Semen amyloids participate in spermatozoa selection and clearance
Figures
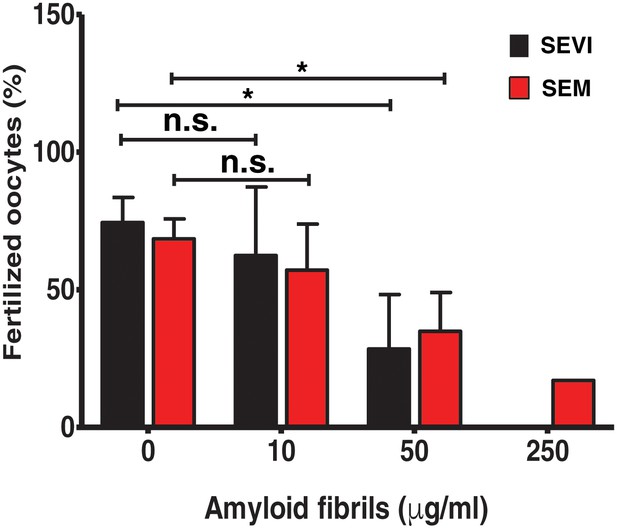
SEVI and SEM fibrils inhibit IVF in a dose-dependent manner.
The indicated concentrations of SEVI and SEM fibrils were added to mouse spermatozoa and oocytes, and monitored for IVF rates as detailed in the Materials and methods section. *p<0.05 (two-tailed Student’s t test). n.s. = non-significant. Error bars reflect variation between different experiments conducted using gametes from different mice, and correspond to data averaged from 3 to 5 experiments. In experiments with SEVI, the number of oocytes fertilized were 197/272 (0 µg/ml SEVI), 112/179 (10 µg/ml SEVI), 67/219 (50 µg/ml SEVI), and 0/77 (250 µg/ml SEVI). In experiments with SEM fibrils, the number of oocytes fertilized were 78/116 (0 µg/ml SEM), 91/162 (10 µg/ml SEM), 39/128 (50 µg/ml SEM), and 6/35 (250 µg/ml SEM). The 250 µg/ml condition lacks error bars as it was only tested in two experiments due to limited cell numbers; in both of these experiments treatment with 250 µg/ml SEVI led to complete abrogation of IVF (0% fertilized oocytes).
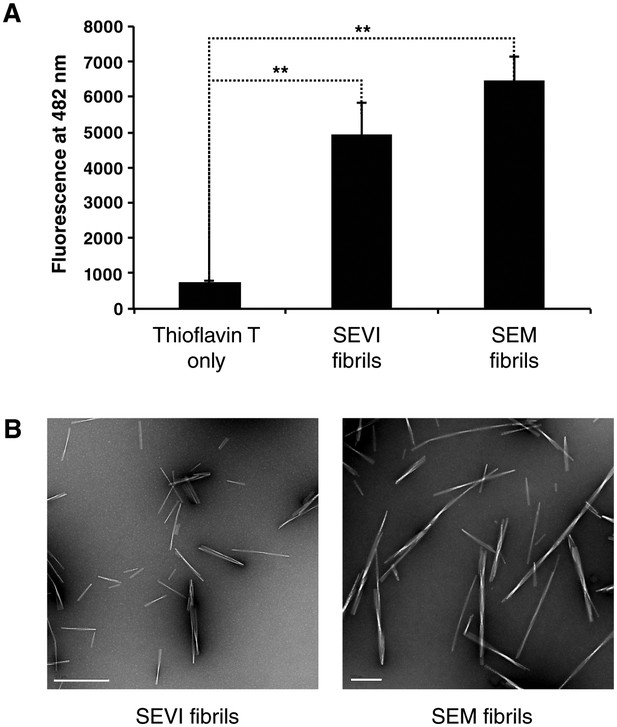
Confirmation of fibril formation by SEVI and SEM peptides.
(A) SEVI and SEM fibrils were mixed with 5 µM thioflavin T, and emission at 482 nm was recorded as a measure of fibril formation. (B) Electron micrograph of SEVI (left) or SEM (right) fibrils. The scale bar on the left image corresponds to 500 nm, and the scale bar on the right image corresponds to 200 nm.
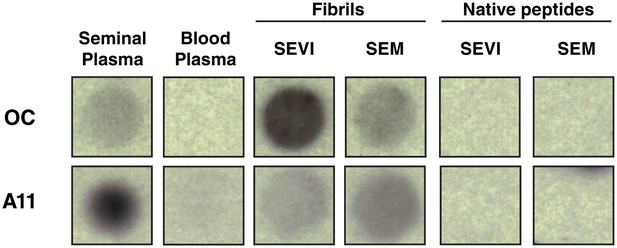
Amyloid conformer analysis of seminal plasma and synthetic semen fibrils.
Seminal plasma (SP), blood plasma, SEVI fibrils, SEM fibrils, or the corresponding native peptides were spotted onto nitrocellulose and then blotted with either OC antibody (which recognizes fibrils and fibrillar oligomers) or A11 antibody (which recognizes prefibrillar oligomers). Following incubation with secondary antibodies, dot blots were developed by chemiluminescence. OC and A11 reacted with seminal plasma and the fibrils, and not with blood plasma or the native peptide precursors of SEVI and SEM.
