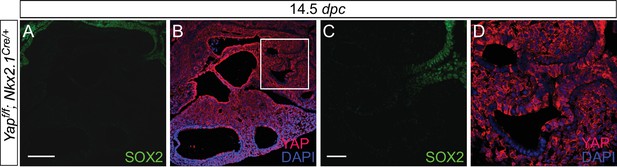
Expression of Nkx2.1Cre is associated with loss of YAP in the upper lobes.
(A–D) Immunostaining of lung sections collected from Yapf/f; Nkx2.1Cre/+ mice at 14.5 days post coitus (dpc). SOX2 expression marks the proximal airway, while the distal airway is distinguished by SOX9 expression (not shown). YAP was lost mainly in the upper lobe in Yapf/f; Nkx2.1Cre/+ lung. Loss of YAP was more apparent in the distal airway, while loss of YAP was sporadic in the proximal airway. Lung cyst formation was primarily observed in the distal airway. The boxed region in (B) indicates areas shown in (C,D). Scale bar = 250 μm for A,B; 250 μm for C,D. Sox2 expression was present in sporadic Yap-deficient cells in the transition zone induced by Sox9Cre, spcCre or Nkx2.1Cre. This suggests that Sox2 expression is not controlled by YAP.
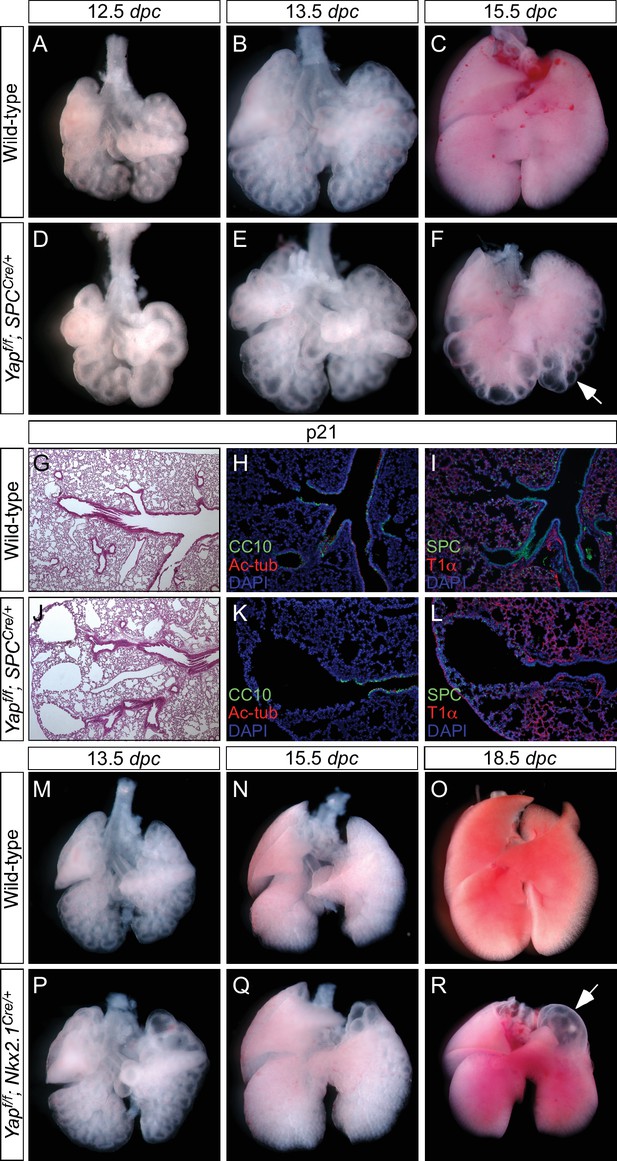
Loss of epithelial Yap at restricted areas leads to localized lung cysts.
(A–F, M–R) Ventral view of dissected lungs from wild-type, Yapf/f; spcCre/+ and Yapf/f; Nkx2.1Cre/+ mice at various developmental stages as indicated. Lung cysts in Yapf/f; spcCre/+ mice were largely confined to the distal airway (arrow in F), while lung cysts were found in the upper lobes (arrow in R) of Yapf/f; Nkx2.1Cre/+ mice. The location of lung cysts is correlated with the sites of active Cre activity. (G, J) Hematoxylin and eosin-stained sections of lungs from wild-type and occasional adult survivors of Yapf/f; spcCre/+ mice. Multiple cysts in the distal airway were present. (H, I, K, L) Immunstaining of lung sections from control and Yapf/f; spcCre/+ mice. Lung cysts expressed markers of cell types in the distal airway albeit at reduced levels. dpc, days post coitus; p, postnatal.
