Plasticity of calcium-permeable AMPA glutamate receptors in Pro-opiomelanocortin neurons
Figures
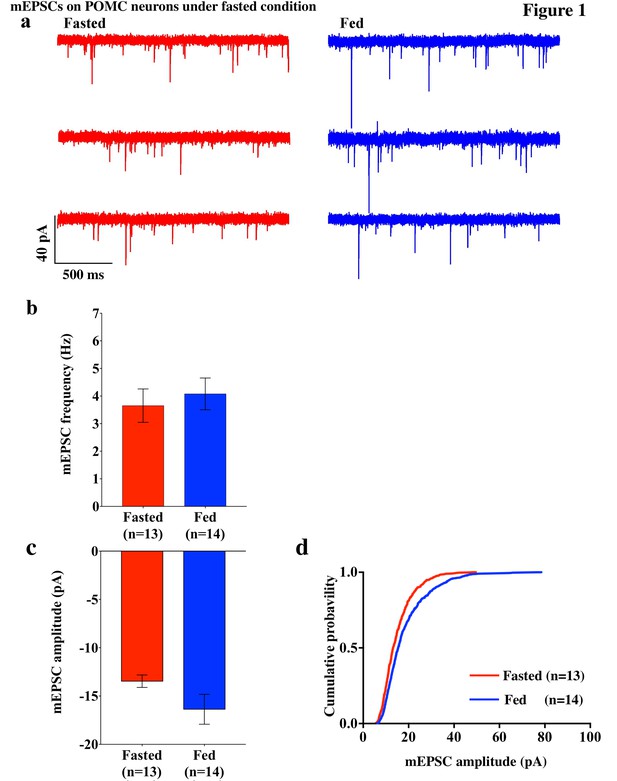
mEPSCs on POMC neurons was decreased by fasting.
(a) Representative trace of mEPSCs on POMC neurons. Scale bar indicates 40 pA on vertical and 500 ms on horizontal. (b) mEPSC frequency (mean ± SEM) did not change significantly between overnight fasting (red) and ad lib feeding conditions (blue). (c) Averaged median mEPSC amplitude (mean ± SEM) of POMC neurons tended to decrease under fasting (red) than feeding (blue) conditions. (d) Cumulative probability of mEPSC amplitude under fasting condition (red) decreased significantly than under feeding condition (blue) (* indicates p<0.01, Kolmogorov-Smirnov test).
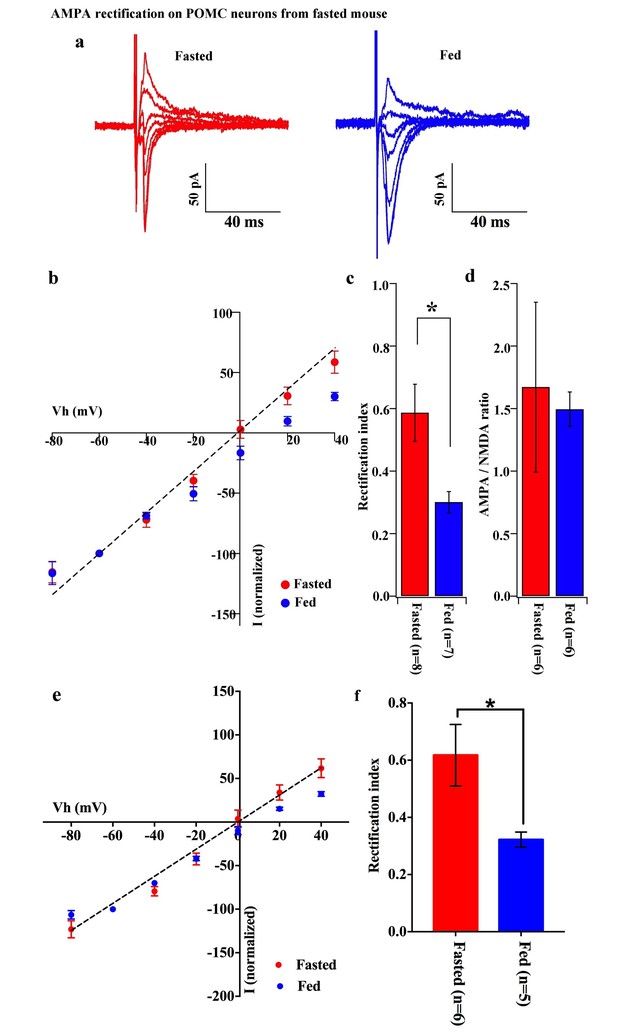
AMPA rectification on POMC neurons disappeared but AMPA/NMDA ratio unchanged by fasting.
