Type XVII collagen coordinates proliferation in the interfollicular epidermis
Figures
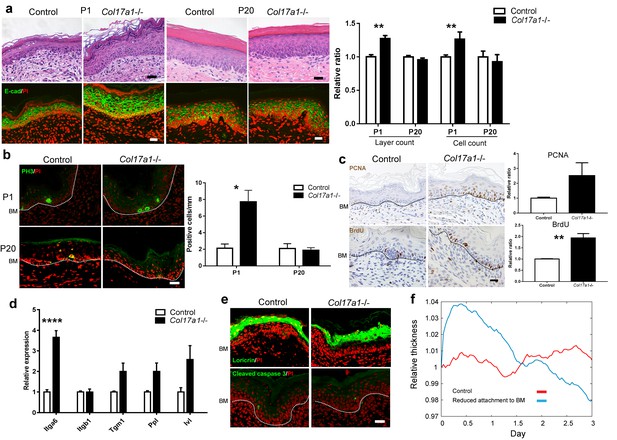
COL17 deletion induces transient IFE hyperproliferation in neonates.
(a) Hematoxylin and eosin (H&E) staining and E-cadherin (E-cad) labeling (with PI nuclear counterstain) of Col17a1−/− and control IFE skin samples from Col17a1+/- or Col17a1+/+ littermates (Control) at P1 (n = 5) and P20 (n = 4). Scale bar: 20 μm. Quantitation of the number of epidermal layers and epidermal cell counts. The values are shown as relative ratios to the controls. (b) PH3 staining at P1 and P20. Scale bar: 20 μm. The number of epidermal basal cells positively labeled for PH3 per mm epidermis (n = 4). BM, basement membrane. (c) PCNA and BrdU labeling at P1. Scale bar: 20 μm. Quantitation of PCNA- (n = 5) and BrdU-positive basal cells (n = 4). The values are shown as relative ratios to the controls. (d) Quantitative RT-PCR (qRT-PCR) of Itga6, Itgb1, Tgm1, Ppl and Ivl mRNAs (n = 5). (e) Loricrin and cleaved caspase-3 staining (representative images from 3 mice). Scale bar: 20 μm. BM, basement membrane. (f) An in silico model of the epidermal cell proliferation upon the reduced adhesion of committed progenitor cells to the BMZ. The details are described in the Material and Methods. The data in all of the histograms are the means ± SE. *0.01<p<0.05, **0.001<p<0.01, ****p<0.0001. Student’s t-tests.
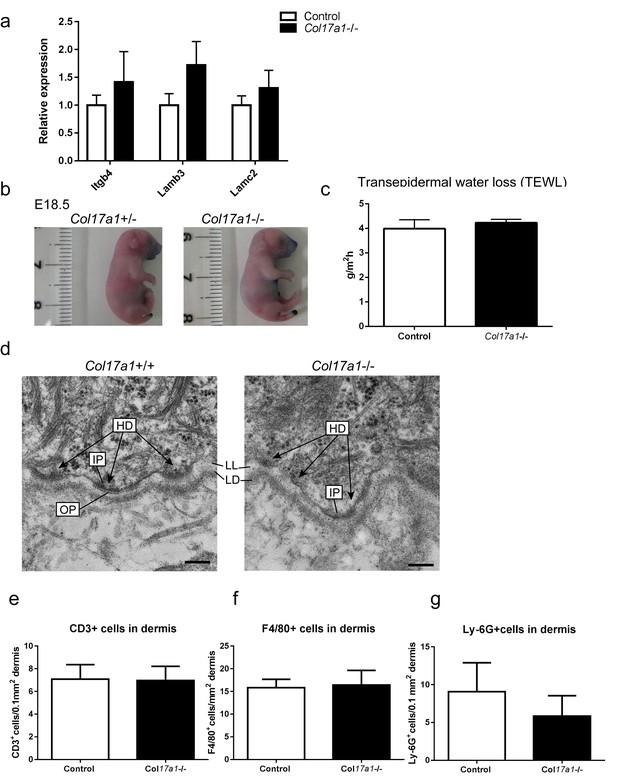
Barrier function assay of Col17a1−/− and littermate controls (E18.5).