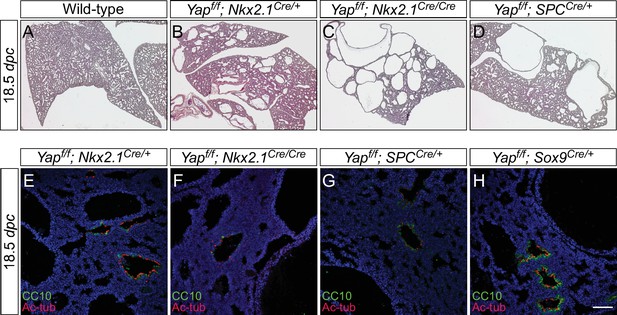
The dosage of Nkx2.1Cre and spcCre affects the severity of lung phenotypes.
(A–D) Hematoxylin and eosin-stained sections of lungs from wild-type, Yapf/f; Nkx2.1Cre/+ (one copy of Nkx2.1Cre), Yapf/f; Nkx2.1Cre/Cre (two copies of Nkx2.1Cre) and Yapf/f; spcCre/+ (one copy of spcCre) mice at 18.5 dpc. Removal of Yap was more efficient when two copies of Nkx2.1Cre or spcCre were present and lungs phenotypes were more severe. Data not shown for Yapf/f; spcCre/Cre (two copies of spcCre). (E–H) Immunostaining of lung sections collected from Yapf/f; Nkx2.1Cre/+, Yapf/f; Nkx2.1Cre/Cre, Yapf/f; spcCre/+ and Yapf/f; Sox9Cre/+ mice at 18.5 dpc. While more lung cysts were detected in Yapf/f; Nkx2.1Cre/Cre mice than Yapf/f; Nkx2.1Cre/+ mice, cell types in the proximal airway or regions where no cysts were present appeared to be properly specified as indicated by their expression of CC10 (Clara cell marker) and acetylated-tubulin [Ac-tub] (ciliated cell marker). Similarly, cell types in the proximal airway were specified in Yapf/f; spcCre/+ and Yapf/f; Sox9Cre/+ mice. This suggests that lung cyst formation is correlated with Cre activity and is independent of proximal airway specification. Scale bar = 75 μm for E–H. dpc, days post coitus.
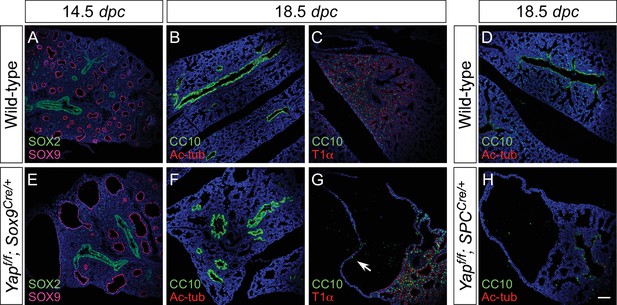
Proximal airway development is unaffected by loss of YAP in the distal airway.
(A–H) Immunostaining of lung sections collected from wild-type, Yapf/f; Sox9Cre/+ and Yapf/f; spcCre/+ mice at 14.5 and 18.5 dpc as indicated. Proximal-distal airway specification was unaffected in Yapf/f; Sox9Cre/+ lungs as revealed by proper expression of the proximal (SOX2) and distal (SOX9) airway markers despite cyst formation in the SOX9+ lung epithelium. Moreover, cell types in the proximal airway of Yapf/f; Sox9Cre/+ and Yapf/f; spcCre/+ mice were specified. For example, cells expressing CC10 (Clara cell marker) and acetylated-tubulin [Ac-tub] (ciliated cell marker) were detected in a similar pattern between control and mutant lungs. Scale bar = 100 μm for A–H.
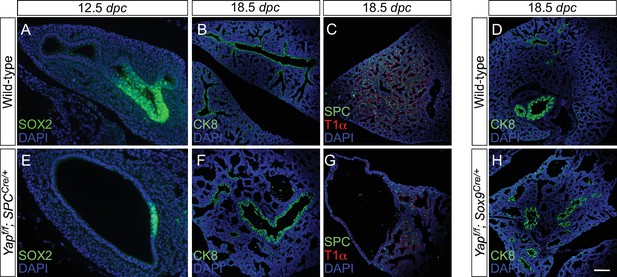
Disruption of the distal airway in Yapf/f; spcCre/+ and Yapf/f; Sox9Cre/+ mouse lungs.
