Chronic social isolation reduces 5-HT neuronal activity via upregulated SK3 calcium-activated potassium channels
Figures
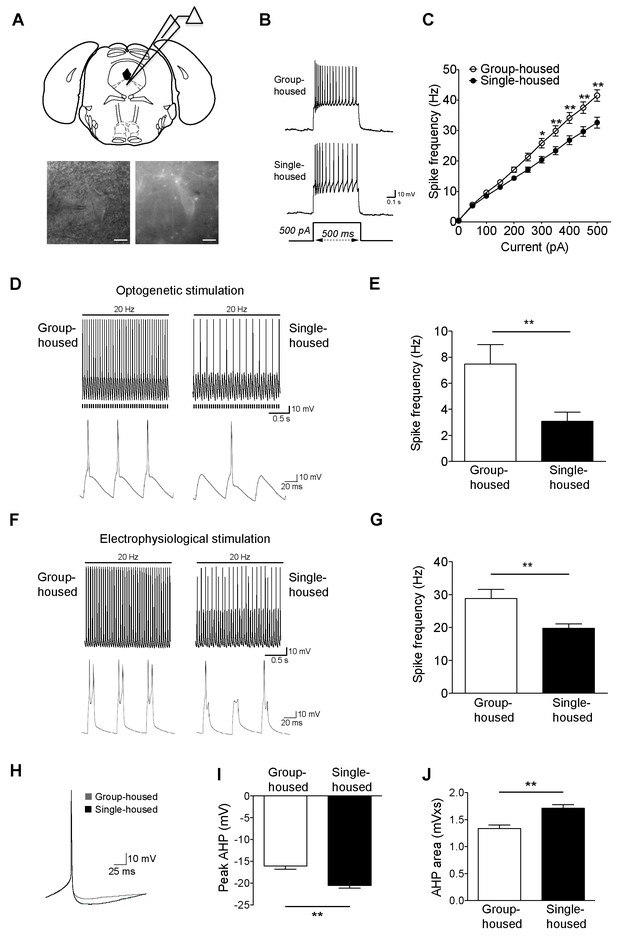
Reduced firing of dorsal raphe 5-HT neurons after chronic social isolation is accompanied by an increase in afterhyperpolarization (AHP).
(A) Schematic representation of a coronal brainstem section (above) comprising dorsal raphe nucleus (adapted from Paxinos and Franklin, 2001) where 5-HT neurons are recorded. IR-DIC and EYFP fluorescence images (below) of a dorsal raphe 5-HT neuron being approached by a recording pipette. Scale bars, 10 μM. (B) Current-clamp traces of a 5-HT neuron from a group-housed (above) and a single-housed (below) mouse in response to a 500 pA depolarizing step. (C) Input-output curve showing spike frequency (Hz) of 5-HT neurons in response to a series of depolarizing current (pA) injections. The firing frequency (Hz) of 5-HT neurons from single-housed mice (n = 38 neurons) is reduced compared to 5-HT neurons from group-housed mice (n = 33 neurons) indicating reduced excitability (two-way repeated-measures ANOVA, effect of housing, F (1690) = 9.19, p=0.003; Newman-Keuls posthoc test, *p<0.05, **p<0.01) (D) Current-clamp recordings of a 5-HT neuron from a group-housed mouse (left, inset below) and a single-housed mouse (right, inset below) showing action potential firing in response to 20 Hz optogenetic stimulation by blue light. Note that the neuron from the group-housed mouse is able to respond with 20 Hz firing frequency to blue light whereas the neuron from the single-housed mouse responds with a 7 Hz firing frequency. (E) The frequency (Hz) of action potentials in response to optogenetic stimulation is reduced in 5-HT neurons from single-housed mice (n = 20 neurons) compared to neurons from group-housed mice (n = 17 neurons) (unpaired t-test, *p<0.05). (photocurrents; group-housed: −343 ± 32.3 pA, single-housed: −269 ± 29.4 pA, p=0.1) (F) Current-clamp recordings of a 5-HT neuron from a group-housed mouse (left, inset below) and a single-housed mouse (right, inset below) showing action potential firing in response to 20 Hz electrophysiological stimulation. The neuron from the group-housed mouse produced spike doublets in response to the strong electrophysiological stimulation (left inset). The neuron from the single-housed mouse responded with missing spikes (right inset). (G) The frequency (Hz) of action potentials in response to electrophysiological stimulation is reduced in 5-HT neurons from single-housed mice (n = 14 neurons) compared to neurons from group-housed mice (n = 13 neurons) (unpaired t-test, **p<0.01). (H) The first spike of the 25 pA depolarizing step of a current-clamp trace from each 5-HT neuron from group-housed (n = 33 neurons) and single-housed (n = 37 neurons) mice is averaged in order to obtain the resulting trace showing the AHP difference. The peak value of the AHP (mV) (I) and AHP area (mVxs) (J) are greater in 5-HT neurons from single-housed mice, relative to group-housed mice (unpaired t-test, **p<0.01). Data are represented as mean ± S.E.M.