Inflammatory cell infiltration in the dermis of Col17a1−/− and littermate controls (P1) and ultrastructural findings of the basement membrane zone in the paw epidermis of Col17a1−/− and littermate controls (P1). (a) mRNA expression levels of Itgb4, Lamb3 and Lamc3 (n = 4). (b) Dye permeabilization with Toluidine blue (representative images from three Col17a1−/− mice and eight control mice). (c) Transepidermal water loss (TEWL) (n = 3 for Col17a1−/− mice; n = 8 for control). (d) In the Col17a1−/− paw epidermis, hemidesmosomes (HD) comprising inner plaques (IP), outer plaques (OP) and anchoring fibrils were blurred compared with those of the Col17a1-/- mice. LL: Lamina Lucida, LD: Lamina densa. Scale bar = 0.2 μm. Representative images from two mice. (eg) Quantification of immune cells in the paw skin dermis of Col17a1−/− and control mice (n = 4). The data are the means±SE. *0.01<p<0.05, **0.001<p<0.01, Student’s t-tests.
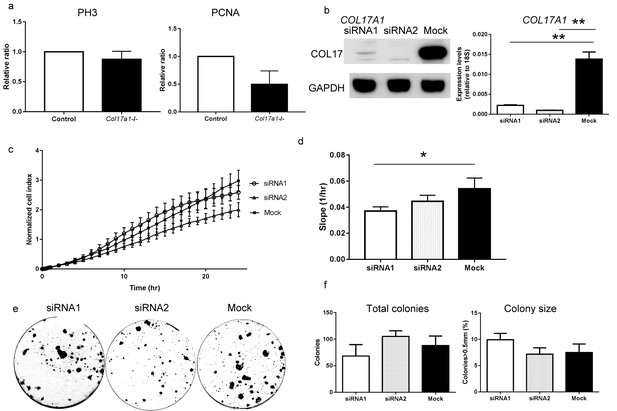
Proliferative ability of the back skin IFE from Col17a1−/− mice and NHEKs treated with COL17A1 siRNAs.
(a) PH3- and PCNA-positive cells in the Col17a1−/− back skin (n = 4 for Col17a1−/−; n = 5 for control). (b) COL17A1 knockdown efficiency in NHEKs. The left panel shows COL17 immunoblotting of lysates from NHEKs treated with siRNAs. The right panel shows the qRT-PCR results of COL17A1 (n = 3). (c–d) Cell proliferation curve (c) and slope (d) of NHEKs treated with siRNAs (n = 3). (e–f) Colony formation assay of NHEKs treated with siRNAs. Gross appearance (e), total colony number (f-left) and the percentage of colonies that were larger than 0.5 mm (f-right) (n = 3). The data are presented as the means±SE. *0.01<p<0.05, **0.001<p<0.01, Student’s t-tests.
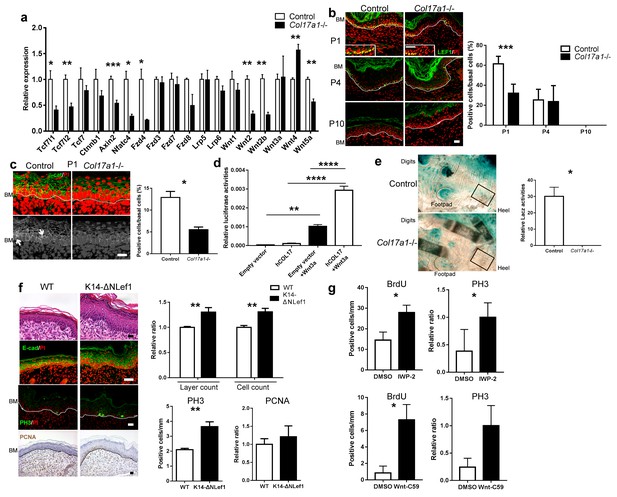
COL17 deficiency destabilizes Wnt-β-catenin signaling in neonates.