Representative trace of AMPA rectification on POMC neurons under fasting (red) and feeding (blue) conditions. Scale bar indicates 50 pA on vertical and 40 ms on horizontal. (b) I-V relation of AMPARs on POMC neurons under fasted (red) and fed (blue) conditions. The value of peak amplitude of AMPAR-mediated eEPSC was normalized as percent by the value at −60 mV. The dotted line represents a linear regression of AMPAR-EPSCs in POMC neurons between −80 and 0 mV in fasted mice. (c) Rectification index (RI) was calculated by the peak amplitude of AMPAR-EPSC at 40 mV / peak amplitude at −60 mV. RI of fasting condition (red) increased more than feeding condition (blue). (d) AMPA-NMDA ratio did not show a difference between fasting (red) and feeding (blue) conditions. (e) I-V relationship of AMPAR-EPSCs and (f) RI in POMC neurons excluded poor space clamp recordings did not change from original results.
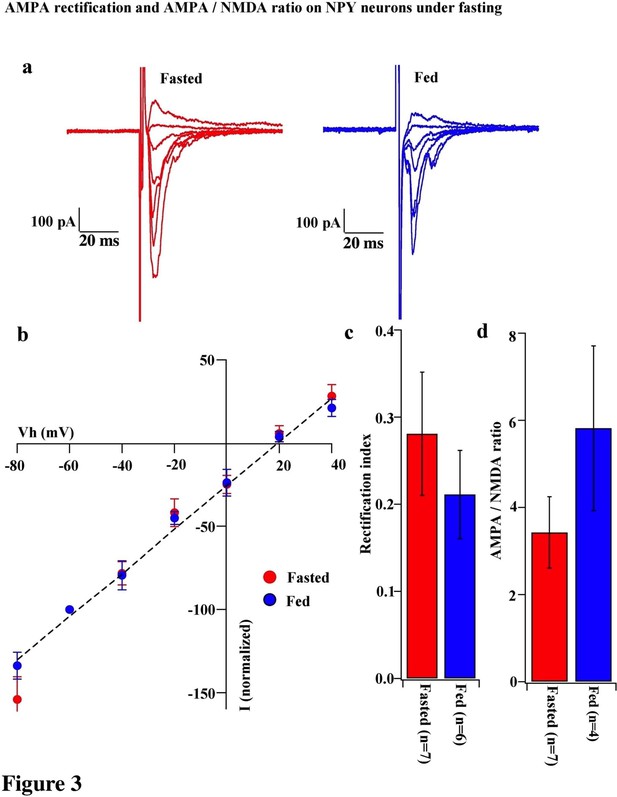
AMPA rectification and AMPA / NMDA ratio did not change on NPY neurons during fasting.
(a) Representative trace of AMPA rectification in NPY neurons in fasted (red) and fed (blue) mice. Scale bar indicates 100 pA on vertical and 20 ms on horizontal. (b) I-V relationship of AMPAR-EPSCs in POMC neurons under fasting (red) and feeding (blue). The value of peak amplitude of AMPAR-mediated eEPSC was normalized as percent by the value at −60 mV. Linear regression (dotted line) represents between −80 and 0 mV under fasting. (c) RI of fasted group (red) did not change compared with the fed group (blue). (d) AMPA-NMDA ratio did not show a difference between fasted and fed groups.