Membrane characteristics of dorsal raphe 5-HT neurons from group-housed and single-housed mice are similar.
Current-clamp traces of a 5-HT neuron from a group-housed (A) and a single-housed (B) mouse in response to depolarizing and hyperpolarizing currents injected in a stepwise manner (25 pA steps). (C) Membrane properties of dorsal raphe 5-HT neurons from group- (n = 6 mice, 35 neurons) and single-housed (n = 5 mice, 41 neurons) mice. (D) Input-output curve showing spike frequency (Hz) of 5-HT neurons in response to a series of depolarizing current (pA) injections before and after application of GABAA and GABAB receptor blockers (picrotoxin, abbreviated as PTX, and CGP52432). The firing frequency (Hz) of 5-HT neurons from single-housed mice (Baseline; n = 10, +PTX+CGP52432; n = 13 neurons) is reduced compared to 5-HT neurons from group-housed mice (Baseline; n = 14, +PTX+CGP52432; n = 7 neurons) in the absence or presence of GABA receptor blockers (two-way repeated-measures ANOVA, main effect of group, F (3400) = 9.2, p<0.001). Posthoc tests showed that there was a significant difference between group- and single-housed mice in the presence or absence of GABA receptor blockers (p<0.01). Blocking GABA receptors did not cause a significant change in neurons from either group- or single-housed mice (p’s > 0.05). Data are mean ± S.E.M. RMP, resting membrane potential; Rinput, input resistance; Cm, membrane capacitance; AP Amp, action potential amplitude; AP Thr, action potential threshold.
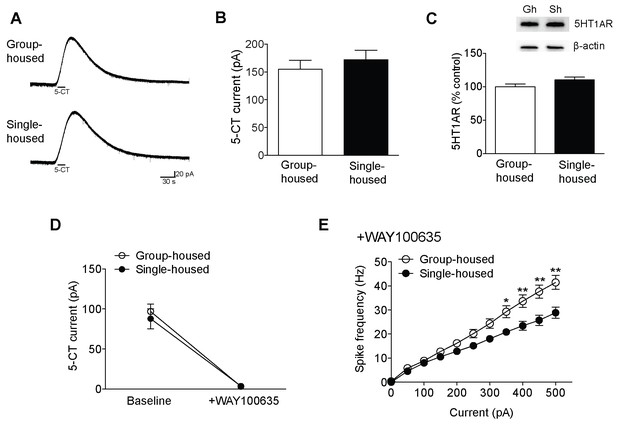
Reduced excitability of 5-HT neurons of single-housed mice is not due to changes in the serotonergic tone.
(A) Averaged voltage-clamp recordings in response to 15 s bath application of 100 nM 5-CT from 5-HT neurons of group- (n = 21 neurons) and single-housed (n = 21 neurons) mice. (B) The amplitude (pA) of the inhibitory outward current in response to 5-CT is similar between 5-HT neurons of group- and single-housed mice (unpaired t-test, p>0.05). (C) Representative immunoblots (top) and quantification (bottom) showing 5-HT1AR protein levels in the DRN of group- (n = 6) and single-housed (n = 6) mice (unpaired t-test, p>0.05). Gh; group-housed, Sh; single-housed. (D) 5-CT current responses before and after application of the 5-HT1A antagonist WAY100635 (30 nM) from 5-HT neurons of group-housed (baseline, n = 12 neurons; +WAY100635, n = 11 neurons) and single-housed (baseline, n = 13 neurons; +WAY100635, n = 8 neurons) mice (two-way ANOVA, effect of drug, F (1,40) = 94.24, p<0.001). (E) The difference in the excitability of 5-HT neurons from group- (n = 11 neurons) and single-housed (n = 7 neurons) mice is maintained in the presence of WAY100635 (two-way repeated-measures ANOVA, effect of housing, F (1160) = 7.11, p=0.017; Newman-Keuls posthoc test, *p<0.05, **p<0.01). Data are mean ± S.E.M.