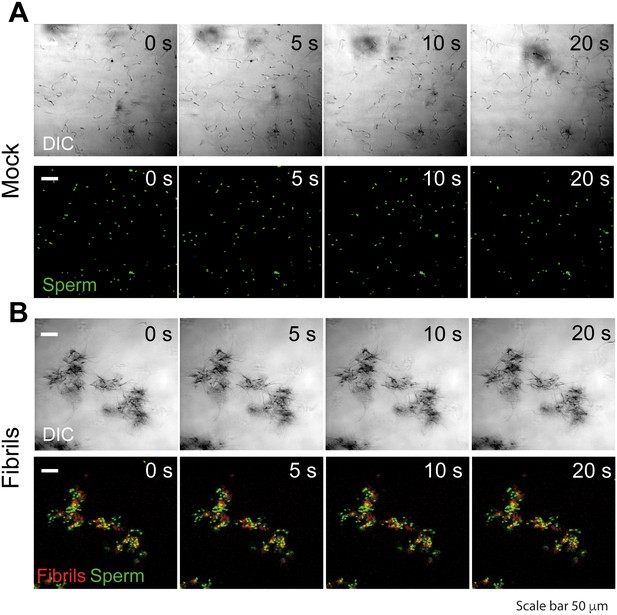
Semen fibrils trap mouse spermatozoa and inhibit their progressive motility.
Spermatozoa were allowed to swim out of epididymis of euthanized C57Bl/6N mice into buffer and allowed to capacitate at 37°C for 1 hr. Spermatozoa were stained with nuclear stain Hoechst 33342 (green) and amyloid fibrils were stained with Proteostat amyloid staining dye (red). Spermatozoa (107/ml) were mixed with the fibrils and images were acquired for 20 s with an interval of 1 s on a laser scanning confocal microscope using a 20X air objective. Shown are still images from a representative time-lapse experiment with mock (A) or 50 µg/ml SEVI-treated (B) mouse spermatozoa. Scale bar corresponds to 50 µm. Results are representative of two independent experiments. See also Video 1.
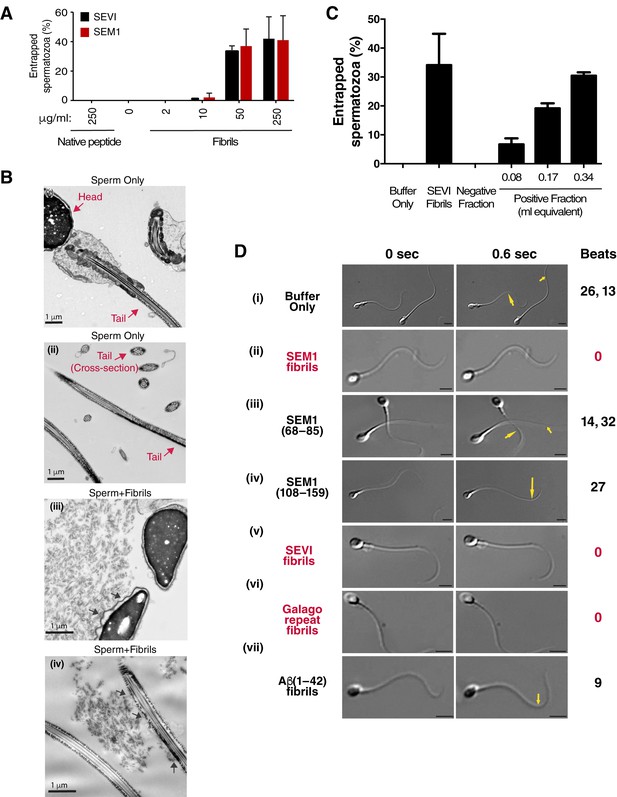
Semen fibrils directly bind and immobilize human spermatozoa.
(A) Human spermatozoa incubated with semen fibrils were imaged at 37°C for 5–10 min and then assessed for % entrapped spermatozoa as described in the Materials and methods section. Native peptide corresponds to monomeric, non-fibrillized peptide. (B) Spermatozoa were incubated in the absence (i, ii) or presence (iii, iv) of SEM fibrils and then imaged by sectioning electron microscopy. Image in panel (iii) shows two sperm heads and image in panel (iv) shows two sperm tails, with arrows highlighting examples of interactions between the fibrils and the tail. (C) Spermatozoa treated with fractions containing (Positive Fraction) or lacking (Negative Fraction) endogenous semen amyloids were imaged for 5–10 min at 37°C and then assessed for % entrapped spermatozoa as described in the Materials and methods section. Treatment of spermatozoa with synthetic SEVI fibrils was used as a positive control for entrapment. The buffer only and negative fraction controls exhibited 0% entrapment. (D) Sperm motility was assessed before (i) or after (ii–vii) perfusion with 50 µg/ml of SEM fibrils (ii), SEM1(68–85) (iii), SEM1(108–159) (iv), SEVI (v), the O. garnettii (Galago) SEM2 repeat amyloid fibrils (vi), or Aβ(1–42) (vii). Within each pair of images, the first corresponds to t = 0, whereas the second corresponds to t = 0.6 s. Numbers correspond to the number of beats that occurred within the length of each movie (total time = 2 s). In instances where two numbers are shown, the first corresponds to the spermatozoon on the left and the second to the spermatozoon on the right. Red text highlights samples where spermatozoa were immobilized. Yellow arrows highlight tail regions within the second frame that moved relative to the first frame. Scale bars = 5 µm. Data for each treatment are representative of at least two independent experiments examining >5 individual spermatozoa per treatment.