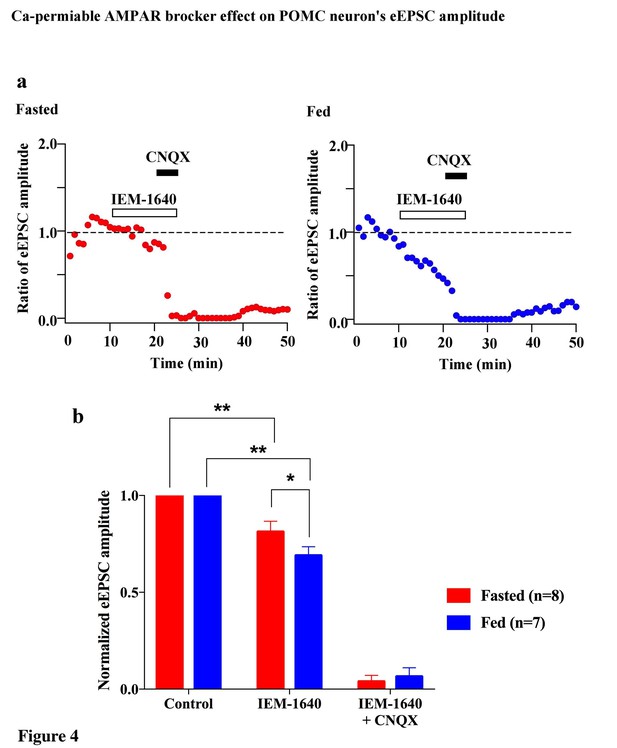
Cp-AMPARs blocker, IEM-1460, inhibited evoked EPSCs under feeding but not fasting.
(a) Representative trace of normalized eEPSC amplitude under fasting (red) and feeding (blue). 100 µM IEM-1640, competitive Cp-AMPARs blocker, was applied to bath solution at 10–25 min. 10 µM CNQX, AMPARs blocker, was also applied to bath solution at 20–25 min. (b) Average normalized eEPSC amplitude onto POMC neurons under fed condition was decreased by IEM 1460 but not under fasted condition.
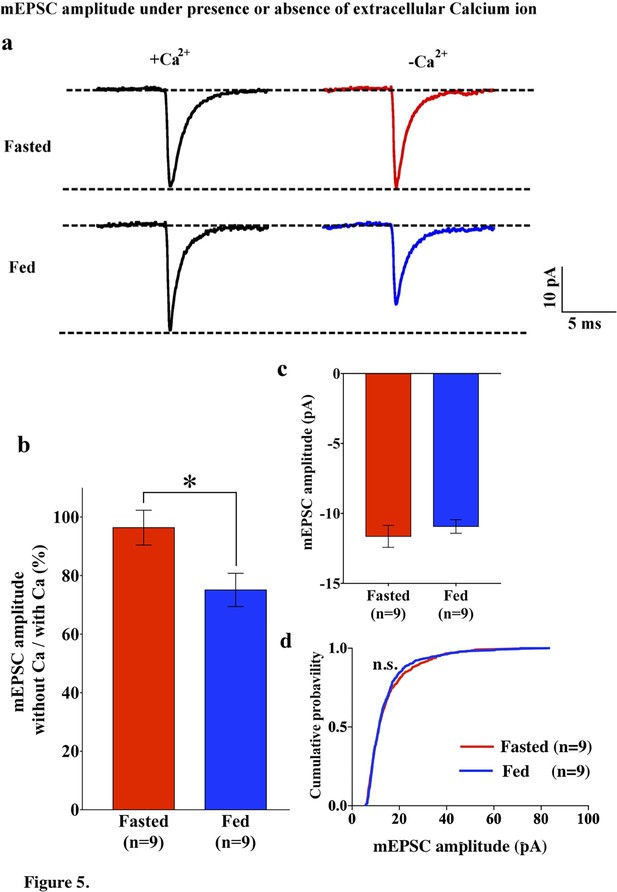
mEPSC without extracellular calcium did not change under fasting, whereas it decreased under feeding conditions.
(a) Representative traces of mEPSC with calcium (left) and without calcium (right) under fasting (top) and feeding (bottom) conditions. Scale bar indicates 10 pA on vertical and 5 ms on horizontal. (b) The ratio of mEPSC amplitude without calcium/with calcium decreased under the feeding (blue) but remained unchanged under fasting (red). (c) mEPSC distribution did not change between fasting and feeding (blue) in Ca2+-free ACSF (p>0.05, Kolmogorov-Smirnov test) (d) Averaged median mEPSC amplitude did not change between fasting and feeding in Ca2+-free ACSF.