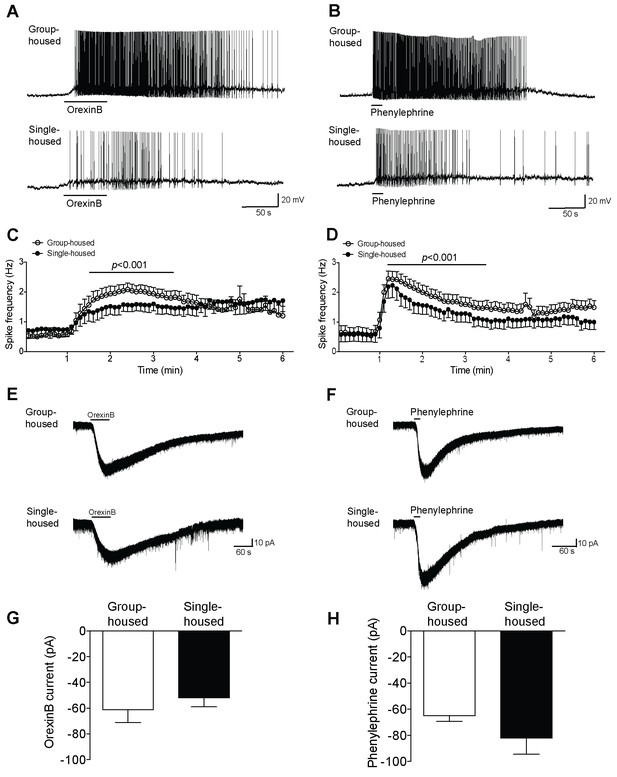
5-HT neurons in dorsal raphe show reduced firing yet similar inward currents in response to excitatory neuromodulators after chronic social isolation.
(A, B) Current-clamp recordings of a 5-HT neuron from a group-housed (above) and a single-housed (below) mouse in response to orexinB (300 nM) or phenylephrine (10 μM). (C, D) The frequency (Hz) of action potentials in response to orexinB and phenylephrine application is shown over a 6 min period. (C) 5-HT neurons of single-housed mice (n = 15) show reduced firing in response to orexinB compared to neurons from group-housed mice (n = 20) (two-way ANOVA, effect of housing, F (1660) = 28.43, p<0.001). (D) In response to phenylephrine, 5-HT neurons from single-housed mice (n = 19 neurons) also show reduced firing compared with neurons of group-housed mice (n = 23 neurons) (two-way ANOVA, effect of housing, F (1920) = 23.17, p<0.001). (E, F) Averaged voltage-clamp recordings of 5-HT neurons from group-housed (above) and single-housed (below) mice in response to orexinB (300 nM) or phenylephrine (10 μM). The amplitude (pA) of the excitatory inward currents in response to application of orexinB (G) or phenylephrine (H) is similar between 5-HT neurons of group-housed (orexinB and phenylephrine, n = 18 neurons) and single-housed (orexinB, n = 20; phenylephrine, n = 14 neurons) mice (unpaired t-test, p>0.05). Data are represented as mean ± S.E.M.
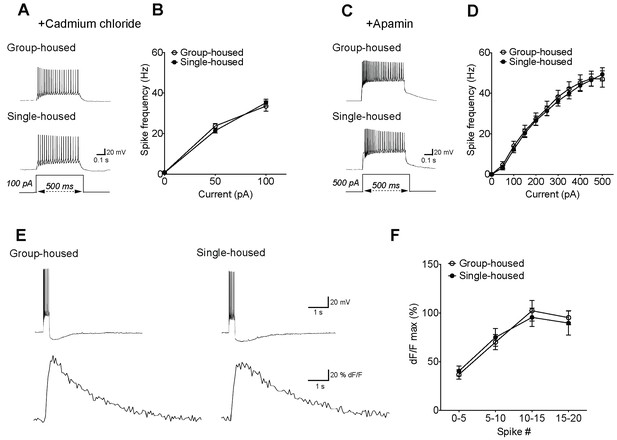
The reduced firing of 5-HT neurons after chronic social isolation can be normalized by blockers of voltage-gated calcium channels and SK channels, despite the lack of calcium differences.