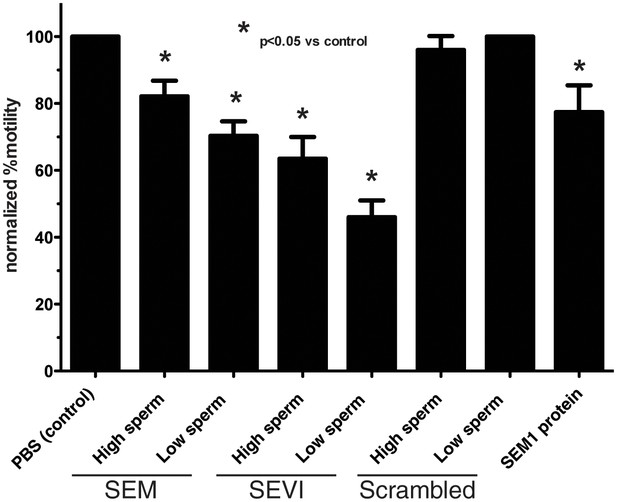
Semen fibrils immobilize spermatozoa in CASA.
Spermatozoa, at a concentration of 5,000/µl (‘High Sperm’) or 500/µl (‘Low Sperm’), were treated with 50 µg/ml of SEM or SEVI fibrils, or scrambled SEM peptide control, and assessed for the % of motile spermatozoa following normalization to a PBS control. SEM1 protein, also known to inhibit sperm motility (Silva et al., 2013), served as a positive control for motility inhibition (sperm concentration = 5000/µl). The data demonstrate that semen fibrils immobilize spermatozoa, and higher ratios of sperm:fibril lower the proportion of immobilized spermatozoa. Shown are cumulative results from eight experiments. Bonferroni’s multiple comparisons test was used for statistical analysis.
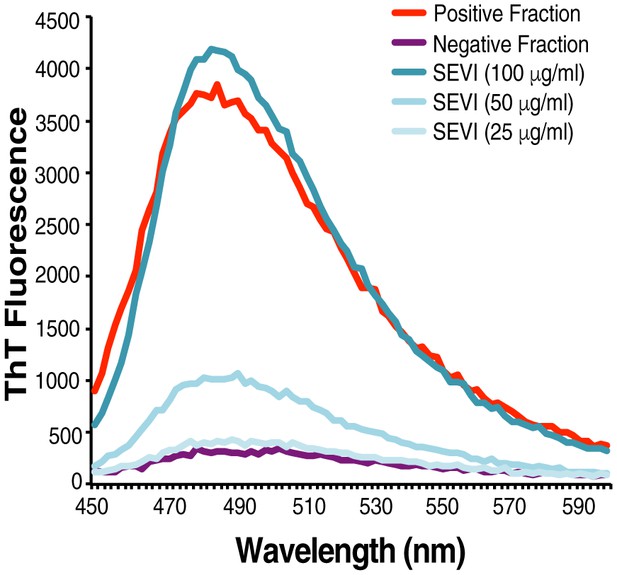
Confirmation of fibrillar nature of purified endogenous amyloids by thioflavin T.
SP was fractionated and the amyloid-containing fraction (positive fraction) and an amyloid-deficient fraction (negative fraction) were stained with thioflavin T (ThT), and emission was measured at the indicated wavelengths. Synthetic SEVI fibrils were used as a positive control. Emission at 482 nm is reflective of the presence of amyloids.
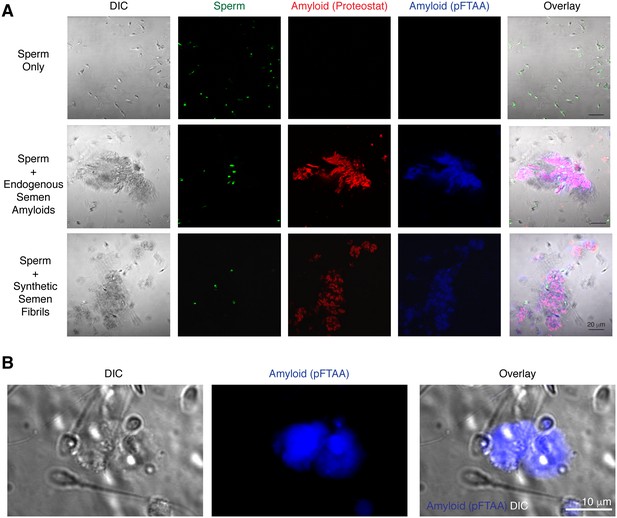
Spermatozoa interact with endogenous amyloids in human semen.
