Motoneurons regulate the central pattern generator during drug-induced locomotor-like activity in the neonatal mouse
Figures
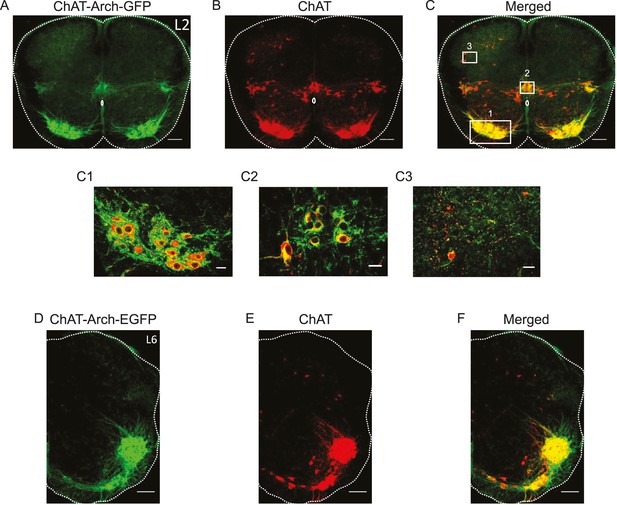
Archaerhodopsin is expressed in all ChAT-positive neurons.
(A–B–C) Z-stack projection of 10X images (2.05 μm) of a 60 μm section of the L2 segment of a P3 ChAT-Arch mouse spinal cord showing archaerhodopsin (green, A and C) and ChAT-positive (red, B and C) neurons and the merged image (C). The white scale bars represent 100 μm. Insets from the white rectangles in the merged image show that motoneurons (C1), sympathetic pre-ganglionic neurons (C2) and dorsal cholinergic neurons (C3) all express archaerhodopsin and ChAT (1 μm optical section). The white scale bars measure 20 μm. (D–E–F) 10X z-stack projection (4 μm) of a 60 μm section in the L6 segment of a P3 ChAT-Arch mouse hemi-cord showing archaerhodopsin (green, D–F) and ChAT-positive (red, E–F) neurons and the merged image (F). The white scale bar is 100 μm.
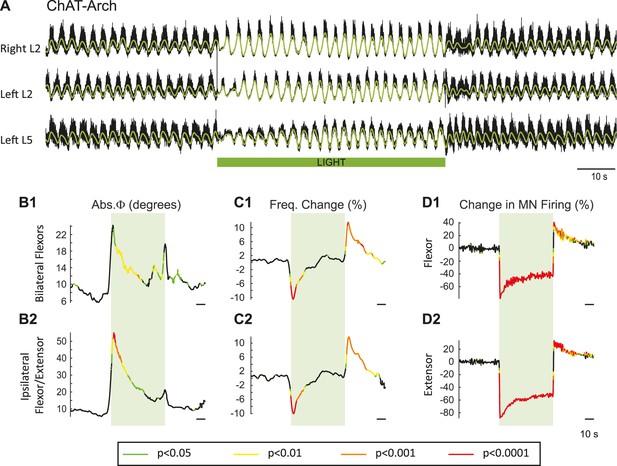
Light-induced hyperpolarization of cholinergic neurons transiently decreases the frequency and alters the phasing of drug-induced locomotor-like activity.
(A) Locomotor-like activity recorded from the right L2 and left L2 and L5 ventral roots (black traces) of a P1 isolated spinal cord of a ChAT-Arch mouse. The superimposed green traces are the slow potentials obtained by low pass filtering the raw signals. Locomotor-like activity was evoked by applying 5 μM NMDA and 10 μM 5-HT. The duration of the light (60 s) is indicated by the green bar. (B–C) Time series showing the change in absolute phase (B) and frequency (C) averaged for all experiments for the bilateral flexor (B1–C1) and the ipsilateral flexor/extensor roots (B2–C2). (D) Change (%) in the averaged integrated ventral root discharge (Change in MN firing) for the ipsilateral flexor (D1) and extensor (D2) ventral roots. The statistics were obtained using a bootstrap t-test between ChAT-Archaerhodopsin (n = 25) and wild type cords (n = 28) and are color-coded as indicated in the box below the records.
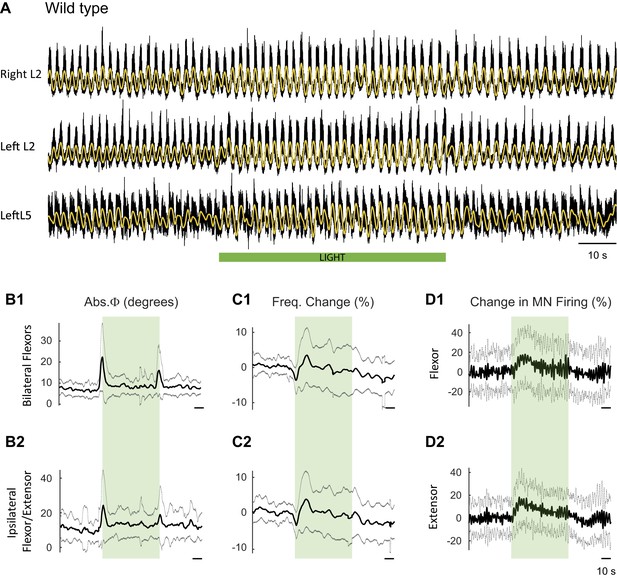
Effect of green light on drug-induced locomotor-like activity in wild type cords.
(A) Ventral root recordings (Right L2, and Left L2 and L5) showing the effect of light on the locomotor-like rhythm evoked by 5 μM NMDA and 10 μM 5-HT in a wild type cord. The superimposed yellow traces are the slow potentials obtained by low pass filtering the raw signal. The green bar indicates the duration of the light (60 s). (B–C) Time series of the change in absolute phase (B) and frequency (C) averaged for all experiments for the bilateral flexors (B1 and C1) and the ipsilateral flexor-extensors (B2 and C2) in 28 wild-type cords. (D) Averaged integrated signals (Change in MN firing) for the ipsilateral flexor (D1) and extensor (D2) ventral roots. The dotted lines on the traces represent ±1 standard deviation of the mean. The green light (green rectangles) causes an increase in the firing of motoneurons in both the bilateral flexors and the ipsilateral flexor/extensor roots. Accompanying this is a small increase in the frequency of the locomotor-like rhythm.
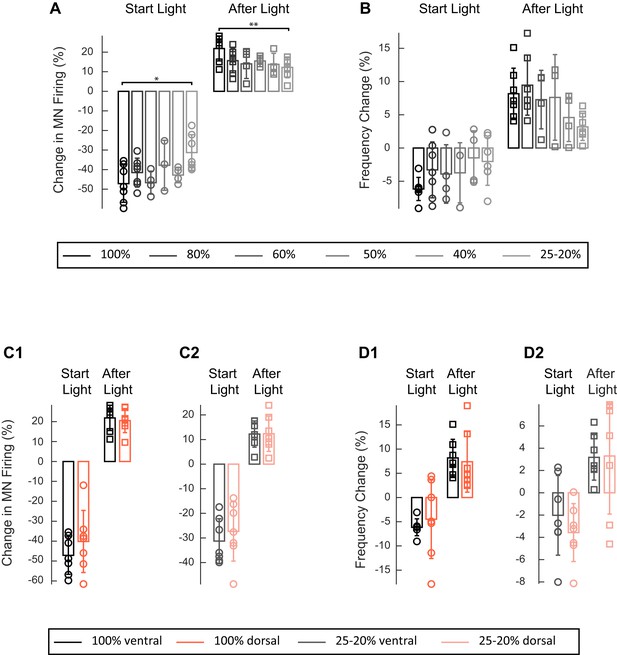
Effect of varying the green light intensity on the integrated firing of the right L2 ventral root and on the frequency of the locomotor-like rhythm in ChAT-Arch cords.