(A–H) Immunostaining of lung sections collected from wild-type, Yapf/f; spcCre/+ and Yapf/f; Sox9Cre/+ mice at 12.5 and 18.5 days post coitus (dpc) as indicated. Lung cysts were detected in the distal airway of Yapf/f; spcCre/+ and Yapf/f; Sox9Cre/+ mice. Cell types in the proximal airway of Yapf/f; spcCre/+ and Yapf/f; Sox9Cre/+ mice appeared to be properly specified as indicated by their expression of CC10 (Clara cell marker) and acetylated-tubulin [Ac-tub] (ciliated cell marker). SOX2 expression, which marks the proximal airway, was detected in a localized region of the lung cyst in Yapf/f; spcCre/+ mice at 12.5 dpc. This suggests that while lung branching morphogenesis was disrupted in Yapf/f; spcCre/+ lungs, specification of the proximal-distal airway was unaffected. Scale bar = 50 μm for A, E; 100 μm for B–D, F–H.
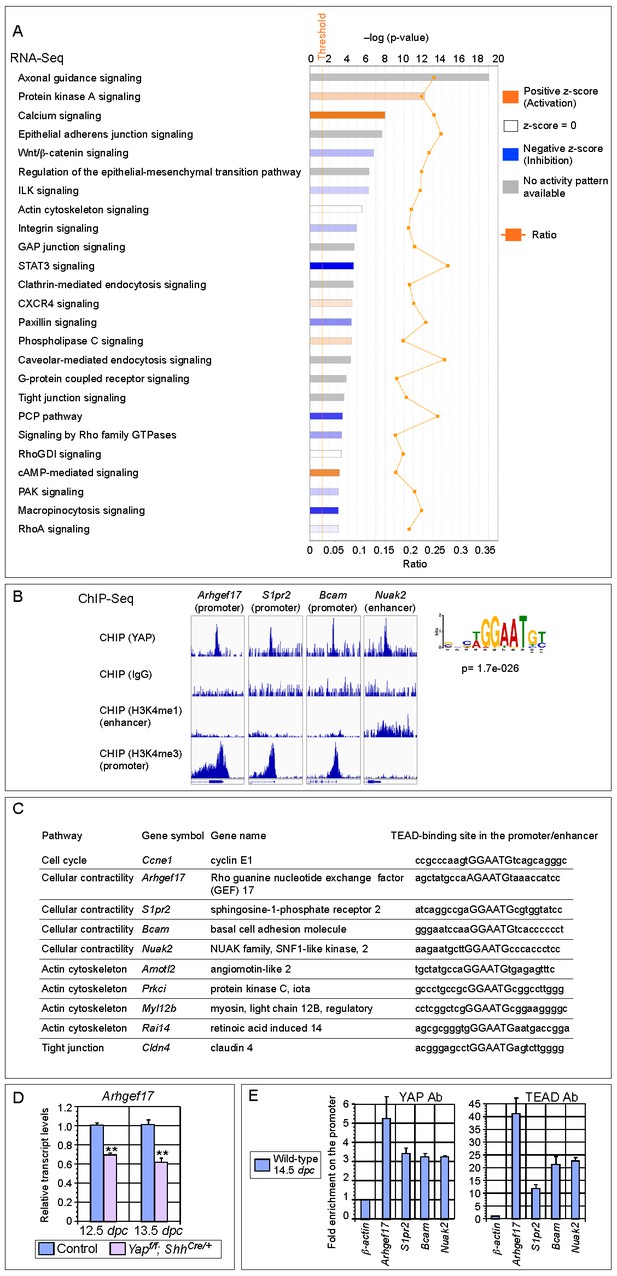
Genome-wide expression analysis identifies YAP targets that are involved in regulating cell proliferation and cellular contractility.