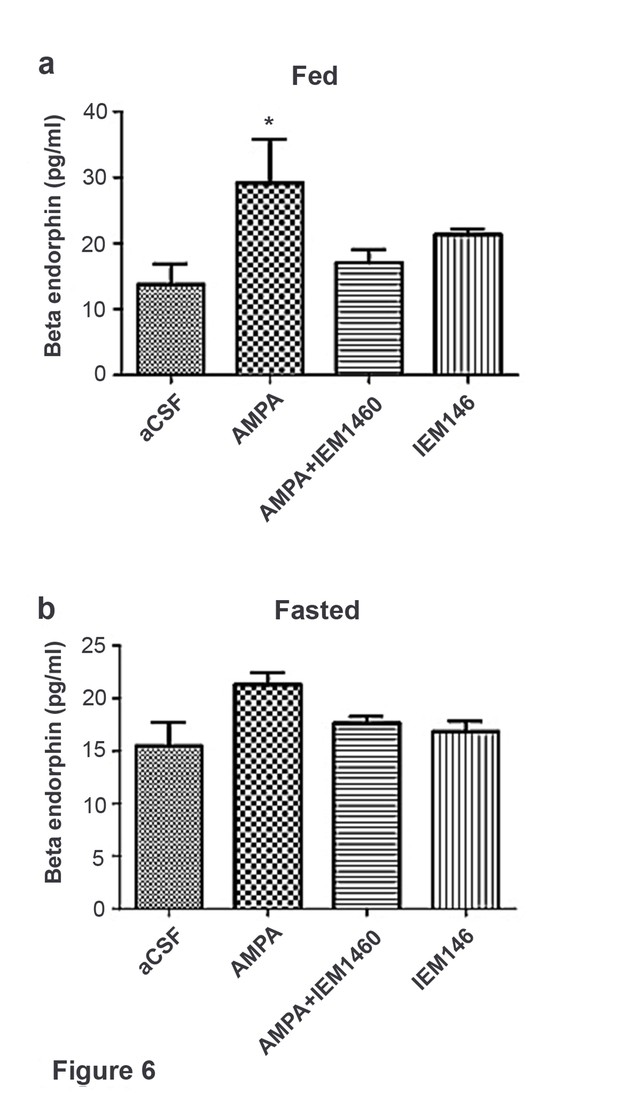
Beta-endorphin secreted by AMPA application was inhibited by Cp-AMPAR blocker, IEM 1460 under fed but not under fasted conditions.
(a) Under fed condition, beta-endorphin secretion from hypothalamus slice was significantly increased by AMPA application compared to aCSF and AMPA + IEM 1460. (b) Under fasted condition, beta-endorphin secretion was not increased by AMPA, aCSF and AMPA + IEM 1460 treatment. Results are expressed mean ± SEM. * indicates p<0.05, two-way analysis of variance (ANOVA) and Tukey multiple comparison posthoc test.
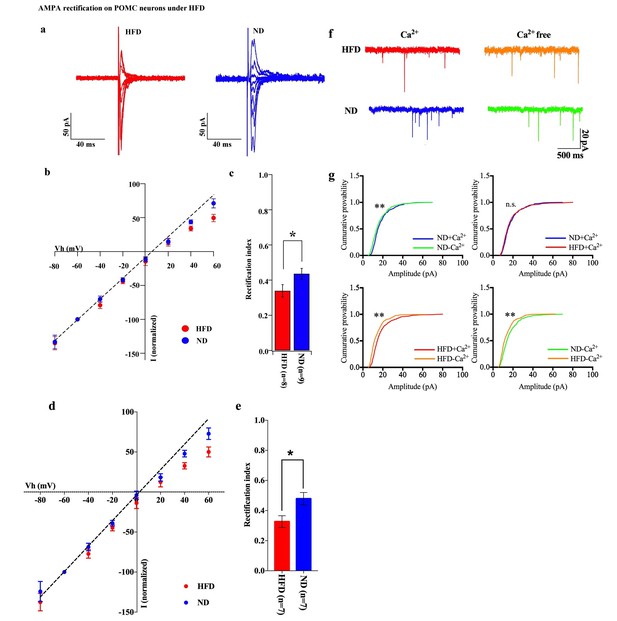
HFD increases AMPA rectification in POMC neurons.