(A–B) Bar plots showing the average change in motoneuron firing (A, Change in MN firing) and frequency of the right L2 (B) for the 10 s just before and just after the light is turned on (Start Light, circles) and the 10 s just before and just after the light is turned off (After Light, squares). The color gradient of the bars indicates the intensity of the light from black 100%, to light grey 20–25%. (C–D) Bar plots showing the averaged change in motoneuron firing (C, Change in MN firing) and in the frequency of the right L2 (D) for the 10 s just before and just after the light is turned on (Start Light, circles) and the 10 s just before and just after the light is turned off (After Light, squares). Either the ventral (black and grey bars) or the dorsal (orange and light orange bars) part of the cord was illuminated either at 100% intensity (C1, D1, black and orange respectively) or at 20–25% intensity (C2, D2, grey and light orange respectively). Using a two-way ANOVA, we calculated the statistical differences between the different light intensities (shown above the bars in A, *p<0.05, **p<0.01) and site of illumination (dorsal or ventral in C-D). ANOVA for the changes in frequency at the beginning of the light (Site of illumination: F(1,52)=0.1705, p=0.6814, Light intensity: F (5,52)=0.932, p<0.4680, Interaction F(5,52)=0.3916, p=0.8523) and after the light is turned off (Site of illumination: F(1,52)=0.5472, p=0.4628, Light intensity: F (5,52)=2.823, p<0.4680, Interaction F(5,52)=0.5886, p=0.7086) and the two-way ANOVA results for the change in motoneuron firing at the beginning of the light were (Site of illumination: F(1,52)=5.276, p=0.0257, Light intensity: F(5,52)=2.695, p<0.0307, Interaction F(5,52)=0.2178, p=0.9534) and after the light (Site of illumination: F(1,52)=0.3265, p=0.5702, Light intensity: F(5,52)=3.216, p<0.0132, Interaction F(5,52)=0.2178, p=0.9534).
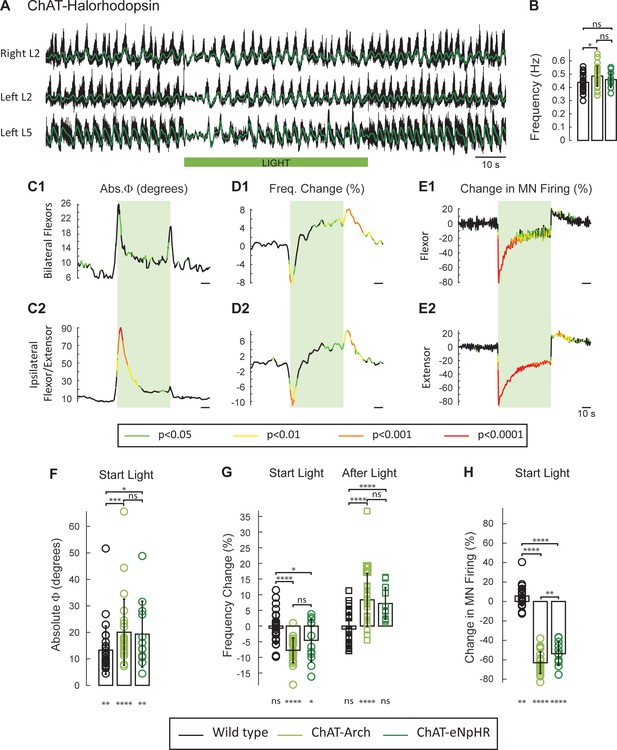
The light-induced decrease in the frequency of the rhythm and the phase changes are not due to changes in pH.
(A) Locomotor-like activity recorded from the right and left L2 and the left L5 ventral roots (black traces) in a P1 ChAT-eNpHR animal. The superimposed green traces are the slow potentials obtained by low pass filtering the raw signals. The activity was evoked by applying 6 μM NMDA and 8 μM 5-HT and the green bar indicates the duration of the light (60 s). (B) Bar plot showing the average locomotor-like frequency in wild type (n = 28, black circles), ChAT-Arch (n = 25, light green circles) and ChAT- eNpHR (n = 12, dark green circles) cords before the light (ANOVA, p=0.0533, two-stage linear step-up procedure, *: p=0.0141). (C–D) Time series of the change in absolute phase (C) and frequency (D) averaged for all experiments for the bilateral flexor (C1–D1) and ipsilateral flexor-extensor roots (C2–D2). (E) Averaged integrated ventral root discharge (Change in MN firing) for the ipsilateral flexor (E1) and extensor (E2) ventral roots. The statistics are obtained using a bootstrap t-test between ChAT- eNpHR (n = 12) and wild type cords (n = 28). The statistics are color-coded as indicated in the box below the records. The green rectangles indicate the duration of the light (60 s). (F–G–H) Bar plots showing the average change in the absolute phase (F), frequency of the bilateral flexors (G) for the 10 s just before and just after the light is turned on (Start Light, circles) and the 10 s just before and just after the light is turned off (After Light, squares) for wild type (black), ChAT-Arch (light green) and ChAT- eNpHR (dark green) animals. (H) Bar plots showing the average change of the ipsilateral flexor root for the 10 s just before and just after the light is turned on (Start Light, circles). Using a two-way ANOVA, we calculated the statistical differences between the three groups of animals (genetic Identity, shown above the bars) and the differences between light on and light off (light status; shown below the bars). The results of the ANOVA for the frequency changes were (Light status: F(3, 248) p<0.0001, Genetic identity F(2,248) p=0.0276, Interaction F(6,248): p<0.0001), for the absolute phase changes were (Light status: F(3, 248) p<0.0001, Genetic identity F(2,248) p<0.0001, Interaction F(6,248): p=0.8341), and for the changes in motoneuron firing were (Light status: F(3, 248) p<0.0001, Genetic identity F(2,248) p<0.0001, Interaction F(6,248): p<0.0001*p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
-
Figure 3—source data 1
Source data for Bar plots in Figure 3B.
- https://doi.org/10.7554/eLife.26622.007
-
Figure 3—source data 2
Source data for Bar plots in Figure 3F.
- https://doi.org/10.7554/eLife.26622.008
-
Figure 3—source data 3
Source data for Bar plots in Figure 3G.
- https://doi.org/10.7554/eLife.26622.009
-
Figure 3—source data 4
Source data for Bar plots in Figure 3H.