(A) Pathway analysis of differentially expressed genes identified in RNA-Seq analysis of control and Yap-deficient lungs at 12.5 dpc. p-value and z-score were shown. The calculated z-score indicates the prediction of overall increase or decrease in the activity of a pathway. For z-score >0, the pathway is predicted to be activated; for z-score <0, the pathway is predicted to be inhibited. The ratio indicates the ratio of genes from the dataset that map to the pathway divided by the total number of genes that map to the same pathway. The orange line indicates a threshold of -log(p-value) = 1.30 (p<0.05) and the cutoff was set at -log(p-value) = 3 (p<0.001). Several pathways involved in regulating the cell cycle, cellular contractility and cell adhesion were uncovered. (B) Visualization of YAP-enriched peaks in ChIP-Seq for the indicated genes using Integrative Genomics Viewer (IGV). The peaks were associated with the promoter or enhancer as determined by ChIP-Seq for histone modifications. The conserved TEAD-binding site that is present in YAP-enriched peaks was also shown. (C) A list of bona fide YAP targets in the lung. These YAP targets not only contain a TEAD-binding site in their promoter/enhancer but their expression was also reduced in Yap-deficient lungs by RNA-Seq or qPCR analysis. Many of these YAP targets are involved in regulating cell proliferation and cellular contractility. (D) qPCR analysis of Arhgef17 in wild-type and Yapf/f; ShhCre/+ lungs (n ≥ 3 for each group) at 12.5 and 13.5 dpc. The mRNA levels of Arhgef17 were significantly reduced in the absence of Yap. Note that mRNA from the whole lung was used for qPCR analysis and Arhgef17 in the mesenchyme was presumably unaffected by ShhCre. All values are means ± SEM. (**) p<0.01 (unpaired Student’s t-test). (E) ChIP-qPCR of YAP targets identified from ChIP-Seq of wild-type lungs. Both YAP and TEAD were found to reside at the promoter of YAP targets. We also found that neither YAP nor TEAD was enriched on the Sox2 promoter (not shown), suggesting that YAP does not directly regulate Sox2 expression in the developing lung.
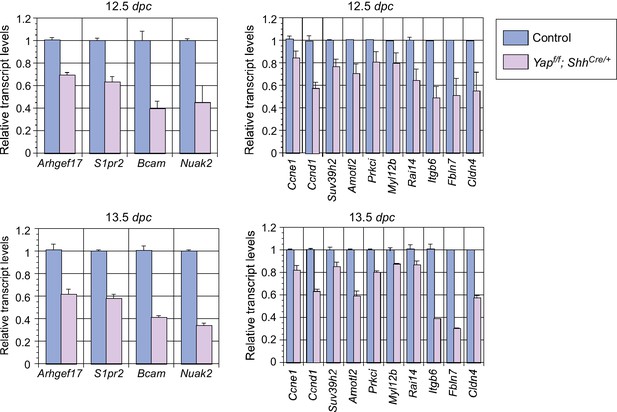
Analysis of the transcript levels of YAP targets in the mouse lung.
qPCR analysis of YAP targets identified from RNA-Seq analysis of wild-type and Yap-deficient lungs at the stages indicated. The mRNA levels of various YAP targets that control cell proliferation and cellular contractility were significantly reduced in the absence of YAP. All values are means ± SEM. dpc, days post coitus.
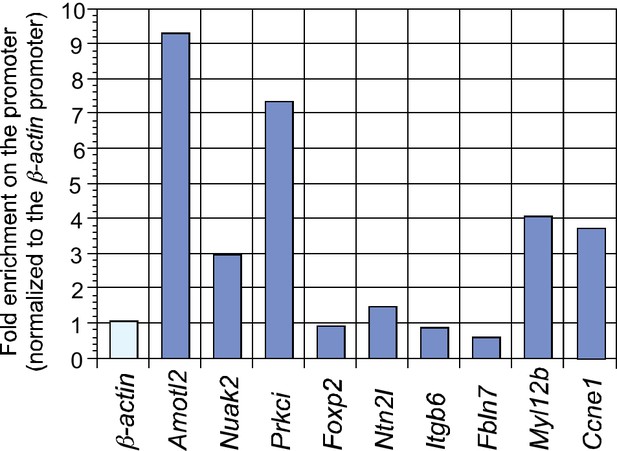
ChIP analysis identifies new YAP target genes in the mouse lung.
ChIP analysis of a select group of genes that were downregulated in Yap-deficient lungs by RNA-Seq and qPCR. YAP was significantly enriched at the promoters Amotl2, Nuak2, Prkci, Myl12b and Ccne1 at 14.5 days post coitus (dpc), indicating that they are bona fide YAP target genes. By contrast, no enrichment of YAP or TEAD was found at the Sox2 promoter (data not shown).
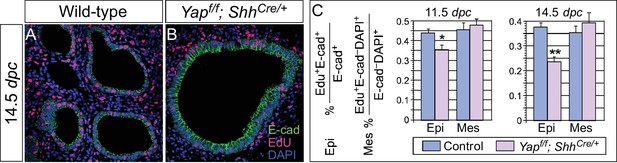
Cell proliferation is reduced in the epithelium of Yap-deficient lungs.