(a) qRT-PCR of Wnt-related molecules in Col17a1−/− and control IFE skin samples at P1 (n = 5). Student’s t-test. (b) LEF1 staining of Col17a1−/− and control IFE skin at P1 (n = 5), P4 (n = 4) and P10 (n = 4). Inlet: higher magnification of LEF1-positive basal cells at P1. Scale bar: 20 μm. Quantitation of LEF1-positive basal cells as a percentage of all basal cells (%). Student’s t-test. (c) β-catenin staining of Col17a1−/− and control IFE skin at P1. Nuclear β-catenin accumulation is indicated with arrows. The quantification of nuclear β-catenin-positive cells (n = 3). Student’s t-test. Scale bar: 20 μm. (d) Wnt activity in STF293 cells expressing hCOL17 treated with Wnt3a CM (n = 3). One-way ANOVA test, followed by Tukey’s test. (e) Wnt activities in the hindpaw IFE from ins-Topgal+ (Control: left) and ins-Topgal+:Col17a1−/− (Col17a1−/−: right) mice. Calculated areas devoid of hair follicles or sweat glands are indicated with squares in the representative figures. The results are quantified as the Wnt-activated area per unit (n = 4). Scale bar: 100 μm. Mann-Whitney test. (f) H&E, E-cad, PH3 and PCNA staining of IFE skin samples from K14-ΔNLef and littermate controls at P1. Scale bar: 20 μm. The numbers of epidermal layers, epidermal cell counts and PCNA- and PH3-positive basal cells (n = 4). Student’s t-test. (g) Quantification of BrdU- and PH3-positive cells in WT paw skin IFE treated with Wnt inhibitors (IWP-2 (n = 6) vs DMSO (n = 5) or Wnt-C59 (n = 6) vs DMSO (n = 4)). The data are presented as the means ± SE. Student’s t-test. *0.01<p<0.05, **0.001<p<0.01, ***0.0001<p<0.001, ****p<0.0001.

mRNA profiles of signaling molecules and TGF-β staining.
(a) qRT-PCR screening of genes involved in the Wnt, TGF-β/BMP, Notch, Hedgehog, and FGF signaling pathways. mRNA samples from IFE skin of Col17a1−/− and littermate controls at P1 were analyzed (n = 5). *0.01<p<0.05, Student’s t-tests. (b) TGF-β-stained IFE of Col17a1−/− and littermate controls at P1 (representative images from three mice). (c) p-Smad2 staining of IFE from Col17a1−/− and littermate controls at P1 (representative images from three mice). Scale bar: 20 μm.
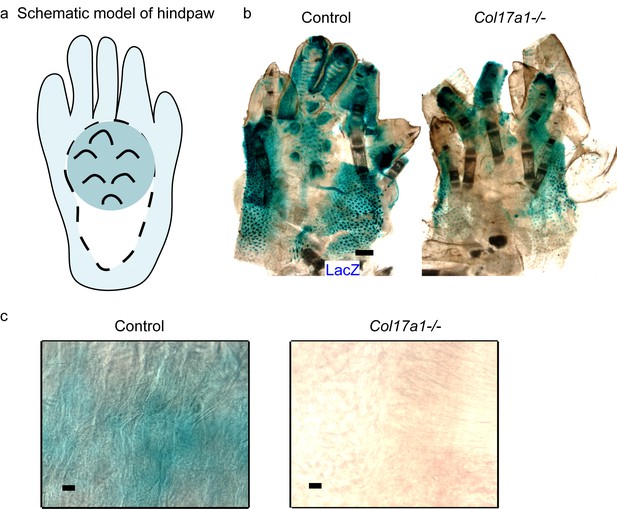
The details of LacZ staining in ins-Topgal+ mice with COL17 deficiency.
(a) Schematic model of murine hindpaw on LacZ staining. The dark blue area indicates sweat glands-abundant regions and the light blue area represents to regions with hair follicles. Sweat glands and hair follicles are major sources of Wnt activities as marked with LacZ. The white area, without sweat glands or hair follicles, shows the regions used for the quantification. (b) Gross appearance of LacZ staining on ins-Topgal+ (Control: left) and ins-Topgal+:Col17a1−/− (Col17a1−/−: right) mice hind paw. Scale bar: 500 μm. (c) LacZ staining on ins-Topgal+ (Control: left) and ins-Topgal+:Col17a1−/− (Col17a1−/−: right) mice in high magnification for quantification. Scale bar: 100 μm.
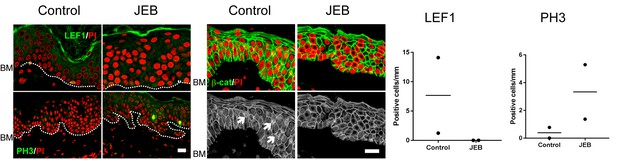
Wnt signaling and proliferation profile in JEB patients epidermis with COL17 deficiency.