(A) Current-clamp traces of a 5-HT neuron from a group-housed (above) and a single-housed (below) mouse in response to a 100 pA depolarizing step in the presence of cadmium chloride (100 μM), a blocker of voltage gated calcium channels. (B) In the presence of cadmium chloride, the frequency (Hz) of action potentials in response to a series of depolarizing current steps is similar between 5-HT neurons of group- (n = 33 neurons) and single-housed (n = 33 neurons) mice (two-way repeated-measures ANOVA, effect of housing, F (1128) = 0.03, p=0.871). (C) Current-clamp traces of a 5-HT neuron from a group-housed (above) and a single-housed (below) mouse in response to a 500 pA depolarizing step in the presence of apamin (200 nM), a blocker of SK channels. (D) In the presence of apamin, the firing frequency (Hz) of 5-HT neurons from group-housed (n = 10 neurons) and single-housed (n = 12 neurons) mice is comparable (two-way repeated-measures ANOVA, effect of housing, F (1200) = 0.08, p=0.778). (E) Sample traces showing Fluo5F fluorescence increases (below) in response to current input producing 11 spikes in neurons (above). (F) The peak dF/F detected by the calcium indicator Fluo5F in response to a set number of spikes is not different between 5-HT neurons of group-housed (n = 9 neurons) and single-housed (n = 12 neurons) mice (two-way repeated-measures ANOVA, effect of housing, F (1,76) = 0.5, p=0.488). Neurons were held at −70 mV by current injection, given depolarizing steps to elicit spikes, and Ca2+ responses were measured in cells firing with identical numbers of action potentials in both groups.
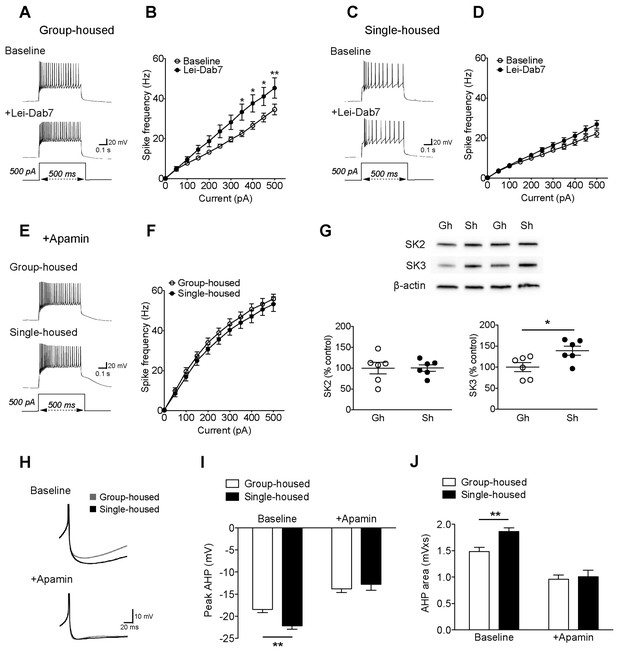
The reduced excitability of 5-HT neurons upon chronic social isolation can be restored by blocking SK3 channels.