(A,B) Immunostaining of lung sections collected from wild-type and Yapf/f; ShhCre/+ mice injected with EdU at 14.5 dpc. Lung epithelial cells were distinguished by E-cadherin (E-cad) staining. (C) Quantification of cell proliferation rate in the epithelium (Epi) and mesenchyme (Mes) of control and Yapf/f; ShhCre/+ lungs at 11.5 and 14.5 dpc. The rate of epithelial cell proliferation was calculated as the ratio of the number of EdU+ epithelial cells (EdU+E-cad+) to the number of epithelial cells (E-cad+). An apparent reduction in the percentage of EdU+ cells was detected in the epithelium of Yapf/f; ShhCre/+ lungs compared to controls (n ≥ 8 for each group). By contrast, cell proliferation in the mesenchyme, where YAP was untouched by ShhCre, was indistinguishable between control and Yapf/f; ShhCre/+ lungs. The rate of mesenchymal cell proliferation was calculated as the ratio of the number of EdU+ mesenchymal cells (EdU+E-cad– DAPI+) to the number of mesenchymal cells (E-cad– DAPI+). All values are means ± SEM. (*) p<0.05; (**) p<0.01 (unpaired Student’s t-test). We found that most epithelial cells in control or Yap-deficient lungs at 11.5 dpc expressed Ki67. This is likely due to a short cell cycle at this stage, which makes Ki67 (as well as other commonly used markers) unsuitable for accurate detection of differences in cell proliferation.
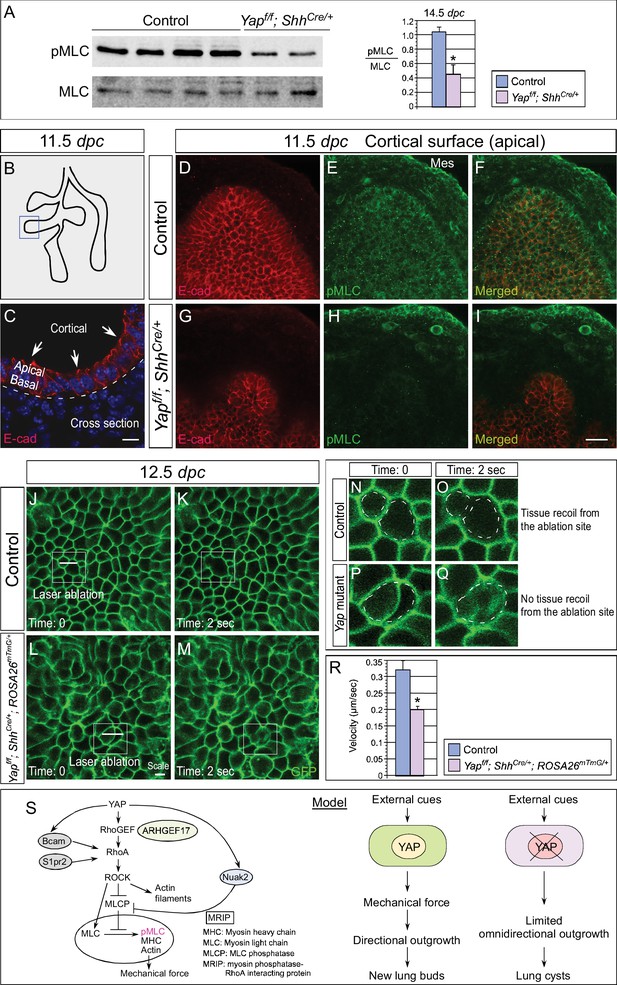
Cortical pMLC is greatly reduced in the epithelium of Yap-deficient lungs and mechanical force production is compromised.