- https://doi.org/10.7554/eLife.26622.010
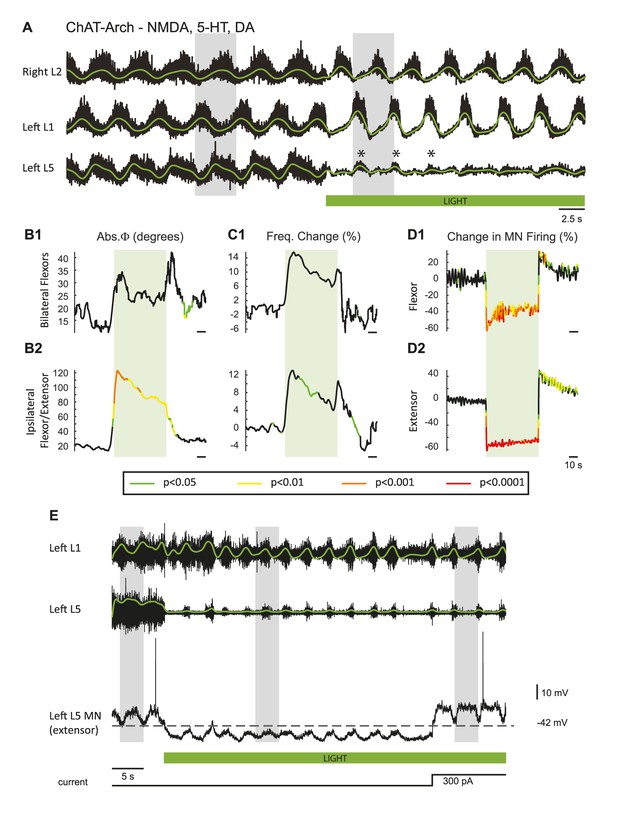
Effect of dopamine of the light-induced effects during drug-induced locomotor-like activity in ChAT-Arch animals.
(A) Ventral root recordings (Right L2 and Left L1 and L5) showing the effect of hyperpolarizing ChAT-positive neurons during the locomotor-like rhythm evoked by 5 μM NMDA, 10 μM 5-HT and 50 μM DA. The superimposed green traces are the slow potentials obtained by low pass filtering the raw signal. The green bar indicates the timing of the light. Before the light, the left L5 slow potentials are alternating with those in the left L1 ventral root. Once the light is turned on, they are reduced in amplitude but are now in phase (*) with the left L1 ventral root. (B–C) Time series of the changes in phase (Abs φ) and frequency for the bilateral flexors (B1–C1) and the ipsilateral flexor/extensors (B2–B3). The color-coded regions on the traces (key beneath the records) indicate the statistically different portions of the record when compared to wild-type animals using a bootstrap t-test (ChAT-Arch: n = 14, WT: n = 8). Note that, except for the phase changes in the ipsilateral flexor/extensor roots, most of the changes are not significant. Note also that the frequency increase during the light contrasts with the light-induced decrease in the frequency of the locomotor-like rhythm when dopamine is omitted from the drug cocktail, consistent with the earlier finding that dopamine blocks ventral root activation of the locomotor rhythm (Humphreys and Whelan (2012). (D1-D2) Changes in the integrated neurogram (Change in MN Firing %) during the light. (E) Ventral root recordings (Left L1 and Left L5) together with an intracellular recording from an extensor motoneuron (Vm = −42 mV) firing in phase with the left L5 ventral root during the locomotor-like rhythm evoked by 5 μM NMDA, 10 μM 5-HT and 50 μM DA. The superimposed green traces show the integrated neurogram. The green bar indicates the timing of the light. In both the L5 ventral root and the intracellular recording, the integrated neurogram and the locomotor drive potentials - which are alternating with the Left L1 ventral root activity - change their phasing and become synchronous with the L1 ventral root activity during the light. However, when the intracellular membrane potential is restored to the pre-light level by current injection (300 pA) as indicated by the current trace beneath the green bar, the intracellularly recorded drive potentials return to their pre-light out of phase activity whereas the Left L5 root recording does not. The grey boxes highlight the phasing between the roots and the intracellular recording. This suggests that during the light the membrane potential falls below the chloride equilibrium potential thereby rendering the inhibitory locomotor drive potentials depolarizing.
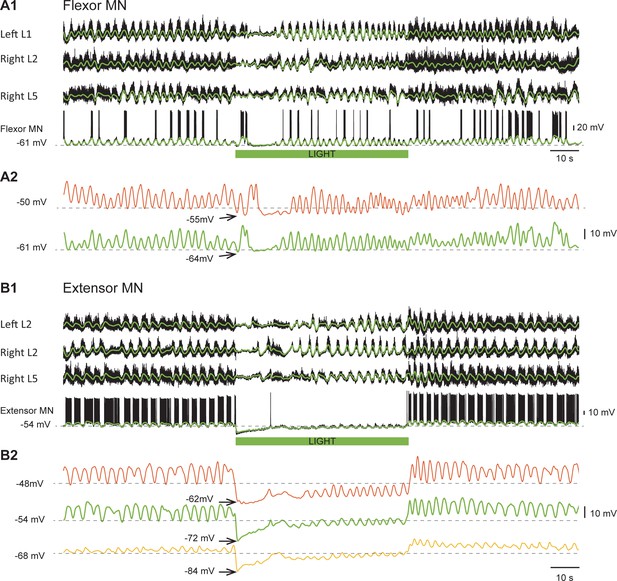
Light-induced hyperpolarization of cholinergic neurons affects the rhythmic synaptic drive to motoneurons.
(A1) Locomotor-like activity recorded from the left L1 and the right L2 and L5 ventral roots together with a flexor motoneuron in the left L1 segment (black traces). The superimposed green traces are the slow potentials obtained by low pass filtering the raw signal. (A2) Low pass filtered records of the membrane potential of the same motoneuron held at two different membrane potentials (−50 mV - red trace; −61 mV - green trace) show that the locomotor drive potentials transiently disappeared for ~10 s after the light. (B1) Locomotor-like activity recorded from the left L2 and the right L2 and L5 ventral roots together with an extensor motoneuron in the right L5 segment (black traces). The superimposed green traces are the slow potentials obtained by low pass filtering the raw signals. (B2) Low pass filtered records of the membrane potential of the same motoneuron held at three different membrane potentials (−48 mV - red trace; −54 mV - green trace; −68 mV – yellow trace) show that the locomotor drive potentials transiently disappeared for 10–15 s after the light. The green bar below the intracellular recording indicates the duration of the light.
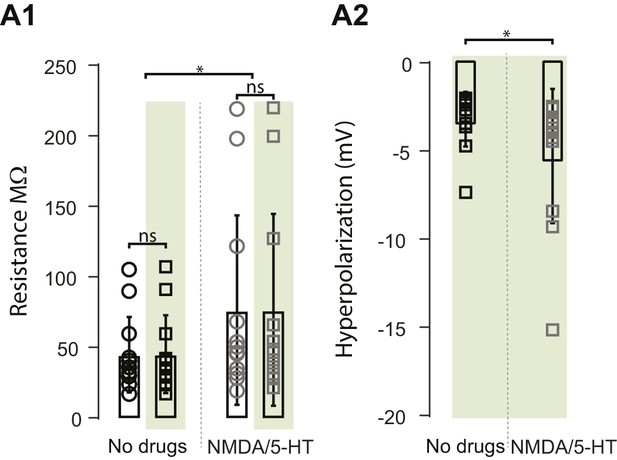
Properties of Motoneurons in ChAT-Arch mice.