LEF1, β-catenin and PH3 labeling of skin specimens from two JEB patients and controls. LEF1-positive cells and nuclear β-catenin accumulation are indicated with arrows. Scale bar: 20 μm. Quantification of LEF1- and PH3-positive basal cells per mm epidermis.
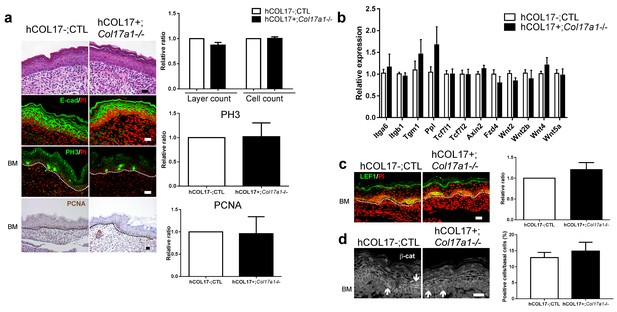
Induction of human COL17 abrogates epidermal hyperproliferation and the expression of Wnt-β-catenin signaling molecules in neonatal Col17a1−/− IFE.
(a) H&E, E-cad, PH3 and PCNA staining of IFE skin specimens from Col17a1+/+ or Col17a1+/- (as hCOL17-; CTL (control)) and hCOL17+; Col17a1−/− littermate mice at P1. Quantification of epidermal layers, epidermal cell counts, and PH3- and PCNA-positive cells (n = 4). Scale bar: 20 μm. (b) Gene expression of Wnt-related molecules in IFE skin samples from hCOL17-; CTL and hCOL17+; Col17a1−/− littermate mice at P1 (n = 4). (c) LEF1 staining of IFE skin samples from hCOL17-; CTL and hCOL17+; Col17a1−/− littermates at P1 (n = 4). Scale bar: 20 μm. (d) β-catenin staining of IFE skin samples from hCOL17-; CTL and hCOL17+; Col17a1−/− littermates at P1 (n = 4). Nuclear β-catenin is indicated with arrows. The number of nuclear β-catenin-positive cells. Scale bar: 20 μm. The data are the means ± SE. Student’s t-tests.
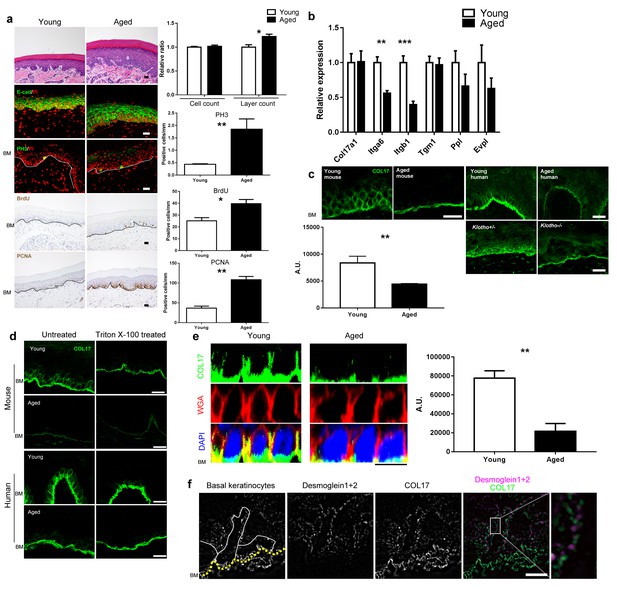
Physical aging affects epidermal proliferation and COL17 distribution.