(A) Western blots of lysates derived from control and Yapf/f; ShhCre/+ lungs at 14.5 dpc. Protein levels of phosphorylated myosin light chain (pMLC) were significantly reduced in the absence of Yap. Moreover, the ratio of pMLC to MLC protein levels was also diminished in Yap mutant lungs. (B) Schematic diagram of wild-type lungs at 11.5 dpc. The boxed region indicates areas shown in D–I. (C) Cross-section of a lung bud that illustrates the apical and basal surface and cortical view along the apical surface. (D–I) Whole-mount immunostaining of control and Yapf/f; ShhCre/+ lungs at 11.5 dpc by two-photon microscopy. This enabled visualization of the distribution of pMLC along the cortical surface of epithelial cells located at the apical surface of the lung bud. Lung epithelium was identified by E-cadherin (E-cad). Cortical pMLC was detected in both the epithelium and mesenchyme (mes) of control lungs. By contrast, cortical pMLC could not be detected in the epithelium but retained wild-type levels in the mesenchyme of Yapf/f; ShhCre/+ lungs. (J–M) Laser ablation of control and Yapf/f; ShhCre/+; ROSA26mTmG/+ lungs. Lung epithelial cells were labeled by GFP from the ROSA26mTmG/+ allele induced by ShhCre. Laser ablation of lung epithelial cells by two-photon microscopy (indicated by bars in J,L) triggered recoiling of non-injured neighboring cells in control but not in Yap-deficient lungs (see Videos 1 and 2). Snapshots of the lung epithelium post-ablation were shown. The boxed regions in (J–M) indicate areas shown in (N–Q). (N–Q) The dotted lines in (N,O) demarcate the cell boundary prior to laser ablation in control lungs. Surrounding cells recoiled from the ablation site as indicated by the space between the dotted line and the new position of cells (O). By contrast, no tissue recoil was found in Yap mutant lungs (Q). (R) The velocity of epithelial recoil was measured in control and Yap-deficient lungs after laser ablation. A reduction in velocity was found in the absence of YAP (n ≥ 6 for each group). All values are means ± SEM. (*) p<0.05 (unpaired Student’s t-test). (S) Schematic diagram of signaling cascades that control the production of pMLC and mechanical force. Our model suggests that YAP regulates multiple pathways to regulate pMLC generation. In addition to a YAP-ARHGEF17-RhoA-ROCK pathway, YAP also induces the expression of Bcam, S1pr2 and Nuak2 to enhance pMLC levels. BCAM and S1PR2 activate RhoA, while NUAK2 inhibits MLCP (myosin light chain phosphatase) in a RhoA-independent manner. The employment of a signaling network ensures the production of appropriate amounts of pMLC required for lung branching. Mechanical force production is perturbed in Yap-deficient lungs due to reduced pMLC. This would contribute to defective lung branching and cyst formation. Scale bar = 10 μm for C; 25 μm for D–I.
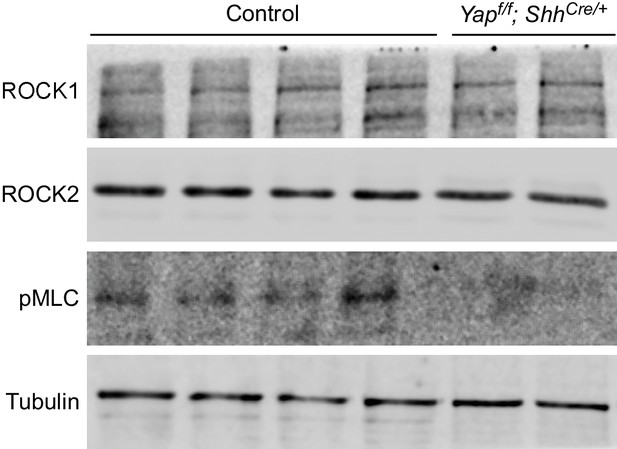
Reduction in pMLC protein levels in Yap mutant lungs.
Western blots of lysates derived from wild-type and Yapf/f; ShhCre/+ lungs at 14.5 dpc. Protein levels of pMLC were significantly reduced in the absence of Yap, while ROCK1 and ROCK2 protein levels were not noticeably altered. This suggests that the activities and not protein levels of ROCK1/2 are reduced in the absence of Yap. Reduction in pMLC protein levels would disrupt mechanical force production. Tubulin was used as the loading control. dpc, days post coitus.
Videos
Movement of surrounding cells after laser ablation of lung epithelial cells in control mice.
Immediately following laser ablation by two-photon microscopy, movement of epithelial cells (labeled by GFP) surrounding the injured cells were imaged at one frame per sec (fps) for 50 s. The movie was played back at 10 pfs. Video 1 is related to Figure 7J,K.
Movement of surrounding cells after laser ablation of lung epithelial cells in Yapf/f; ShhCre/+; ROSA26mTmG/+ mice.
Immediately following laser ablation by two-photon microscopy, movement of epithelial cells (labeled by GFP) surrounding the injured cells were imaged at one frame per sec (fps) for 50 s. The movie was played back at 10 pfs. Video 1 is related to Figure 7L,M.