(A1) Bar plot showing the input resistance of motoneurons in ChAT-Arch cords under quiescent conditions (no drugs) when the light is off (black circles) or on (black squares), and after applying NMDA and 5-HT when the light is off (grey circles) or on (grey squares). ANOVA RM, p=0.0440. (A2) Bar plot showing the hyperpolarization induced by the light without any drugs or with NMDA and 5-HT. A slightly larger, but significant, increase in the hyperpolarization is observed in the drugs (t-test, p=0.0352).
-
Figure 4—figure supplement 1—source data 1
Input resistance MN ChAT-Arch spinal cords.
Related to Figure 4—figure supplement 1 A1.
- https://doi.org/10.7554/eLife.26622.014
-
Figure 4—figure supplement 1—source data 2
Intracellular hyperpolarization MN ChAT-Arch spinal cord.
Related to Figure 4—figure supplement 1 A2.
- https://doi.org/10.7554/eLife.26622.015
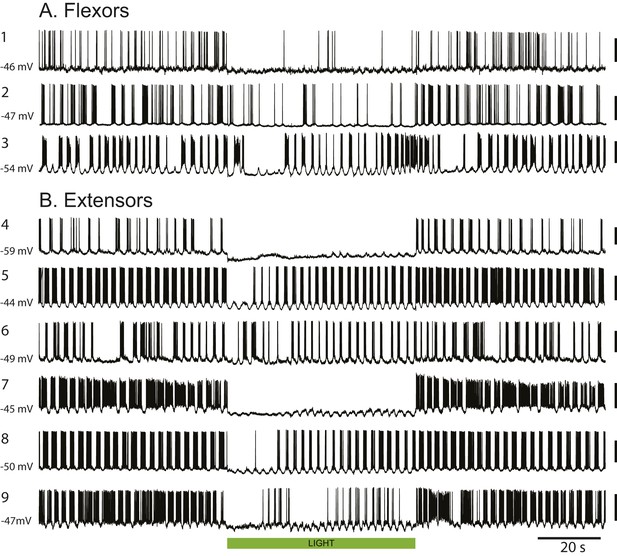
Green light suppresses the firing of individual flexor and extensor motoneurons in ChAT-Arch cords.
(A–B). Examples of 3 flexor (A) and six extensor (B) motoneurons showing the reduced firing during locomotor-like activity. The membrane potential at which the recordings were made is shown on the left of each record. The calibration bar to the right of the records is 40 mV. The green bar indicates the duration of the light (60 s).
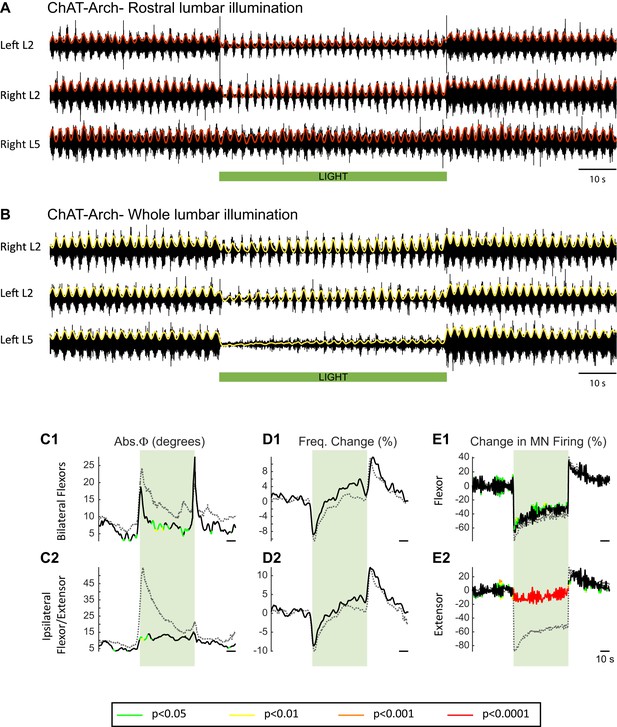
The light-induced changes in the locomotor-like rhythm are not due to light-induced membrane potential changes on the intrinsic motoneuron properties in ChAT-Arch cords.
(A) Ventral root recordings (Left L2, and Right L2 and L5) showing the effect of light on the locomotor-like rhythm evoked by 5 μM NMDA and 10 μM 5-HT when the rostral lumbar segments are illuminated with green light. The black traces show the high-pass (10 Hz) filtered signal and the superimposed red traces are the integrated neurograms. The green bar indicates the duration of the light (60 s). (B) Ventral root recordings (Right L2, and Left L2 and L5) showing the effect of light on the locomotor-like rhythm evoked by 5 μM NMDA and 10 μM 5-HT when the whole lumbar cord is illuminated. The black traces show the high-pass (10 Hz) filtered signal and the superimposed yellow traces are the integrated neurograms. The green bar indicates the duration of the light (60 s). (C–E) Time series of the change in absolute phase (C) and frequency (D) averaged for all experiments for the bilateral flexor (C1–D1) and ipsilateral flexor-extensor roots (C2–D2). (E) Averaged integrated signals (Change in MN Firing) for the ipsilateral flexor (E1) and extensor (E2) ventral roots. The statistics are obtained using a bootstrap t-test between ChAT-Arch whole lumbar illumination (n = 28) and ChAT-Arch rostral lumbar illumination (n = 7) and are color coded as indicated in the box below the records. The black traces represent the data for the partial lumbar illumination, while the grey dotted traces show the average time series for the whole lumbar cord illumination experiments. The timing of the light (60 s) is indicated by the green rectangles.
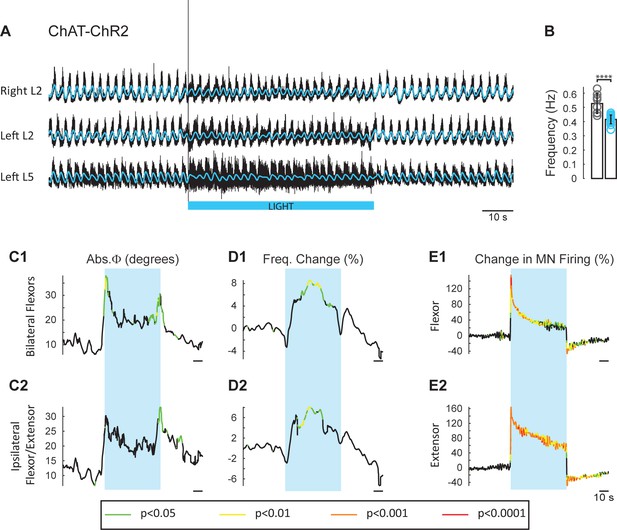
Light-induced depolarization of cholinergic neurons alters the frequency and phasing of drug-induced locomotor-like activity.