(a) H&E, E-cad, PH3, BrdU and PCNA staining of IFE skin from young (6–10 weeks old) and aged (19–27 months old) adult C57BL/6 wild-type (WT) mice. Scale bar: 20 μm. The numbers of epidermal layers, epidermal cell counts, and PH3-, BrdU- and PCNA-positive basal cells (n = 5). Student’s t-test. (b) The gene expression levels of Itga6, Itgb1, Tgm1, Ppl, Evpl, and Col17a1 in IFE skin samples from young and aged WT mice (n = 5 for Itga6, Itgb1, Tgm1 and Col17a1; n = 3 for Ppl and Evpl). Student’s t-test. (c) COL17 staining (antibodies to the juxtamembranous portion) in IFE skin samples from the following groups: young and aged WT mice (n = 5), young (<15 years old) and aged (>85 years old) normal human individuals (representative images from three human samples), and Klotho+/- and Klotho−/− littermates at 6 weeks (representative images from three mice). Scale bar: 20 μm. The quantitative fluorescent intensity of lateral membrane of IFE basal cells from young and aged WT mice (n = 5). Mann-Whitney test. (d) COL17 labeling following the Triton X-100 treatment of IFE skin from young/aged WT mice and human individuals (representative images from three samples). Scale bar: 20 μm. (e) The optical sectioning of 3D reconstructed whole mount COL17-stained skin from young and aged murine WT IFE. The IFE cell membrane was visualized with wheat germ agglutinin (WGA). DAPI (4',6-diamidino-2-phenylindole) was used for nuclear staining. Scale bar: 10 μm. The quantitative fluorescent intensity of COL17 in lateral membrane of IFE basal cells from young and aged WT mice (n = 6). Mann-Whitney test. (f) The distributions of COL17 and desmogleins 1 and 2 in young murine WT IFE using N-SIM (structured illumination microscopy) image reconstruction (representative images from two mice). Basal keratinocytes were depicted by white lines. Scale bar: 5 μm. BM, basement membrane. The data are the means ± SE. *0.01<p<0.05, **0.001<p<0.01, ***0.0001<p<0.001.
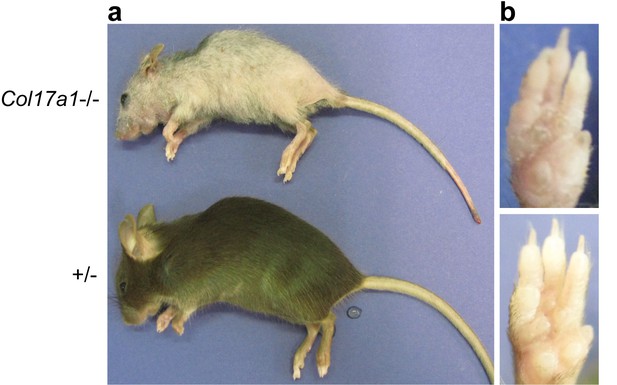
Gross appearance of Col17a1−/− and littermate controls (3 months old).
(a) Gray and sparse hair was noted in Col17a1−/− skin. (b) Scaly paw skin was observed in Col17a1−/− skin (representative images from four mice).
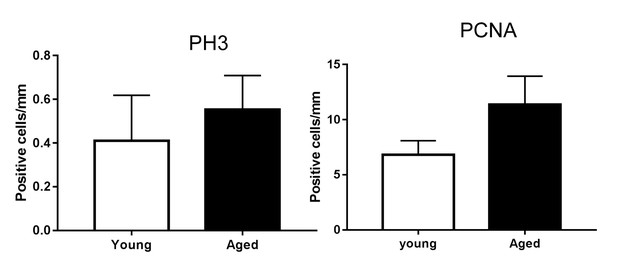
Proliferation markers in back skin from young and aged mice.
Quantification of PH3- and PCNA- positive cells in young and aged paw skin (n = 5). Student’s t-test.

Intracellular COL17 labeling of the IFE from young/aged WT mice and human individuals.
Antibodies against the intracellular portions of COL17, which recognize the full-length protein, were used. The antibodies do not react with the shed ectodomain of COL17. Scale bar: 20 μm. Samples from Klotho+/− and Klotho−/− littermates were obtained at the age of 6 weeks. Representative images from three samples.
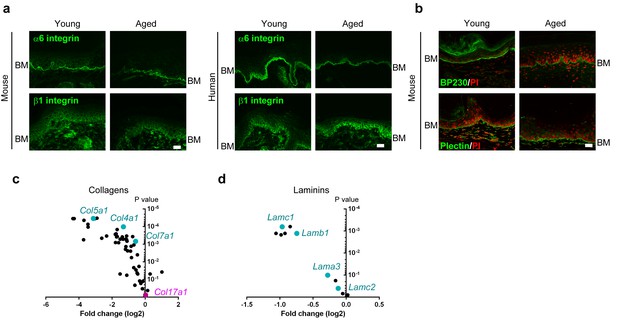
Expression of BMZ and extracellular matrix proteins in the IFE skin of young and aged WT mice and healthy individuals.