(A) Endogenous amyloids purified from SP associate with spermatozoa. Purified human spermatozoa were either imaged alone (top panels), in the presence of purified endogenous amyloids (middle panels), or in the presence of synthetic fibrils (bottom panels). The panels on the left show differential interference contrast (DIC) images. Amyloids were detected by the amyloid-binding dyes Proteostat (red) and pFTAA (blue). Sperm cells were identified by Hoechst 33342 staining (green). In the overlay images, overlaps of Proteostat and pFTAA stains are shown in pink. Scale bar = 20 µm. (B) Endogenous amyloids present in freshly liquefied semen associate with spermatozoa. Liquefied ejaculates were stained with the amyloid binding dye pFTAA and imaged by confocal microscopy. The image on the left shows a DIC image where individual sperm cells can be detected. Scale bar = 10 µm.
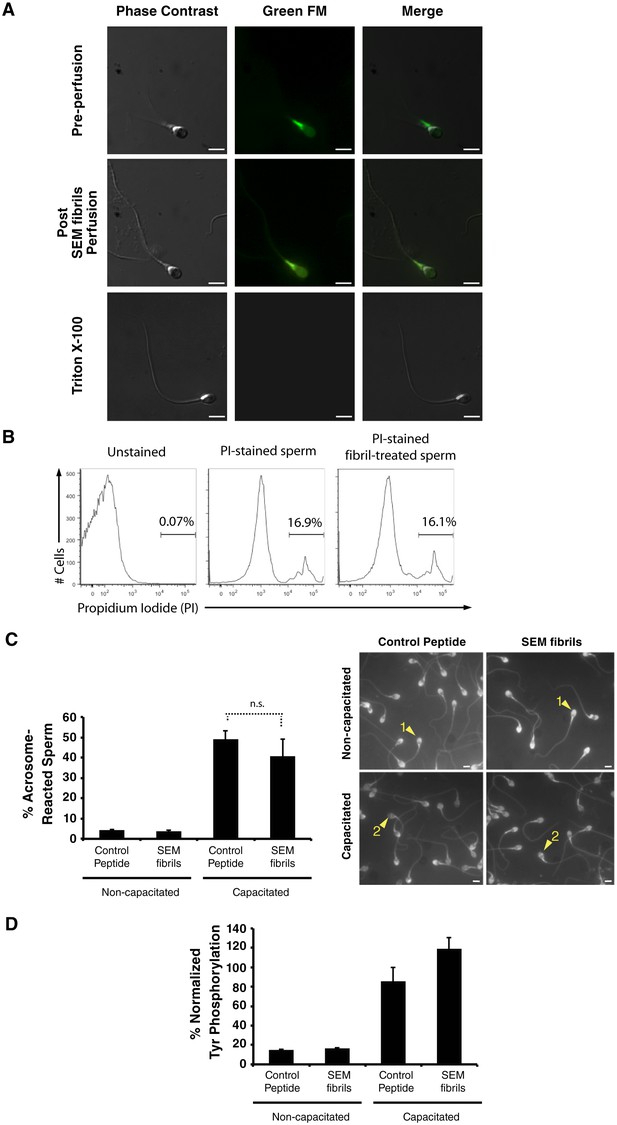
Fibril-immobilized spermatozoa are metabolically active and viable.
(A) Spermatozoa were stained with the mitochondrial activity indicator Mitotracker, attached onto coverslips, and examined by live microscopy. A motile sperm cell with fluorescence localized towards the midpiece region (where most mitochondria are localized) was imaged before perfusion of SEM fibrils (top images). Following perfusion of the fibrils, the sperm cell was immobilized without a loss of fluorescence (middle images). Triton X-100-treated spermatozoa served as a positive control for a loss in sperm viability (bottom images). The channel detecting the Green FM signal in Triton X-100-treated spermatozoa did not detect any fluorescence signal indicating loss of cellular viability. (B) Human spermatozoa were incubated in the absence or presence of semen fibrils for 0.5 hr, stained with propidium iodide (PI), and then assessed for % viable cells by flow cytometry. (C, D) Spermatozoa were treated with control scrambled peptide or SEM fibrils, and then capacitated in vitro. Capacitation was assessed by enumerating acrosome-reacted spermatozoa identified by the Pisum sativum agglutinin staining assay (examples of acrosome-intact sperm (labeled ‘1’) and acrosome-reacted sperm (labeled ‘2’) are indicated by arrowheads) (top panel), or quantitating the levels of phosphotyrosine relative to acetylated tubulin (bottom panel). Scale bars = 5 µm. n.s. = non-significant (two-tailed Student’s t test). Data are representative of at least two independent donors.