Representative traces of AMPA rectification under HFD (red) and ND (blue) conditions. (b) I-V relationship of AMPAR-EPSCs in POMC neurons under HFD (red) and ND (blue). The value of peak amplitude of AMPAR-mediated eEPSC was normalized as percent by the value at −60 mV. The dotted line represents a linear regression of AMPAR-EPSCs in POMC neurons between −80 and 0 mV. (c) The RI of HFD (red) decreased less than ND (blue) significantly (mean ± SEM. * indicates p<0.05, unpaired t-test).) (d) I-V relationship of AMPAR-EPSCs and (e) RI in POMC neurons excluded poor space clamp recordings did not change from original results. (f) Representative trace of mEPSC with or without extracellular calcium under HFD (red) and ND (blue). (g) The distribution of mEPSC amplitude of ND vs. ND without calcium (upper left), ND vs. HFD with calcium (upper right), HFD with or without calcium (lower left), ND vs. HFD without calcium (lower right). (* and ** indicates p<0.05 and p<0.01, respectively, Kolmogorov-Smirnov test.
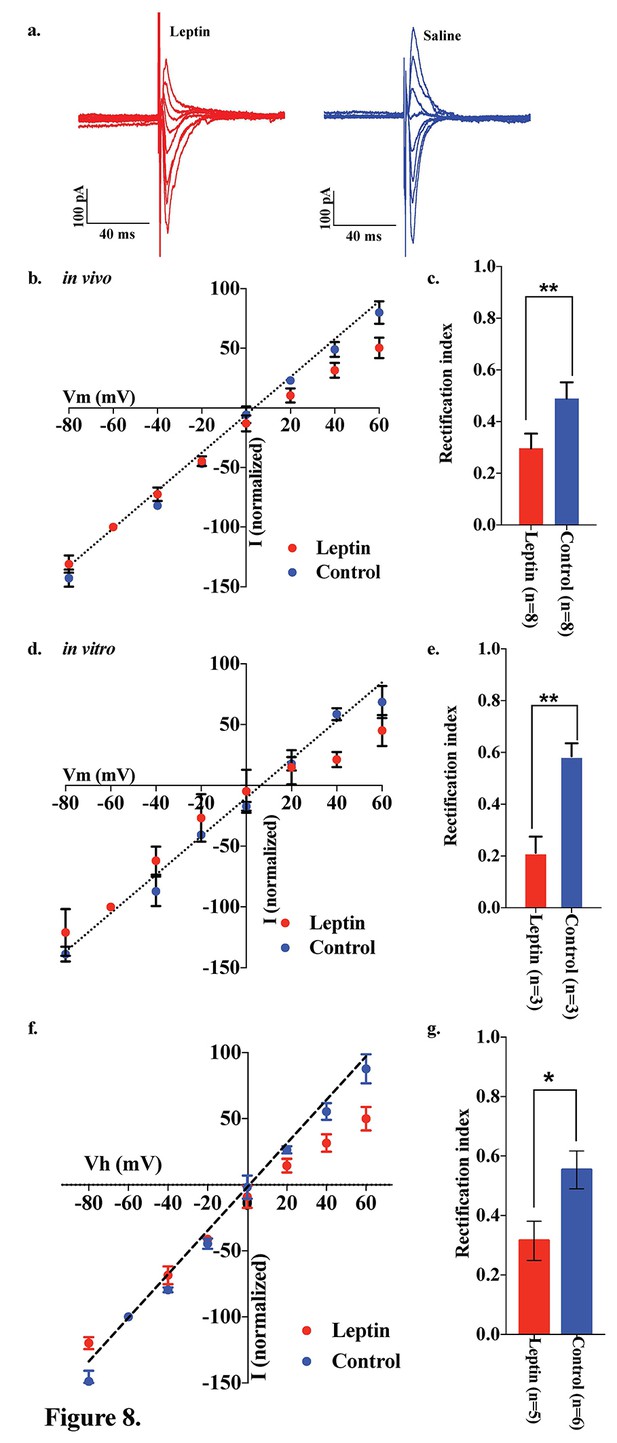
Leptin reversed the elimination of AMPAR rectification in POMC neurons in fasted mice.