(a) Staining of integrin α6 and β1 in young/aged mouse and human IFE samples (representative images from five mice). Scale bar: 20 μm. (b) Labeling of BP230 and plectin in the mice IFE (representative images from five mice). Scale bar: 20 μm. (c, d) Expression data from the microarray (four mice from young and aged groups, respectively) of genes encoding collagens (c) and laminins (d). The fold changes are based on the young group.

Analysis of epidermal proteases in IFE skin samples from young and aged WT mice.
(a) Expression levels from the microarray analysis of genes encoding proteases involved in COL17 digestion and degradation (n = 4). (b) COL17 staining in Serpine1−/− and littermate control mice IFE at 8 weeks (representative images from four mice). The antibody against the juxtamembranous NC16A domain of COL17 was used. (c) ELANE labeling of young and aged IFE (representative images from five mice). Scale bar: 20 μm.
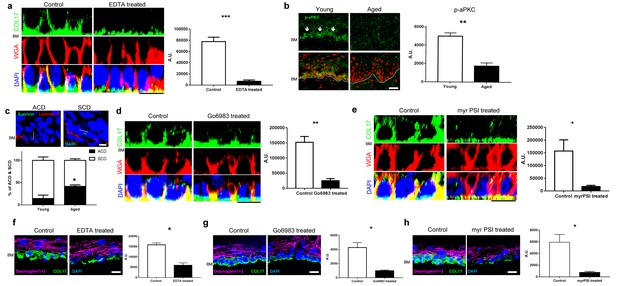
COL17 distribution is modulated by aPKC.
(a) COL17 staining of whole IFE skin treated with 5 mM EDTA. The quantitative fluorescent intensity of COL17 in basal cells of IFE from control (PBS) and 5 mM EDTA treated (n = 4). Mann-Whitney test. Samples were taken from young adult WT mice at 6–10 weeks. Scale bar: 20 μm. (b) Phospho-aPKC labeling (indicated with arrows) and quantitative fluorescent intensity results from young and aged WT IFE skin (n = 4). Mann-Whitney test. Scale bar: 20 μm.(c) Representative figures of asymmetric cell division (ACD; scored as perpendicular to basement membrane) and symmetric cell division (SCD; in parallel to basement membrane) in young IFE. Survivin staining indicates the direction of the cell division. Laminin β1 signifies basement membrane. Scale bar: 10 μm. Graph of percentage of ACD and SCD in young and aged IFE (n = 4). Student’s t-test. (d–e) The pharmacological inhibition of pan-aPKC (d, 1 μM of Go6983; 0.00002% DMSO as control) and aPKCλ/ζ (e, 10 μM of myr PSI; water as control) in whole IFE skin from young adult WT mice, followed by COL17 staining. The quantitative fluorescent intensity of COL17 in lateral membrane of basal cells from control and 1 μM Go6983 treated (d) and 1 μM myr PSI (e) (n = 4). Mann-Whitney test. Scale bar: 10 μm. BM, basement membrane. (f–h) EDTA treatment (5 mM; PBS as control, f) and pharmacological inhibition of pan-aPKC (g, 1 μM of Go6983; 0.00002% DMSO as control) and aPKCλ/ζ (h, 10 μM of myr PSI; water as control) on 3D epidermis. The relative fluorescent intensity of COL17 in lateral membrane of basal cells was measured (n = 4). Mann-Whitney test. BM, basement membrane. Scale bar: 20 μm. The data are the means ± SE. *0.01<p<0.05, **0.001<p<0.01, ***0.0001<p<0.001.
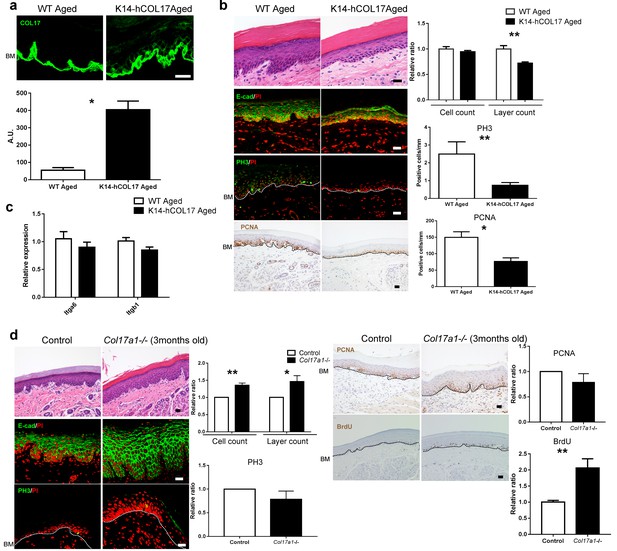
Overexpression of human COL17 ablates hyperproliferation in aged IFE.