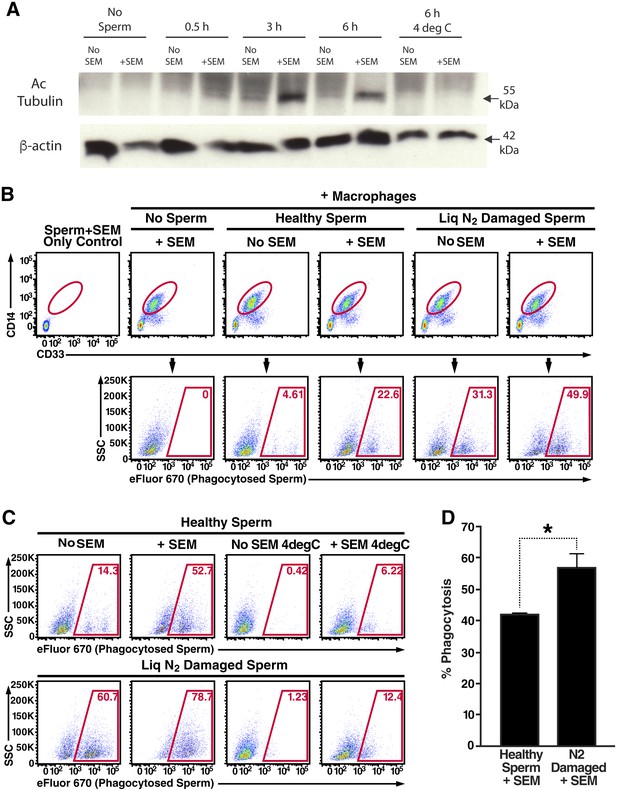
Semen fibrils promote phagocytosis of sperm cells.
(A) Spermatozoa were added to monocyte-derived macrophages for the indicated number of hours in the presence or absence of 100 µg/ml SEM fibrils, washed, and then blotted for acetylated tubulin (to detect spermatozoa) or β-actin (to detect macrophages). Negative controls include macrophages in the absence of spermatozoa, and incubation of macrophages with spermatozoa at 4°C to prevent phagocytosis. (B) Motile sperm cells purified by the swim-up method were labeled with eFluor 670 and then left at room temperature or damaged by five sequential rounds of freeze-thaw with liquid nitrogen. Spermatozoa were then added to monocyte-derived macrophages for 0.5 hr at 37°C in the presence or absence of 100 µg/ml SEM fibrils, washed, and then assessed by flow cytometry. Macrophages were identified by gating on CD14+CD33+ cells, and phagocytosis was assessed by determining the percentages of macrophages that were eFluor 670+. Results are representative of data from five different donors. (C) Comparison of eFluor 670+ macrophages after incubation with labeled spermatozoa at 37°C vs. 4°C (temperature at which phagocytosis is inhibited). These data suggest that in the presence of SEM fibrils, a small number of macrophages have surface-associated spermatozoa. (D) Macrophage-mediated phagocytosis of healthy vs. damaged spermatozoa in the presence of SEM fibrils was compared in triplicates. Shown values are those where the levels of eFluor 670+ macrophages in the presence of the SEM fibrils under 4°C conditions were subtracted out. This normalization was performed to discount surface-associated spermatozoa (which is present to some extent as demonstrated in panel C) from the analysis. *p<0.05 (by 2-tailed t test).
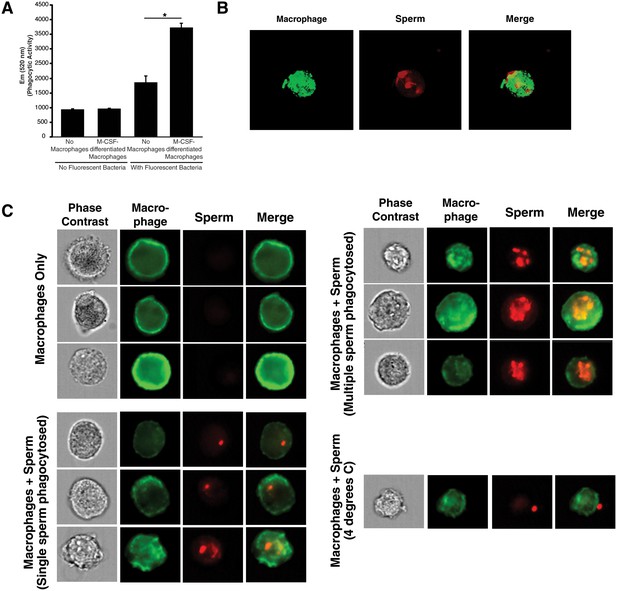
Internalization of spermatozoa by macrophages.
(A) Confirmation of phagocytic activity of monocyte-derived macrophages. The Vybrant kit from Molecular Probes was used to confirm the phagocytic activity of monocyte-derived macrophages. Phagocytosis was assessed by incubating macrophages with killed, fluorescently-labeled bacteria for 2 hr, followed by quenching fluorescence of the extracellular bacteria. Fluorescence (excitation: 480 nm, emission: 520 nm), reflecting the amount of endocytosed bacteria protected from quenching, was then measured. Fluorescence of bacteria in the absence of macrophages corresponds to background signal from incomplete fluorescence quenching. *p<0.05 (two-tailed Student’s t test). (B) Internalization of multiple spermatozoa by a macrophage. Monocyte-derived macrophages labeled with the membrane dye Vybrant DiO (green) were incubated with eFluor 670-stained sperm cells (red) for 3 hr and then imaged on a Nikon Eclipse Ti-E inverted microscope. (C) ImageStream images of primary macrophages incubated with human spermatozoa. Macrophages are shown in green (FITC-conjugated anti-CD14) while spermatozoa are shown in red. Phase contrast images are shown in the first column, followed by each individual channel, followed by merged images. Shown at the bottom is a control where the assay was conducted at 4°C instead of 37°C. Under these conditions, spermatozoa remain on the outside of macrophages. Data are representative of at least two independent donors.