(A) Current-clamp traces of a 5-HT neuron in response to a 500 pA depolarizing step before (Baseline, above) and after application of SK2 blocker, Lei-Dab7 (100 nM) (+Lei-Dab7, below) from a group-housed mouse. (B) Input-output curve showing increased excitability upon application of Lei-Dab7 in 5-HT neurons of group-housed mice (Baseline, n = 17 neurons, +Lei-Dab7, n = 11 neurons) (two-way repeated-measures ANOVA, effect of drug, F (1260) = 5.87, p=0.023; Newman-Keuls posthoc test, *p<0.05, **p<0.01). (C) Current-clamp traces of a 5-HT neuron in response to a 500 pA depolarizing step before (Baseline, above) and after application of SK2 blocker, Lei-Dab7 (100 nM) (+Lei-Dab7, below) from a single-housed mouse. (D) The firing frequency (Hz) of 5-HT neurons from single-housed mice did not change upon Lei-Dab7 (Baseline, n = 16 neurons, +Lei-Dab7, n = 9 neurons) (two-way repeated-measures ANOVA, effect of drug, F (1230) = 2.39, p=0.13). (E) Current-clamp traces of 5-HT neurons in response to 500 pA depolarizing steps after subsequent application of apamin (200 nM) to block SK3 from a group-housed (above) and a single-housed (below) mouse. (F) Although the group difference persisted after SK2 blockade with Lei-Dab7, the subsequent application of apamin to block SK3 rendered the firing frequency (Hz) of neurons from group-housed (n = 15 neurons) and single-housed (n = 13 neurons) mice comparable (two-way repeated-measures ANOVA, effect of housing, F (1260) = 0.67, p=0.42). (G) Representative immunoblots (top) and quantification (bottom) showing protein levels of SK2 and SK3 channels in the DRN of group- and single-housed mice. SK3 channel expression is significantly increased in single-housed mice (n = 6) compared to group-housed mice (n = 6) (unpaired t-test, p=0.027) while SK2 channel expression seems unaltered (unpaired t-test, p=0.98). Gh; group-housed, Sh; single-housed. (H) Averaged superimposed spikes showing the AHP difference of 5-HT neurons before and after application of apamin in group-housed (Baseline; n = 18, +Apamin; n = 10 neurons) and single-housed (Baseline; n = 16, +Apamin; n = 8 neurons) mice. The peak value of the AHP (mV) (I) and AHP area (mVxs) (J) before and after application of apamin are shown (two-way ANOVA, effect of drug, peak AHP: F (1,48) = 60.02, p<0.001, AHP area: F (1,48) = 60.59, p<0.001, Newman-Keuls posthoc test, **p<0.01). Data are represented as mean ± S.E.M.
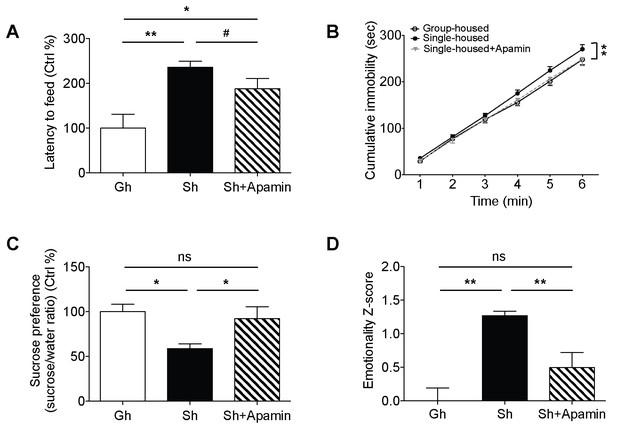
Decreased anxiety/depression-like behaviors in single-housed mice after systemic apamin.
(A) Single-housed mice showed increased latency to feed, which was only partially recovered upon apamin treatment in the novelty suppressed feeding test (one-way ANOVA, group effect, p=0.001, Newman-Keuls posthoc test, *p<0.05, **p<0.01; a priori t-test between Sh and Sh+Apamin, #p=0.09). (B) Single-housed mice showed increased immobility in the tail suspension test suggesting enhanced depressive-like behavior, normalized by apamin treatment (two-way ANOVA, main effect of group, p=0.01, Newman-Keuls posthoc test, **p<0.01). (C) Single-housed mice showed decreased sucrose preference, which was restored by apamin treatment (one-way ANOVA, main effect of group, p=0.02, Newman-Keuls posthoc test, *p<0.05). (D) Single-housed mice showed overall increased emotionality, which was decreased by apamin treatment (one-way ANOVA, main effect of group, p<0.01, Newman-Keuls posthoc test, **p<0.01). Data are represented as mean ± S.E.M. (n = 7–9 per group). Gh; group-housed, Sh; single-housed, ns; not significant.

The amount of food consumed in homecage after novelty suppressed feeding test.
Single-housed mice consumed significantly more food after returning to their homecage following novelty suppressed feeding test. Change in the feeding behavior of single-housed mice was normalized upon apamin treatment (one-way ANOVA, main effect of group, p=0.026, Newman-Keuls posthoc test, *p<0.05). Data are represented as mean ± S.E.M. Gh; group-housed, Sh; single-housed, ns; not significant.