(a) COL17 labeling and its quantitative fluorescent intensity in IFE skin from WT and K14-hCOL17 aged mice (>19 months old) (n = 5). The antibody used in this assay detects both human and murine COL17. Scale bar: 20 μm. Mann-Whitney test. (b) H&E-, E-cad-, PH3- and PCNA-stained skin samples from WT and K14-hCOL17 aged IFE. The numbers of epidermal layers, total epidermal cell counts, and PH3- and PCNA-positive basal cell counts (n = 5 for H&E, E-cad, and PCNA staining; n = 4 for PH3 in aged K14-hCOL17 mice; n = 3 for PH3 of aged WT mice). Student’s t-test. Scale bar: 20 μm. (c) The gene expression levels of Itga6 and Itgb1 in IFE skin samples from WT and K14-hCOL17 aged IFE (n = 5). Student’s t-test. (d) H&E-, E-cad-, PH3- and PCNA-staining; quantifications of epidermal layers; total epidermal cells; and PH3-, PCNA-, and BrdU-positive basal cells in the IFE of Col17a1−/− mice and littermate controls (3 months old) (n = 4). Student’s t-test. Scale bar: 20 μm. The data are the means ± SE. *0.01<p<0.05, **0.001<p<0.01.
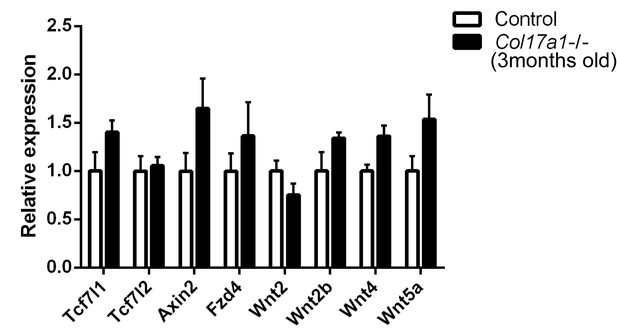
qRT-PCR assessment of Wnt-related molecules in IFE skin samples from Col17a1−/− and littermates at the age of 12 weeks.
The data are the means±SE of three mice per each group. Student’s t-tests.
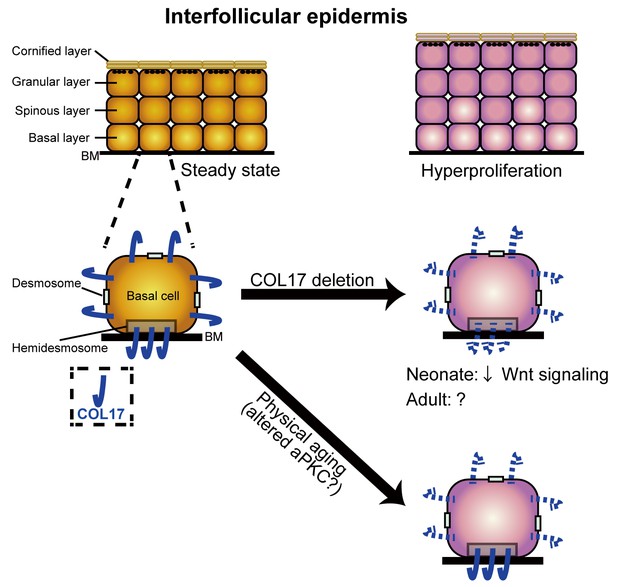
A model of the role of COL17 in maintaining IFE homeostasis.
A graphical abstract of this study. COL17 regulates paw IFE homeostasis in coordination with Wnt signaling at the neonatal stage. Physical aging diminishes non-hemidesmosomal COL17 labeling in IFE keratinocytes, leading to IFE hyperproliferation, associated with altered aPKC activities.