(A) Locomotor-like activity recorded from the right L2 and the left L2 and L5 ventral roots (black traces) of a P1 isolated spinal cord from a ChAT-ChR2 mouse. The superimposed blue traces are the slow potentials obtained by low pass filtering the raw signal. Locomotor-like activity was evoked by applying 5 μM NMDA and 10 μM 5-HT. Activation of channelrhodopsin was achieved using a train of blue stimuli (1 ms at 100 Hz) for 60 s as indicated by the blue bar under the traces. (B) Bar plot showing the average locomotor-like frequency in Chat-EGFP (n = 8, grey circles) and ChAT- Channelrhodopsin (n = 15, blue circles) before the light. Note, that the frequency of the rhythm is significantly slower in the ChAT-ChR2 cords (t-test, ****p<0.0001). (C–D) Time course of the changes in phase (C) and frequency (D) averaged for all experiments for the bilateral flexors (C1–D1) and ipsilateral flexor-extensors (C2–D2). (E) Averaged integrated neurograms (Change in MN firing) for the ipsilateral flexor (E1) and extensor (E2) ventral roots. The statistics are obtained using a bootstrap t-test between ChAT-ChR2 and ChAT-EGFP cords and are color-coded as indicated in the box below the traces. The blue rectangles indicate the duration of the light (60 s).
-
Figure 5—source data 1
Source data for Bar plots in Figure 5B.
- https://doi.org/10.7554/eLife.26622.019
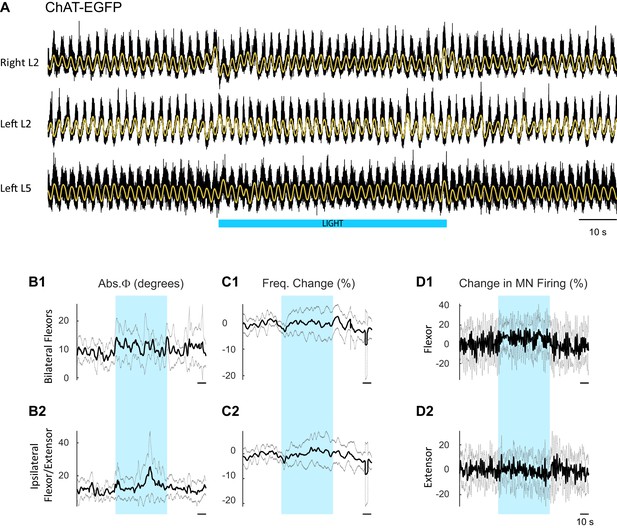
Effect of blue light on drug-induced locomotor-like activity in ChAT-EGFP cords.
(A) Ventral root recordings (Right L2, and Left L2 and L5) showing the effect of blue light on the locomotor-like rhythm evoked by 5 μM NMDA and 10 μM 5-HT in ChAT-EGFP cords (n = 8). The superimposed yellow traces are the slow potentials obtained by low pass filtering the raw signal. The blue bar indicates the duration of the light (60 s). (B–C) Time series of the change in absolute phase (B) and frequency (C) averaged for all experiments for the bilateral flexor roots (B1–C1) and ipsilateral flexor-extensor roots (B2–C2). (D) Averaged integrated signals (Change in MN Firing) for the ipsilateral flexor (D1) and extensor (D2) ventral roots. The grey dotted lines represent one standard deviation of the mean. Note that the blue light (blue rectangles) causes relatively minor changes in each of the measured variables.
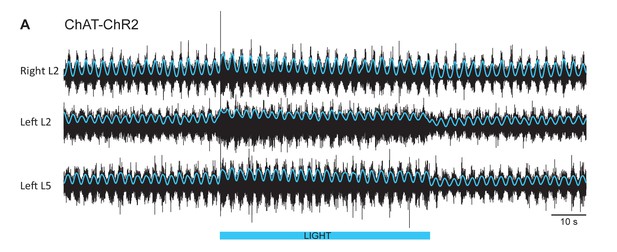
Blue light increases the firing of motoneurons during locomotor-like activity in a ChAT-ChR2 cord.
(A) Ventral root recordings (Right L2, and Left L2 and L5) showing the effect of depolarizing cholinergic neurons during the locomotor-like rhythm evoked by 5 μM NMDA and 10 μM 5-HT in a ChAT-ChR2 cord. The black traces show the high-pass filtered signal (10 Hz) signal, and the superimposed blue traces are the integrated neurograms. The blue bar indicates the duration of the light (60 s).
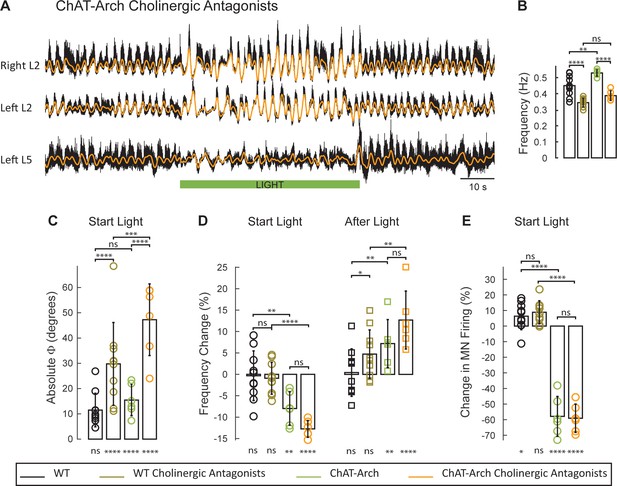
The light-induced disruption of the locomotor-like rhythm is exacerbated in the presence of cholinergic antagonists.
(A) Ventral root recordings (Right L2, and Left L2 and L5) showing the effect of light on the locomotor-like rhythm evoked by 5 μM NMDA and 10 μM in the presence of 50 μM mecamylamine, 50 μM dhβE, and 5 μM atropine. The superimposed orange traces are the slow potentials obtained by low pass filtering the raw signal. The green bar indicates the duration of the light (60 s). (B–E) Comparison between WT (black, brown), ChAT-Arch (green, orange) cords in the absence or presence of cholinergic antagonists. (B) Bar plot showing the average locomotor-like frequency in WT (n = 10) and ChAT-Arch (n = 6) cords under control conditions (no light) in the absence or presence of cholinergic antagonists (ANOVA, p<0.0001). (C–E) Bar plots showing the average change in the absolute phase (C), frequency (D) of the bilateral flexors for the 10 s just before and just after the light is turned on (Start Light, circles) and the 10 s just before and just after the light is turned off (After Light, squares). (E) Bar plot showing the averaged integrated neurogram (Change in MN firing) of the ipsilateral flexor root for the 10 s just before and just after the light is turned on (Start Light, circles) for WT (black, brown), ChAT-Arch (green, orange) cords in the absence or presence of cholinergic antagonists. Using a two-way ANOVA we calculated the statistical differences between the three groups of animals (genetic Identity, shown above the bars) and the differences between light on and light off (light status, shown below the bars). The results of the ANOVA for the changes in the variables were: absolute phase (Light status: F (3,112) p<0.0001, Genetic identity/Drug treatment: F(3,112) p<0.0001, Interaction F(9,112) p=0.0017), frequency (Light status: F (3,112) p<0.0001, Genetic identity/Drug treatment: F(3,112) p=0.3073, Interaction F(9,112) p<0.0001), and motoneuron firing (Light status: F (3,112) p<0.0001, Genetic identity/Drug treatment: F(3,112) p<0.0001, Interaction F(9,112) p<0.0001). *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
-
Figure 6—source data 1
Source data for Bar plots in Figure 6B.