Representative trace of AMPA rectification with (red) or without (blue) IP leptin injection (5 mg/kg body weight) in fasted mice. (b) I-V relationship of AMPARs in POMC neurons after leptin (red) or saline (blue) injection (i.p.) in fasted mice. The value of peak amplitude of AMPAR-mediated eEPSC was normalized as percent by the value at −60 mV. Liner regression (dotted line) represent between −80 and 0 mV under control group. (c) RIs of AMPAR-EPSCs in POMC neurons in ARC slices in fasted mice treated with 50 nM leptin (red) or saline (blue) in vivo. (d) I-V relationship of AMPARs in POMC neurons with (red) or without (blue) leptin treatment to acute brain slices including ARC in fasted mice. (e) RIs of AMPAR-EPSCs in POMC neurons in ARC slices from fasted mice treated with leptin (red) or ASCF (blue) in vitro. (mean ± SEM. ** indicates p<0.01, paired t-test.) (f) I-V relationship of AMPAR-EPSCs and (g) RI in POMC neurons excluded poor space clamp recordings did not change from original results.
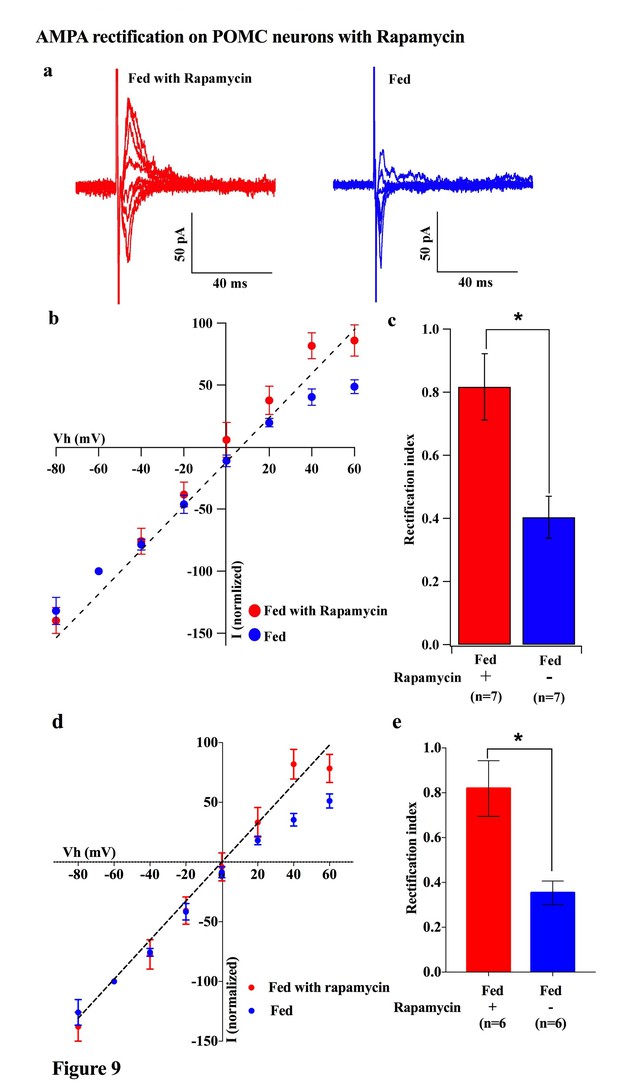
Rapamycin blocked AMPAR rectification in POMC neurons in fed mice.
(a) Representative trace of AMPA rectification with (red) or without (blue) IP rapamycin injection (10 mg/kg body weight) under fed conditions. (b) I-V relationship of AMPARs in POMC neurons in fed mice treated with rapamycin (red) or saline (blue). The value of peak amplitude of AMPAR-mediated eEPSC was normalized as percent by the value at −60 mV. The dotted line represents a linear regression of AMPAR-EPSCs in POMC neurons between −80 and 0 mV in control group. (c) RIs of AMPAR-EPSCs in POMC neurons in fed mice treated with rapamycin (red) or saline (blue). (d) I-V relationship of AMPAR-EPSCs and (e) RI in POMC neurons excluded poor space clamp recordings did not change from original results (mean ± SEM. * indicates p<0.05, paired t-test.).