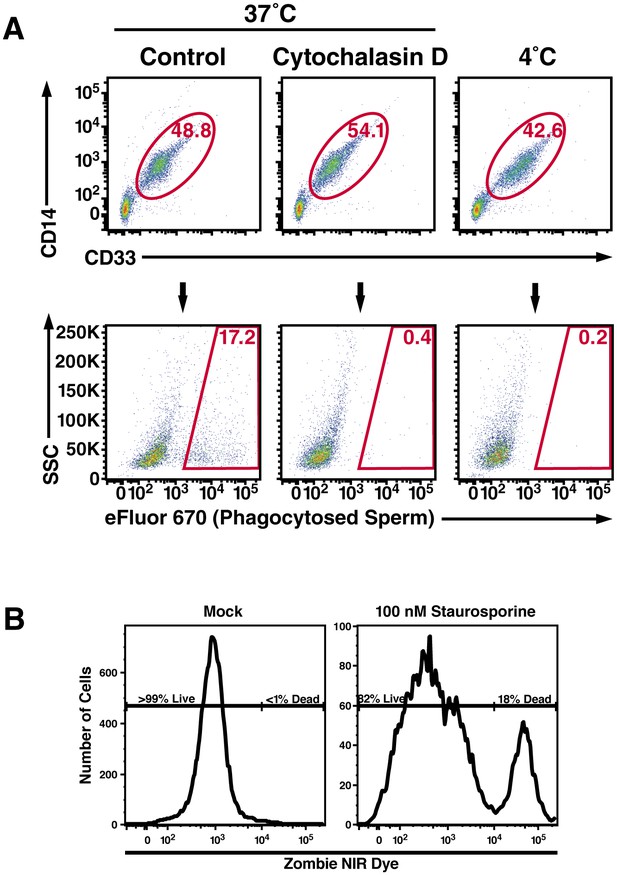
Macrophage-mediated phagocytosis of sperm cells can be detected by flow cytometry.
(A) Monocyte-derived macrophages were assessed for phagocytosis of spermatozoa after 0.5 hr co-incubation at 37°C. A CD14+CD33+ gate was to identify macrophages (top row), which were assessed for phagocytosis of eFluor 670-labeled spermatozoa (bottom row). Cytochalasin D treatment, and 4°C instead of 37°C treatment, were used as conditions where phagocytosis is inhibited. (B) Phagocytic macrophages exhibit high viability. Viability of the monocyte-derived macrophages used in phagocytosis assays was assessed by use of an amine-reactive fluorescent dye. Greater than 99% of the macrophages used in phagocytosis assays were viable, in contrast to the positive control of staurosporine-treated macrophages of which 82% of the cells were viable. Data are representative of at least two independent donors.
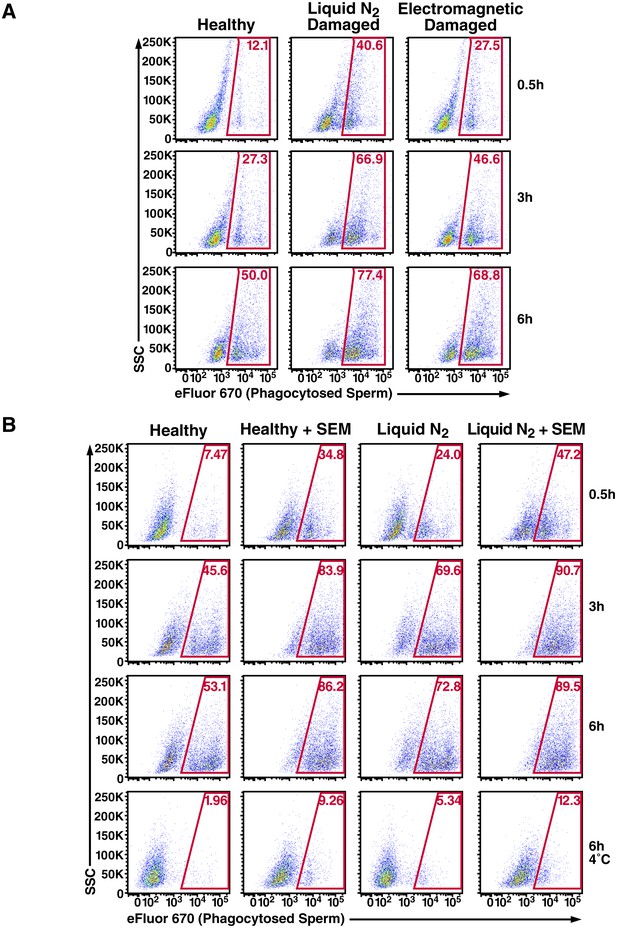
Effect of damage induction and fibrils on sperm phagocytosis.