- https://doi.org/10.7554/eLife.26622.023
-
Figure 6—source data 2
Source data for Bar plots in Figure 6C.
- https://doi.org/10.7554/eLife.26622.024
-
Figure 6—source data 3
Source data for Bar plots in Figure 6D.
- https://doi.org/10.7554/eLife.26622.025
-
Figure 6—source data 4
Source data for Bar plots in Figure 6E.
- https://doi.org/10.7554/eLife.26622.026
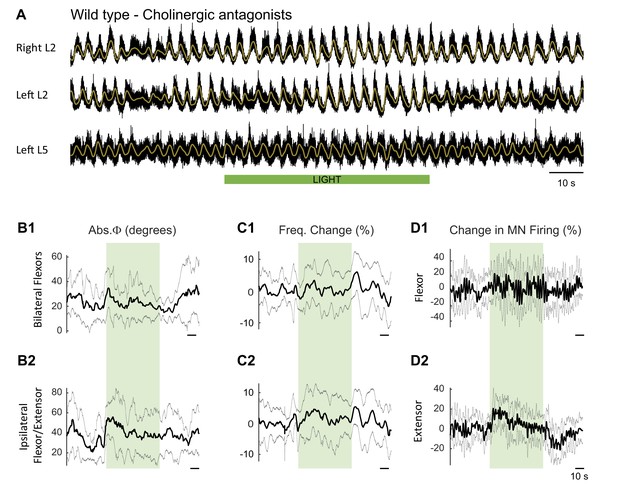
Effect of green light during drug-induced locomotor-like in wild type cords in the presence of cholinergic blockers.
(A) Ventral root recordings (Right L2, and Left L2 and L5) showing the effect of light on the locomotor-like rhythm evoked by 5 μM NMDA and 10 μM 5-HT in the presence of 50 μM mecamylamine, 50 μM dhβE, and 5 μM atropine (n = 10). The superimposed brown traces are the slow potentials obtained by low pass filtering the raw signal. The green bar indicates the duration of the light (60 s). (B–C) Time series of the change in absolute phase (B) and frequency (C) averaged for all experiments for the bilateral flexor (B1–C1) and ipsilateral flexor-extensor roots (B2-C2). (D) Averaged integrated signals (Change in MN Firing) for the ipsilateral flexor (D1) and extensor (D2) ventral roots. The grey dotted line represents one standard deviation of the mean. Except for an increase in the firing of the ipsilateral/flexor extensor roots and a small phase change at light onset, the light (green rectangles) does not result in systematic changes in the other variables.
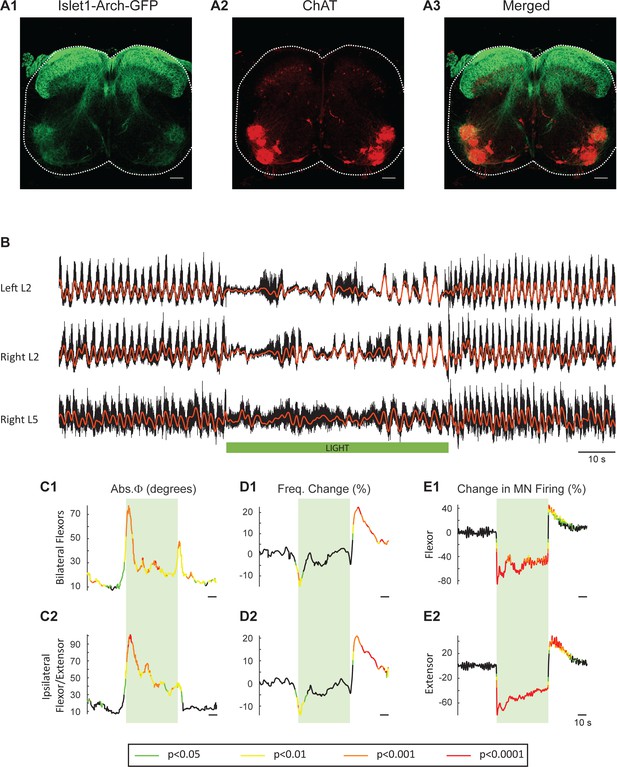
Light-induced hyperpolarization of Isl1 neurons alters the frequency and phasing of locomotor-like activity.
(A) Z-stack projection of 10X images (4 μm) of a 60 μm section of the L5 segment of a P2 Isl1-Arch mouse spinal cord showing archaerhodopsin (green, A1 and A3) and ChAT-positive (red, A2 and A3) neurons and the merged image (A3). The white scale bars represent 100 μm. (B) Locomotor-like activity recorded from the left L2 and the right L2 and L5 ventral roots (black traces) of a P1 isolated spinal cord from an Isl1-Arch mouse. The superimposed red traces are the slow potentials obtained by low pass filtering the raw signal. Locomotor-like activity was evoked by applying 5 μM NMDA and 10 μM 5-HT. The timing of the green light (60 s) is indicated by the green bar below the traces. (C–D) Time series of the change in absolute phase (C) and frequency (D) averaged for all experiments for the bilateral flexor (C1–D1) and ipsilateral flexor-extensor (C2–D2) roots. (E) Averaged integrated signals (Change in MN firing) for the ipsilateral flexor (E1) and extensor (E2) ventral roots. The statistics are obtained using a bootstrap t-test between Isl1-Arch (n = 8) and wild type cords (n = 28) and are color coded as indicated in the box below the records. The timing of the light (60 s) is indicated by the green rectangles.
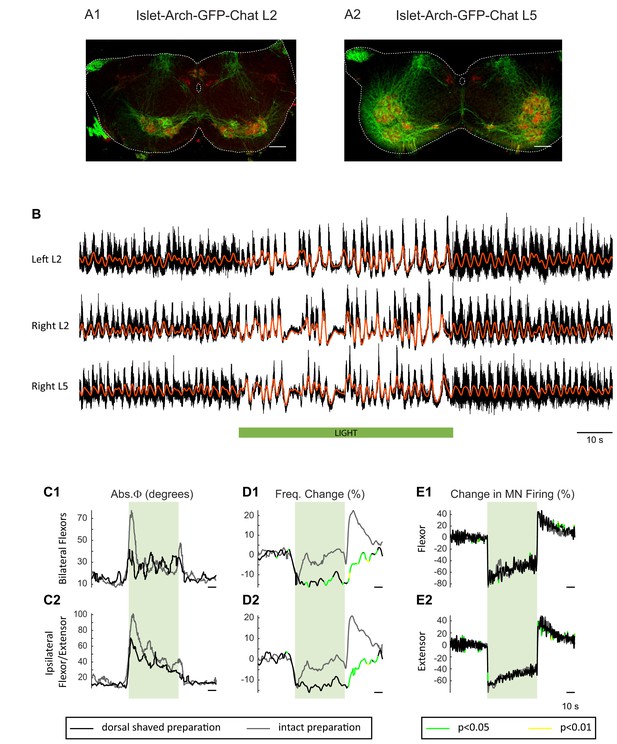
The light-induced perturbation of the locomotor-like rhythm is similar in dorsal-shaved and intact preparations of Isl1-Arch cords.