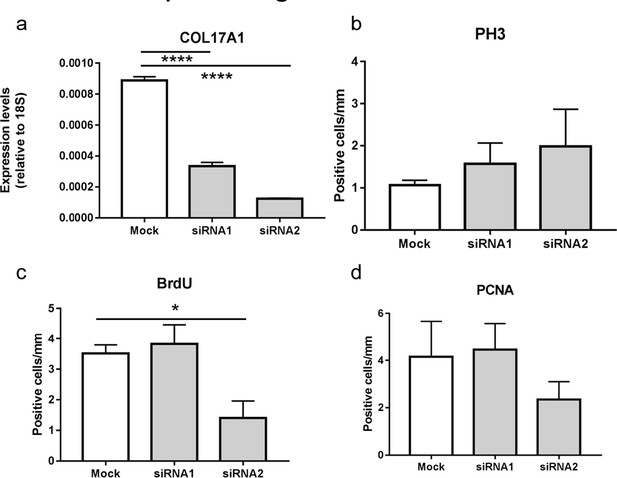
Proliferative ability of 3D reconstituted human epidermis treated with COL17A1 siRNAs
https://doi.org/10.7554/eLife.26635.022Tables
Primers used in qRT-PCR.
| Gene name | Forward (5′→3′) | Reverse (5′→3′) |
|---|---|---|
| mTcf7l1 | TGGTCAACGAATCGGAGAAT | TCACTTCGGCGAAATAGTCG |
| mTcf7l2 | CTCCACAGCTCAAAGCATCA | CACCACCTTCGCTCTCATCT |
| mLef1 | CGCTAAAGGAGAGTGCAGCTA | GCTGTCTCTCTTTCCGTGCT |
| mTcf7 | GCCAGAAGCAAGGAGTTCAC | ACTGGGCCAGCTCACAGTAT |
| mCtnnb1 | AAGGCTTTTCCCAGTCCTTC | CCCTCATCTAGCGTCTCAGG |
| mFzd7 | GACCAAGCCATTCCTCCGTG | CAGGTAGGGAGCAGTAGGGTA |
| mFzd4 | AACCTCGGCTACAACGTGAC | GGCACATAAACCGAACAAAGGAA |
| mNfatc4 | CAAGCTGCGAGGATGAGGAG | ACAGCATGGAGGGGTATCCT |
| mWnt4 | GTCAGGATGCTCGGACAACAT | CACGTCTTTACCTCGCAGGA |
| mWnt5a | GGACCACATGCAGTACATTGG | CGTCTCTCGGCTGCCTATTT |
| mFzd3 | TGATGAGCCATATCCCCGACT | GCCTATGAAATAGCGAGCAAATG |
| mFzd8 | CCGCTGGTGGAGATACAGTG | CGGTTGTAGTCCATGCACAG |
| mItga6 | ATGCCACCTATCACAAGGCT | GCATGGTATCGGGGAATGCT |
| mItgb1 | ATCATGCAGGTTGCGGTTTG | TGGAAAACACCAGCAGTCGT |
| mTgm1 | TTTGATGGGTGGCAGGTTGT | GCCATTCTTGACGGACTCCA |
| mCol17 | GATGGCACTGAAGTCACCGA | TATCCATTGCTGGTGCTCCC |
| mPpl | GCATGCTGAGTGGAAGGAGT | AAGTCTGAGTCCACCTTGCG |
| mEvpl | TCCTACAAGCTGCAAGCACA | TCTAAGGAGCAGCGGTAGGT |
| mIvl | CTCCTGTGAGTTTGTTTGGTCT | CACACAGTCTTGAGAGGTCCC |
| mCyc1 | ATCGTTCGAGCTAGGCATGG | GCCGGGAAAGTAAGGGTTGA |
| mGusb | CAGGGTTTCGAGCAGCAATG | ACCCAGCCAATAAAGTCCCG |
| mGapdh | TCCTGCACCACCAACTGCTTAGC | TGGATGCAGGGATGATGTTCTGG |
| hCOL17 | TCAACCAGAGGACGGAGTCA | TCGACTCCCCTTGAGCAAAC |
| h18S | GGCGCCCCCTCGATGCTCTTAG | GCTCGGGCCTGCTTTGAACACTCT |