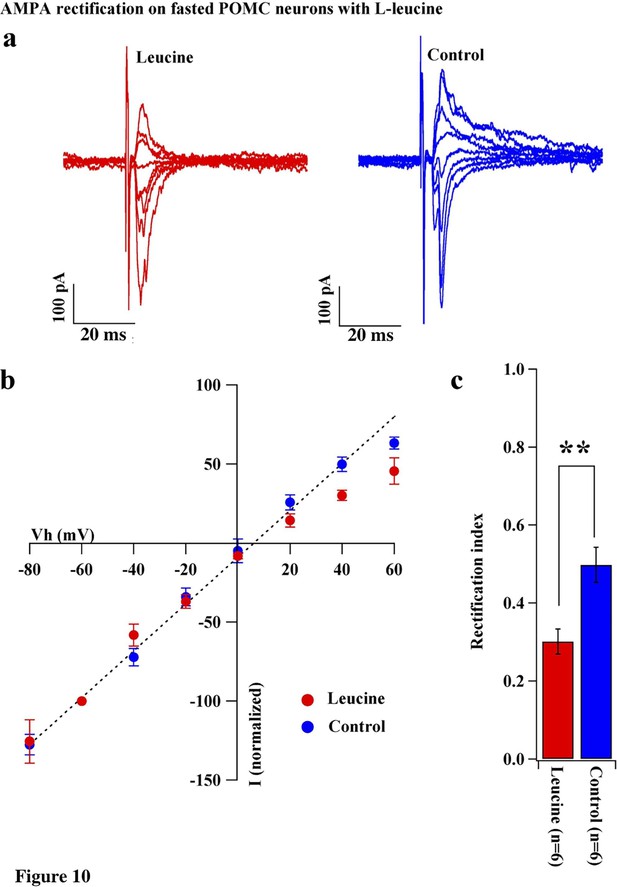
Leucine promoted AMPAR rectification in fasted mice.
(a) Representative trace of AMPA rectification with (red) or without (blue) leucine (100 µM) application to acute brain slice including ARC in fasted mice. (b) I-V relationship of AMPAR-EPSCs in POMC neurons in fasted mice treated with leucine (red) or saline (blue) in vitro. The value of peak amplitude of AMPAR-mediated eEPSC was normalized as percent by the value at −60 mV. The dotted line represents a linear regression of AMPAR-EPSCs in POMC neurons between −80 and 0 mV in fasted mice. (c) RIs of AMPAR-EPSCs in POMC neurons in fasted mice treated with leucine (red) or saline (blue). (mean ± SEM. * indicates p<0.05, paired t-test.).
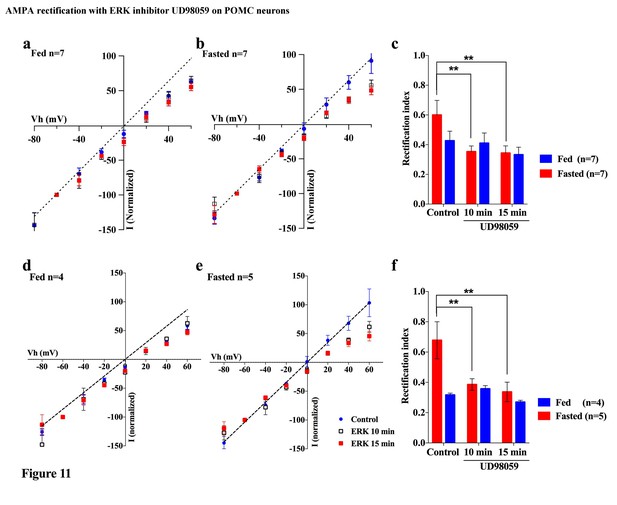
An ERK inhibitor promoted AMPAR rectification in fasted but not fed mice.
(a) I-V relationship of AMPAR-EPSCs in POMC neurons in fed mice at 0 (blue), 10 (box), and 15 (red) min after application (in vitro) of ERK inhibitor, UD98059 (10 µM in recording pipette solution). The value of peak amplitude of AMPAR-mediated eEPSC was normalized as percent by the value at −60 mV. The dotted line represents a linear regression of AMPAR-EPSCs in POMC neurons between −80 and 0 mV at 0 min after application of UD98059. (b) I-V relationship of AMPAR-EPSCs in POMC neurons in fasted mice at 0 (blue), 10 (box), and 15 (red) min after application of UD98059. (c) RIs of AMPAR-EPSCs in POMC neurons in fed (blue) and fasted (red) mice treated with UD98059. (d, e) I-V relationship of AMPAR-EPSCs and (f) RI in POMC neurons excluded poor space clamp recordings did not change from original results. (mean ± SEM. * and ** indicates p<0.05 and 0.01, respectively, paired t-test.).