(A) Z-stack projection of 10X images (4 μm) of a 60 μm section of the L2 (A1) and L5(A2) segments of a P1 Isl1-Arch mouse spinal cord showing archaerhodopsin (green) and ChAT-positive (red) neuronal labelling. The white scale bars represent 100 μm. (B) Ventral root recordings (Left L2, and Right L2 and L5) showing the effect of hyperpolarizing Isl1-positive neurons during the locomotor-like rhythm evoked by 5 μM NMDA and 10 μM 5-HT in a dorsal-shaved Isl1-Arch cord. The black traces show the high-pass filtered signal, and the superimposed red traces are the slow potentials. The green bar indicates the duration of the light (60 s). (C–E) Time series showing the change in absolute phase (C) and frequency (D) averaged for all Isl1-Arch preparations (grey traces) versus Isl1-Arch dorsal shaved preparations (black traces), for the bilateral flexor (C1–D1) and the ipsilateral flexor/extensor roots (C2–D2). (E) Change (%) in the averaged integrated ventral root discharge (Change in MN firing) for the ipsilateral flexor (E1) and extensor (E2) ventral roots. The statistics were obtained using a bootstrap t-test between Isl1-Archaerhodopsin (n = 8) and dorsal shaved Isl1-Archaerhodopsin (n = 5) spinal cords and are color-coded as indicated in the box below the records.
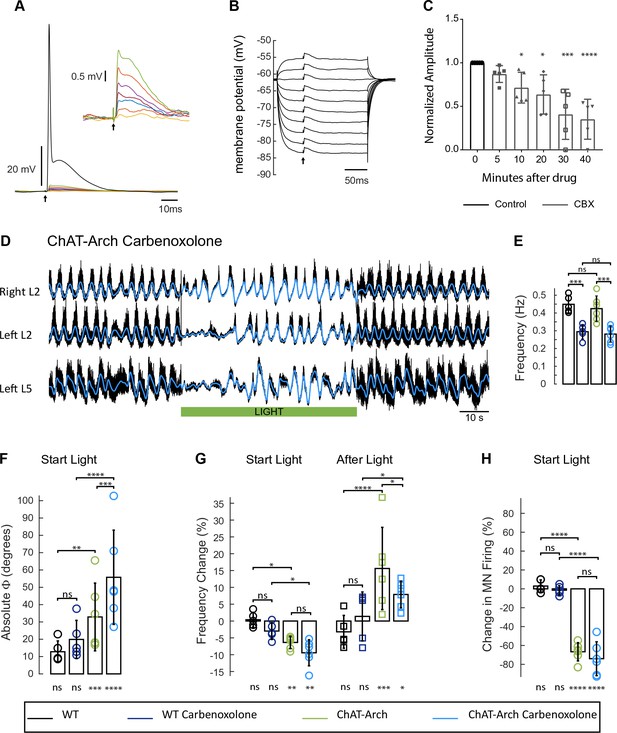
The light-induced disruption of the locomotor-like rhythm is maintained in the presence of the gap junction blocker carbenoxolone.
(A) Superimposed intracellular responses of a motoneuron in a ChAT-Arch mouse to increasing intensity of antidromic stimulation (arrow) of the ventral root. Each trace is the average of 20 trials. The antidromic action potential is shown in black, the short-latency depolarizations (also shown in the inset) are colored and were evoked at stimulus intensities subthreshold for the action potential. (B) The amplitudes of the short-latency depolarizations are independent of membrane potential. The motoneuron was held at different membrane potentials by injecting hyperpolarizing or depolarizing current and a subthreshold stimulus was given every 500 ms (arrow). Each trace is an average of 20 trials. (C) Amplitude of the short latency depolarization in five motoneurons in control (before the drug) and 5, 10, 20, 30 and 40 min after applying 100 μM carbenoxolone. (D) Ventral root recordings (Right L2, and Left L2 and L5) showing the effect of light on the locomotor-like rhythm evoked by 5 μM NMDA and 10 μM 5-HT in the presence of 100 μM carbenoxolone. The superimposed blue traces are the slow potentials obtained by low pass filtering the raw signal. The green bar indicates the duration of the light (60 s). (E) Bar plot showing the average locomotor-like frequency in WT (n = 5) and ChAT-Arch (n = 6) cords under control conditions (no light) in the absence or presence of carbenoxolone. Note that in both the WT and the ChAT-Arch animals the frequency is significantly lower in the presence of carbenoxolone. ANOVA, p<0.0001. (F–H) Bar plots showing the average change in the absolute phase (F) and frequency of the bilateral flexors (G) for the 10 s just before and just after the light is turned on (Start Light, circles) and the 10 s just before and just after the light is turned off (After Light, squares). (H) Bar plot showing the averaged integrated neurogram (Change in MN firing) of the ipsilateral flexor root for the 10 s just before and just after the light is turned on for WT (black, dark blue), ChAT-Arch (light green, light blue) cords in the absence or presence of carbenoxolone. Using a two-way ANOVA, we calculated the statistical differences between the different groups of animals (genetic Identity, shown above the bars) and the differences between light on and light off (light status; shown below the bars). The results of the ANOVA for the changes in the phase were (light status: F (3,72) p<0.0001, Genetic identity/Drug treatment: F(3,72) p<0.0001, Interaction F(9,72) p=0.0379), for the frequency were: F (3,72) p<0.0001, Genetic identity/Drug treatment: F(3,72) p=0.004, Interaction F(9,72) p<0.0001) and for the change in motoneuron firing were: (Light status: F (3,72) p<0.0001, Genetic identity/Drug treatment: F(3,72) p<0.0001, Interaction F(9,72) p<0.0001). *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
-
Figure 8—source data 1
Source data for Bar plots in Figure 8C.
- https://doi.org/10.7554/eLife.26622.031
-
Figure 8—source data 2
Source data for Bar plots in Figure 8E.
- https://doi.org/10.7554/eLife.26622.032
-
Figure 8—source data 3
Source data for Bar plots in Figure 8F.
- https://doi.org/10.7554/eLife.26622.033
-
Figure 8—source data 4
Source data for Bar plots in Figure 8G.
- https://doi.org/10.7554/eLife.26622.034
-
Figure 8—source data 5
Source data for Bar plots in Figure 8H.