(A) Sperm damage induced by liquid nitrogen and electromagnetic radiation both increase susceptibility of spermatozoa to macrophage-mediated phagocytosis. Monocyte-derived macrophages were incubated for the indicated number of hours at 37°C with swim-up sperm cells that were either mock-treated, or damaged by five successive rounds of freeze-thaw in liquid nitrogen, or by radiofrequency electromagnetic radiation by microwave. Results are gated on CD14+CD33+ cells, and the percentages of macrophages with phagocytosed spermatozoa are shown within the gates. (B) SEM fibrils increase the kinetics of sperm phagocytosis. Monocyte-derived macrophages were incubated for the indicated number of hours at 37°C with eFluor 670-labeled swim-up sperm cells that were either mock-treated, or damaged with five successive rounds of freeze-thaw in liquid nitrogen. Results are gated on CD14+CD33+ cells, and the percentages of macrophages with phagocytosed spermatozoa are shown within the gates. After 6 hr, the percentages of macrophages that have phagocytosed healthy vs. damaged spermatozoa in the presence of SEM fibrils are similar, suggesting that the fibrils increase the kinetics of damaged sperm phagocytosis. The bottom row presents results obtained at 4°C to show percentages of eFluor 670+ macrophages that are surface-associated after 6 hr. Data are representative of at least two independent donors.
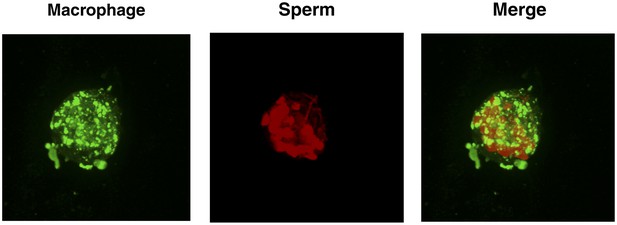
High number of spermatozoa internalized by a single macrophage in the presence of semen fibrils.
Monocyte-derived macrophages labeled with the membrane dye Vybrant DiO (green) were incubated with eFluor 670-stained sperm cells (red) in the presence of 100 µg/ml SEM fibrils for 3 hr and then imaged on a Nikon Eclipse Ti-E inverted microscope. More than a dozen sperm heads can be detected in the macrophage shown.
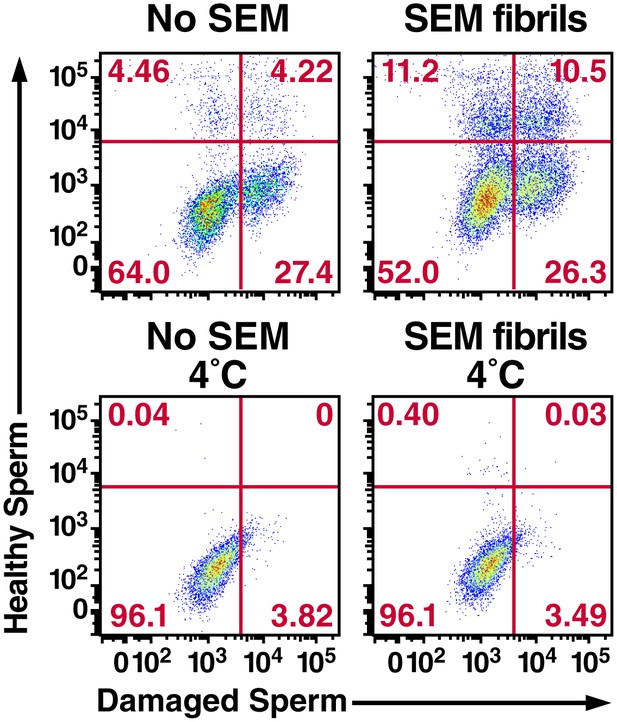
Semen fibrils increase the percentage of macrophages that have phagocytosed both healthy and damaged spermatozoa.
Monocyte-derived macrophages were incubated for 0.5 hr at 37°C with a 1:1 ratio of eFluor 670-labeled healthy spermatozoa and Celltracker Blue CMAC-labeled liquid nitrogen-damaged spermatozoa in the absence or presence of SEM fibrils. CD14+ macrophages were then analyzed for the presence of phagocytosed healthy (eFluor 670+) or damaged (CMAC+) spermatozoa. The data reveal that SEM fibrils increase the percentage of macrophages that have taken up both healthy and damaged spermatozoa (upper right hand quadrant), resulting in an overall increase in the proportions of macrophages that have taken up damaged spermatozoa. Data are representative of at least two independent donors.
