Optical control of pain in vivo with a photoactive mGlu5 receptor negative allosteric modulator
Figures
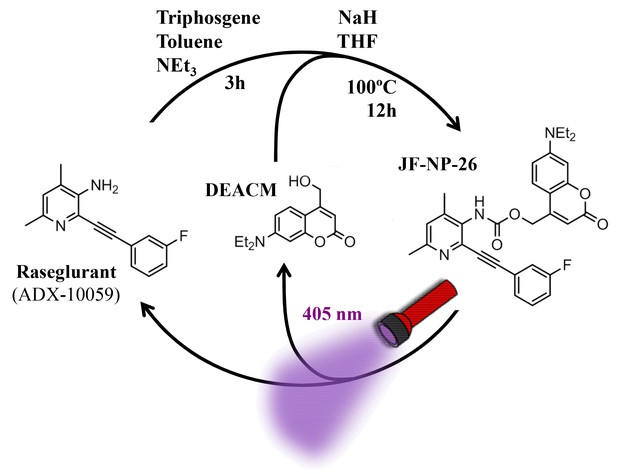
Design and synthesis of JF-NP-26.
The synthesis of JF-NP-26 from raseglurant involves a one-pot procedure using raseglurant and 4-hydroxymethyl-7-diethylaminocoumarin (DEACM). In brief, a first reaction with triphosgene, NEt3 and toluene for 3 hr was followed by an incubation with DEACM, NaH and THF at 100°C for 12 hr (see Materials and methods). Upon irradiation with 405 nm visible light the irreversible photolytic reaction produced raseglurant. The following figure supplements are available for Figure 1.
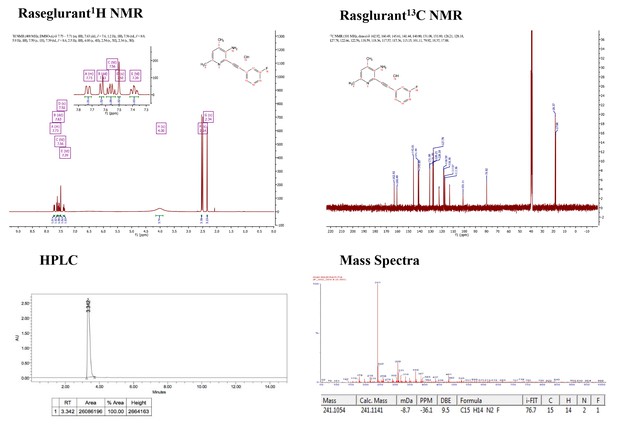
Supporting spectra for the synthesis of raseglurant.
https://doi.org/10.7554/eLife.23545.003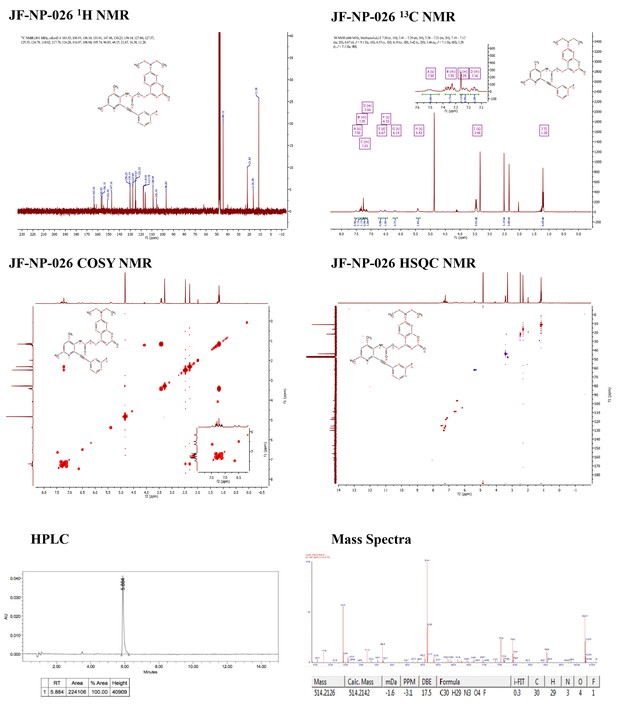
Supporting spectra for the synthesis of JF-NP-26.