- https://doi.org/10.7554/eLife.26622.035
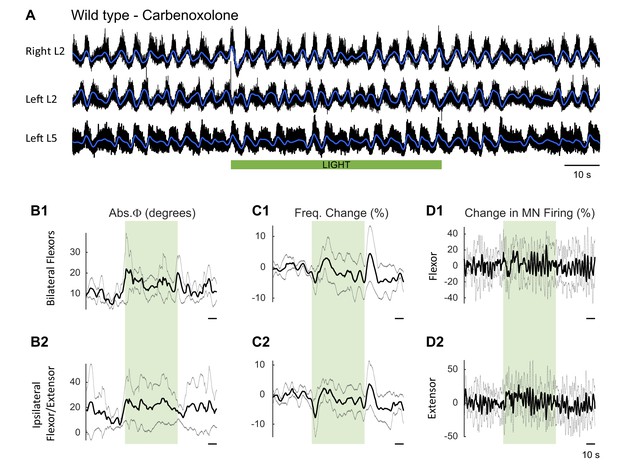
Effect of green light on drug-induced locomotor-like activity in wild type cords in the presence of carbenoxolone to block gap junctions.
(A) Ventral root recordings (Right L2, and Left L2 and L5) showing the effect of light on the locomotor-like rhythm evoked by 5 μM NMDA and 10 μM 5-HT in the presence of 100 μM carbenoxolone in wild type cords (n = 5). The superimposed dark blue traces are the slow potentials obtained by low pass filtering the raw signal. The green bar indicates the duration of the light (60 s). (B–C) Time series of the changes in absolute phase (B) and frequency (C) averaged for all experiments for the bilateral flexor (B1–C1) and ipsilateral flexor-extensor roots (B2–C2). (D) Averaged integrated signals (Change in MN Firing) for the ipsilateral flexor (D1) and extensor (D2) ventral roots. The grey dotted line represents one standard deviation of the mean. The light (green rectangles) results in a small increase in the firing of the flexor/extensor roots and a small change in the phasing of the activity in the roots. In addition, there is a transient decrease in frequency when the light is turned on and a brief increase when the light is turned off.
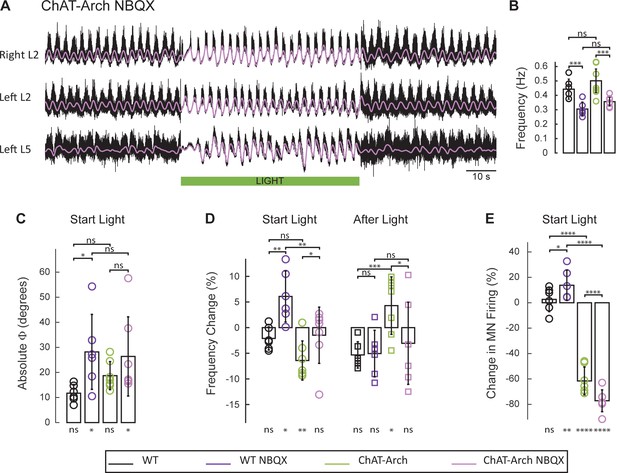
The light-induced disruption of the locomotor-like rhythm is attenuated in the presence of the AMPA-receptor antagonist NBQX.
(A) Ventral root recordings (Right L2, and Left L2 and L5) showing the effect of green light on the locomotor-like rhythm evoked by 5 μM NMDA and 10 μM 5-HT in the presence of 10 μM NBQX. The superimposed light purple traces are the slow potentials obtained by low pass filtering the raw signal. The green bar indicates the duration of the light. (B) Bar plot showing the average locomotor-like frequency in WT (n = 6) and ChAT-Arch (n = 7) cords under control conditions (no light) in the absence or presence of NBQX. For both WT and ChAT-Arch animals the frequency of the rhythm is significantly slower in the presence of NBQX. ANOVA, p<0.0001 (C–E) Bar plots showing the average change in the absolute phase (C) and frequency (D) of the bilateral flexors for the 10 s just before and just after the light is turned on (Start Light, circles) and the 10 s just before and just after the light is turned off (After Light, squares). (E) Bar plot showing the averaged integrated neurogram (Change in MN firing) of the ipsilateral flexor root for the 10 s just before and just after the light is turned on for WT (black, dark purple), ChAT-Arch (green, light purple) cords in the absence or presence of NBQX. Using a two-way ANOVA, we calculated the statistical differences between the different groups of animals (genetic identity, shown above the bars) and the differences between light on and light off (light status; shown below the bars). (B–C–D–E) Comparison between WT (black, dark purple), ChAT-Arch (green, light purple) cords in the absence or presence of NBQX. The results of the ANOVA for the changes in the phase were (Light status: F (3,88) p=0.2436, genetic identity/Drug treatment: F(3,88) p<0.0001, interaction F(9,88) p=0.3893), for the frequency were (Light status: F (3,88) p=0.0630, Genetic identity/Drug treatment: F(3,88) p=0.1335, interaction F(9,88) p<0.0001) and for the motoneuron firing were (Light status: F (3,88) p<0.0001, Genetic identity/Drug treatment: F(3,88) p=0.0001, interaction F(9,88) p<0.0001). *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
-
Figure 9—source data 1
Source data for Bar plots in Figure 9B.
- https://doi.org/10.7554/eLife.26622.038
-
Figure 9—source data 2
Source data for Bar plots in Figure 9C.
- https://doi.org/10.7554/eLife.26622.039
-
Figure 9—source data 3
Source data for Bar plots in Figure 9D.
- https://doi.org/10.7554/eLife.26622.040
-
Figure 9—source data 4
Source data for Bar plots in Figure 9E.
- https://doi.org/10.7554/eLife.26622.041
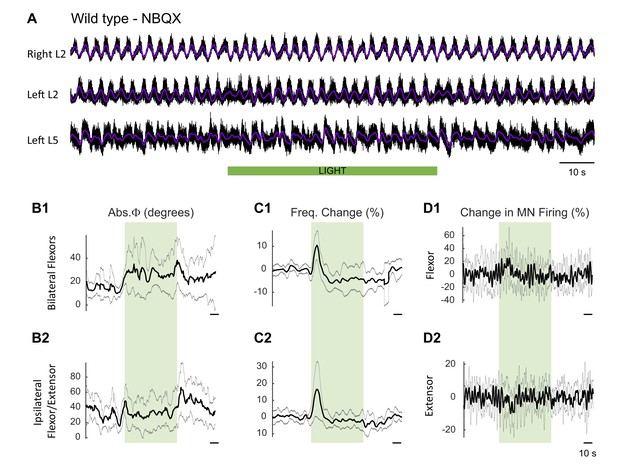
Effect of green light on drug-induced locomotor-like activity in wild type cords in the presence of NBQX.
(A) Ventral root recordings (Right L2, and Left L2 and L5) showing the effect of light on the locomotor-like rhythm evoked by 5 μM NMDA and 10 μM 5-HT in the presence of 10 μM NBQX in wild type cords (n = 6). The superimposed dark purple traces are the slow potentials obtained by low pass filtering the raw signal. The green bar indicates the duration of the light (60 s). (B–C) Time series of the change in absolute phase (B) and frequency (C) averaged for all experiments for the bilateral flexor (B1–C1) and ipsilateral flexor-extensor roots (B2–C2). (D) Averaged integrated signals (Change in MN Firing) for the ipsilateral flexor (D1) and extensor (D2) ventral roots. The grey dotted lines represent one standard deviation of the mean. The most prominent effect of the light (green rectangles) is a transient increase in frequency at light onset.