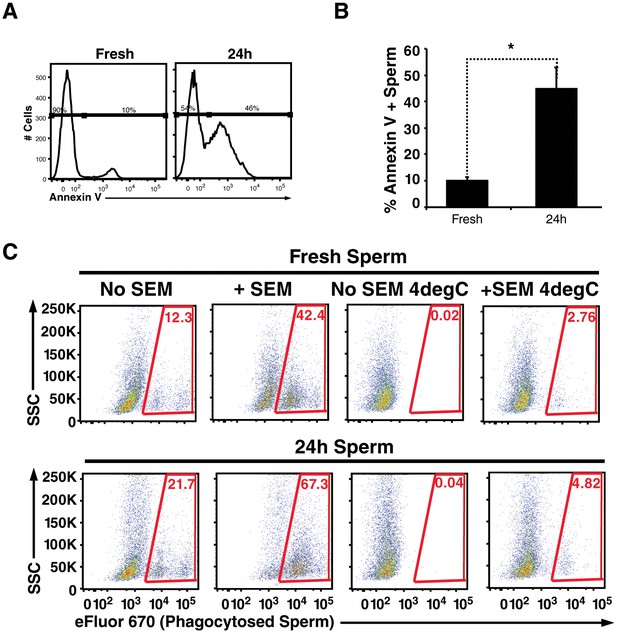
Apoptotic sperm cells are efficiently phagocytosed in the presence of fibrils.
(A) Incubation of spermatozoa for 24 hr at 25°C increases the proportion of apoptotic sperm cells. Spermatozoa from fresh ejaculates were purified by the swim-up technique and then assessed immediately for cell surface expression of the apoptotic marker Annexin V by flow cytometry, or incubated for 24 hr at 25°C prior to staining and analysis. Results are representative of 3 independent donors. Presented are flow cytometric plots showing the percentages of Annexin-negative (non-apoptotic) and Annexin-positive (apoptotic) spermatozoa as indicated. (B) Results from experimental triplicates of each condition described in panel A. *p<0.05 (two-tailed Student’s t test). Results are representative of 3 independent donors. (C) Phagocytosis of fresh spermatozoa or spermatozoa incubated for 24 hr at 25°C. Motile spermatozoa purified by the swim-up method were labeled with eFluor 670 and then fed immediately to macrophages or incubated for 24 hr at 25°C to induce apoptosis prior to incubation with macrophages. Assays were conducted in the presence or absence of 100 µg/ml SEM fibrils, and in all cases phagocytosis was allowed to proceed for 0.5 hr prior to flow cytometric analysis. Macrophages were identified by gating on CD14+CD33+ cells, and phagocytosis was assessed by determining the percentages of macrophages that were eFluor 670+. Results are representative of data from three different donors.
Videos
Semen fibrils entrap mouse spermatozoa.
105 mouse spermatozoa stained with Hoechst 33342 (green) were incubated in the absence (A) or presence (B) of 50 µg/ml of semen fibrils at 37°C in a final volume of 100 µl for 15–20 min. Images were acquired for 20 s with an interval of 1 s on an LSM710 confocal microscope (Zeiss) using a 20X air objective.
Spermatozoa from fresh ejaculates are immobilized by SEM fibrils.
(A) Spermatozoa were isolated as detailed in the supplemental experimental procedures, attached onto coverslips, and examined for motility by live microscopy. (B) Cells were then perfused with 50 µg/ml SEM1(86–107) fibrils and motility was assessed by video microscopy after 10 min. Of note, the same four spermatozoa are shown in the two panels.
Immobilization of surface-associated sperm cells by SEM1(86–107) fibrils is reversible.
Motile spermatozoa were examined before (A) and 10 min after (B) perfusion with SEM1(86–107) amyloid fibrils. 0.45 µm-filtered seminal plasma was then perfused in at a concentration of 20% as a source of seminal proteases, and 20 min later sperm cells were assessed for motility (C). Data are representative of n = 6 experiments from two sperm donors.
PSA-generated non-fibrillar fragments SEM1(68–85) and SEM1(108–159) do not inhibit sperm motility.
Motile spermatozoa were examined for motility before and 10 min after perfusion with 50 µg/ml SEM1(68–85) (A) or SEM1(108–159) (B).
SEVI and O. garnettii SEM2 repeat fibrils inhibit sperm motility, whereas Aβ(1–42) fibrils do not.
Motile spermatozoa were examined for motility before and 10 min after perfusion with 50 µg/ml SEVI fibrils (A), O. garnettii (Galago) SEM2 repeat fibrils (B), or Aβ(1–42) fibrils (C). An Aβ(1–42) fibril concentration of 100 µg/ml, corresponding to an equimolar amount of 50 µg/ml SEM1(86–107), was also tested and did not inhibit sperm motility (data not shown).
Internalization of spermatozoa by macrophage (rotational view).
Monocyte-derived macrophages labeled with a membrane dye (green) were incubated with sperm cells (red) for 3 hr and then imaged by confocal microscopy.
Internalization of spermatozoa by macrophage (z-stacks view).
Monocyte-derived macrophages labeled with a membrane dye (green) were incubated with sperm cells (red) for 3 hr and then imaged by confocal microscopy.
High number of spermatozoa internalized by a single macrophage in the presence of semen fibrils (rotational view).
Monocyte-derived macrophages labeled with a membrane dye (green) were incubated with sperm cells (red) for 3 hr in the presence of 100 µg/ml SEM fibrils and then imaged by confocal microscopy.
High number of spermatozoa internalized by a single macrophage in the presence of semen fibrils (z-stacks view).
Monocyte-derived macrophages labeled with a membrane dye (green) were incubated with sperm cells (red) for 3 hr in the presence of 100 µg/ml SEM fibrils and then imaged by confocal microscopy.