https://doi.org/10.7554/eLife.23545.004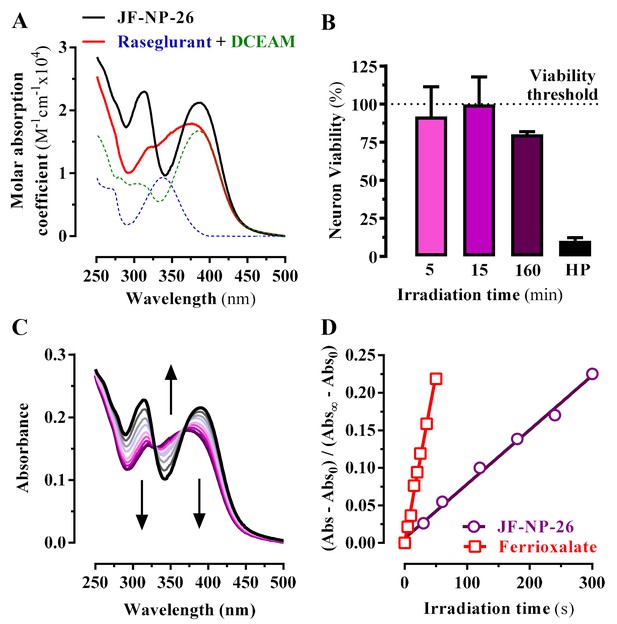
Photochemical properties of JF-NP-26.
(A) UV-visible absorption spectra of raseglurant (Ras), the coumarine DEACM, and JF-NP-26 (JF) in PBS. The sum of the free Ras and DEACM spectra shows important differences relative to that of their conjugate JF (B) Neuronal viability upon 405 nm irradiation. The neuronal viability was assessed using the 3-(4,5-dimethylthiazol-2-yl)−2,5-diphenyltetrazolium bromide (MTT) assay (see Supplementary Information). Cultured neurons were irradiated with 405 nm light during 5, 15 and 160 min before the MTT incubation 3% hydrogen peroxide (HP) was used a positive control of cell death. All values were normalized using intact neurons as a 100% of viability threshold and expressed as mean ± S.E.M. of three independent experiments performed in triplicate. (C) Changes in the absorption spectrum of JF-NP-26 upon illumination in PBS with 405 nm light. The spectrum of the irradiated sample shows an excellent match with the sum of the free Ras and DEACM spectra in panel A. (D) Determination of the quantum yield of raseglurant photouncaging by comparison of the rate of this process with the rate of a standard photochemical reaction, namely ferrioxalate photoreduction (φr = 1.14 at 405 nm, see Experimental Section).
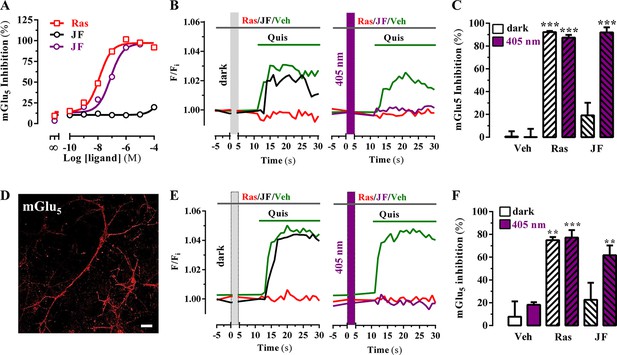
Optical modulation of mGlu5 receptor activity in cultured cells and in primary striatal neurons.
(A) Determination of mGlu5 receptor-mediated inositol phosphate accumulation in transiently transfected HEK-293T cells. Normalized dose-response of raseglurant (Ras)- and JF-NP-26 (JF)-mediated inhibition of quisqualate (1 µM)-induced IP accumulation in dark or upon irradiation at 405 nm (violet trace) in mGlu5 receptor expressing cells. Each data point corresponds to mean± SEM of three independent experiments performed in triplicate. The pEC50 value determined for quisqualate was 7.52. (B) Transiently transfected HEK-293T cells with the mGlu5 receptor were pretreated with vehicle (veh), raseglurant (Ras) and JF-NP-26 (JF) at 1 µM in the dark (left panel) or upon 405 nm irradiation (right), and then challenged with quisqualate (Quis, 100 μM). (C) Quantification of mGlu5 receptor inhibition. The percentage of receptor inhibition is calculated as described in Materials and methods and expressed as mean ± S.E.M. (n = 10). ***p<0.001, one-way ANOVA with Dunnett’s multiple comparison test using the Veh+dark condition as a control. (D) Expression of mGlu5 receptor in primary striatal neurons. Primary striatal neurons were fixed, permeabilized and immunostained using a rabbit anti-mGlu5 receptor (1 µg/ml) antibody. The primary antibody was detected using Cy3-conjugated donkey anti-rabbit antibody (1/200). Neurons were analyzed by single immunofluorescence with a confocal microscopy. Scale bar: 10 μm. (E) Determination of mGlu5 receptor-mediated intracellular calcium accumulation in primary striatal neurons. Neurons were incubated with vehicle (veh), raseglurant (Ras) or JF-NP-26 (JF), then kept in the dark (left panel) or irradiated at 405 nm (right panel) before being challenged with quisqualate (Quis) (see Materials and methods). (F) Quantification of mGlu5 receptor inhibition. The percentage of receptor inhibition is calculated as described in the Material and methods section and expressed as mean ± S.E.M. (n = 5–10). **p<0.005 and ***p<0.001, one-way ANOVA with Dunnett’s multiple comparison test using the Veh+dark condition as a control.
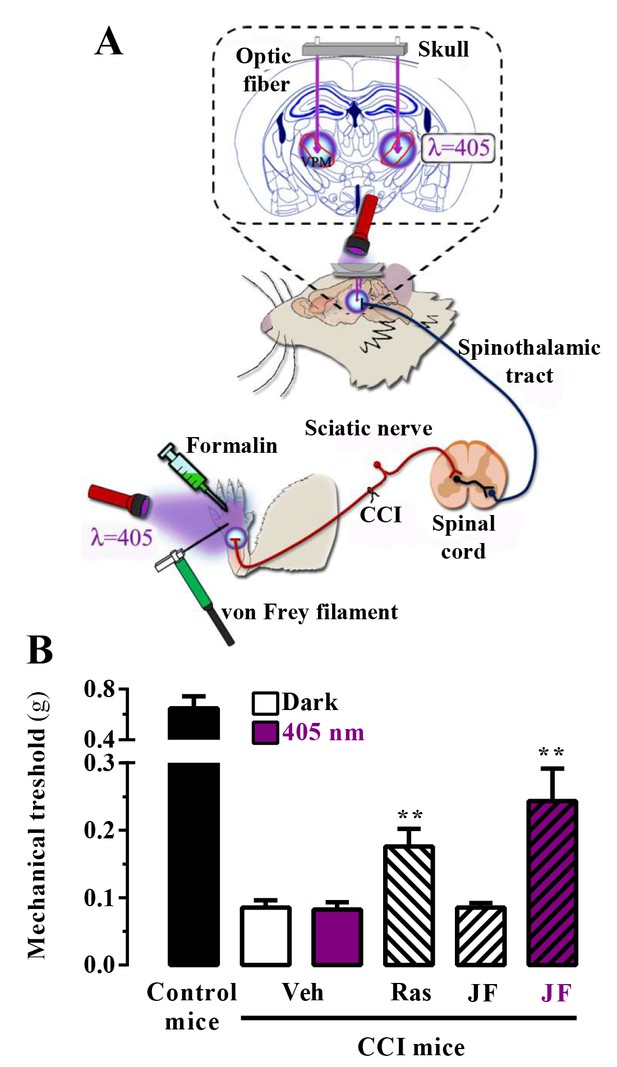
In vivo assessment of JF-NP-26 light-dependent analgesic efficacy in neuropathic pain.
(A) Scheme showing the different LED-mediated irradiation points within the pain neuraxis (i.e. hind paw and thalamus) and pain triggering (i.e. hind paw formalin administration and chronic constriction injury -CCI- of the sciatic nerve). VPM, ventral posteromedial nucleus. (B) Chronic constriction injury (CCI) of the sciatic nerve causing mechanical allodynia. Mechanical thresholds were measured in 21 days post-surgery CCI mice. Thus, animals were intraperitoneally injected with vehicle (Veh, saline), raseglurant (Ras, 10 mg/kg) or JF-NP-026 (JF, 10 mg/kg) 20 min before the thalamus was irradiated at 405 nm (or dark) for 5 min. Subsequently, the mechanical thresholds were assessed before and after light irradiation. Values are means ± S.E.M. of 7–9 mice per group. **p<0.05, Student t test, JF-NP-26 dark vs. JF-NP-26 irradiated and raseglurant dark vs. Veh dark.
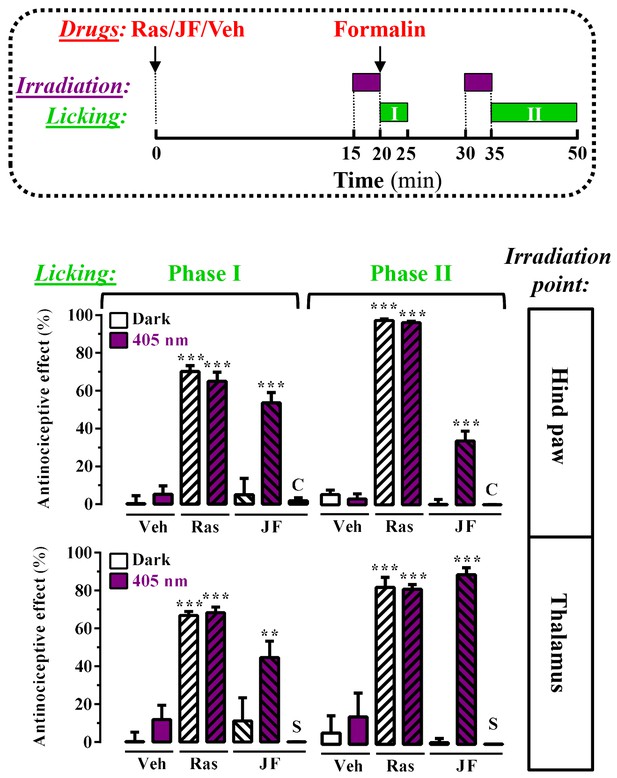
Peripheral and central light-dependent JF-NP-26-mediated antinociception in mice.
In the upper panel a scheme of the irradiation regime at 405 nm light (violet rectangles) and liking recordings (green rectangles - Phase I and Phase II) in the formalin animal model of pain is shown. Thus, animals were intraperitoneally injected with vehicle (Veh, 20%DMSO + 20%tween80 in saline), raseglurant (Ras, 10 mg/kg) or JF-NP-026 (JF, 10 mg/kg) 20 min before the hind paw or the thalamus was irradiated at 405 nm (or dark) for 5 min. As a control, the contralateral hind paw (C) or the striatum (S) were also irradiated. Subsequently, the total hind paw licking was measured for 0–5 min (Phase I) and 15–30 min (Phase II) after intraplantar injection of 20 µl of formalin solution (2.5% paraformaldehide). The antinociceptive effect was calculated as the percentage of the maximum possible effect (mean ± S.E.M., n = 5–6 mice per group). **p<0.01 and ***p<0.001, one-way ANOVA with Dunnett’s multiple comparison test using Veh+dark as a control.
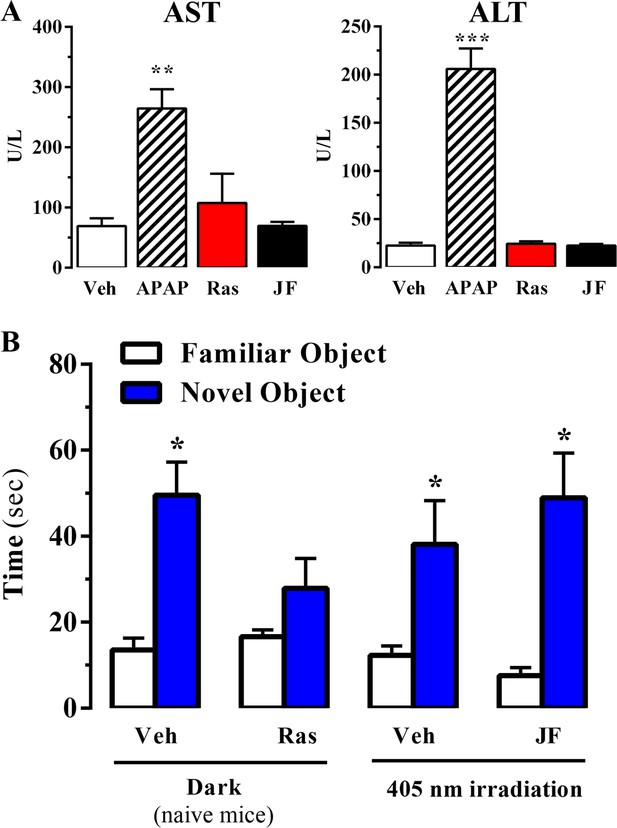
Effect of systemic administration and in vivo photoactivation of JF-NP-26 in liver toxicity and memory in mouse.
(A) Effect of Raseglurant and JF-NP-26 on serum transaminases. Mice were treated with vehicle (veh), acetaminophen treatment (APAP; 300 mg/kg, ip), raseglurant (Ras; 50 mg/kg, ip) or JF-NP-26 (JF; 50 mg/kg, ip) for three consecutive days. Serum levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were determined as described in Materials and methods. The results are expressed as means ± S.E.M. of three mice per group. **p<0.01, ***p<0.001 (One-Way ANOVA followed by Dunnett's post hoc test using the vehicle condition as a control. (B) Novel object recognition test. The time spent in exploring novel and familiar objects were measured 35 min after drug administration. Naive mice were intraperitoneally injected with vehicle (6% DMSO +6% tween80 in saline) or raseglurant (10 or 20 mg/kg). Mice bilaterally implanted with optic fibers in the thalamus were injected with vehicle or JF-NP-26 3 (10 or 20 mg/kg) 15 min before light irradiatiation (405 nm for 5 min). Values are means ± S.E.M. of 4–7 mice per group. *p<0.05 (One-Way ANOVA + Fisher's LSD test) vs. the respective values of the time spent with the familiar object (F(7,38) = 7.28). Doses of 10 mg/kg of either raseglurant in naive mice or JF-NP-26 three in light-irradiated mice did not affect recognition memory. These data are not included in the graph.
Videos
Video showing formalin-induced hind paw licking in a JF-NP-26 -treated mouse upon dark conditions from Figure 5.
Mouse, in a glass beaker (15 cm ø x 20 cm height), was administered with JF-NP-26 (10 mg/kg, i.p.). After 20 min, the LED-based irradiation system was attached to the thalamic implanted optic fiber and mock irradiated (dark) during 5 min. Then, formalin solution (20 μL) was intraplantarly (i.pl.) injected and total licking or biting time of the hind paw was recorded. A 30 s’ video fragment exemplifying the formalin test is shown.
Video showing formalin-induced hind paw licking in a JF-NP-26-treated mouse upon 405 nm irradiation from Figure 5.
Mouse, in a glass beaker (15 cm ø x 20 cm height), was administered with JF-NP-26 (10 mg/kg, i.p.). After 20 min, the LED-based irradiation system was attached to the thalamic implanted optic fiber and irradiated with 405 nm visible light during 5 min. Then, formalin solution (20 μL) was intraplantarly (i.pl.) injected and total licking or biting time of the hind paw was recorded. A 30 s’ video fragment exemplifying the formalin test is shown.





