Unfolded protein response transducer IRE1-mediated signaling independent of XBP1 mRNA splicing is not required for growth and development of medaka fish
Figures
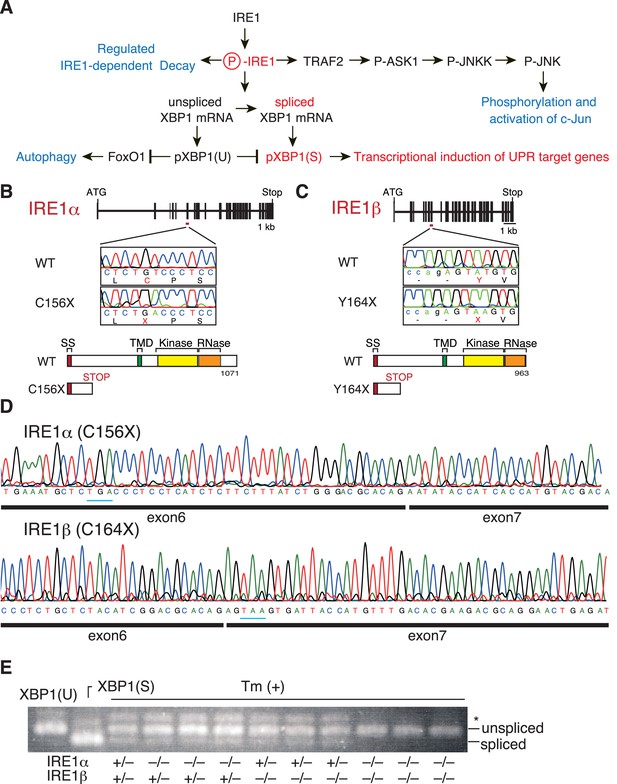
Construction and initial characterization of IRE1α-KO, IRE1β-KO and IRE1α/β-DKO medaka.
(A) Schematic representation of the IRE1-mediated signaling pathways. (B) Schematic representation of the WT and C156X-mutant IRE1α genes and proteins. SS, TMD, Kinase and RNase denote the signal sequence, transmembrane domain, protein kinase domain and ribonuclease domain, respectively, which also apply to (C). (C) Schematic representation of the WT and Y164X-mutant IRE1β genes and proteins. (D) RT-PCR products corresponding to a part of IRE1α mRNA expressed in embryo at 5 dpf of IRE1α-KO medaka (top) and a part of IRE1β mRNA expressed in embryo at 5 dpf of IRE1β-KO medaka (bottom) were sequenced. Expected stop codons are underlined with blue. Note that they do not have an intron. (E) Total RNA prepared from embryos at 4 dpf of the indicated genotypes which had been treated (+) with 4 μg/ml tunicamycin (Tm) for 4 hr was subjected to RT-PCR. XBP1(U) and XBP1(S) indicate RT-PCR product corresponding to unspliced and spliced XBP1 mRNA, respectively. The asterisk denotes a heteroduplex of unspliced and spliced XBP1 mRNA.
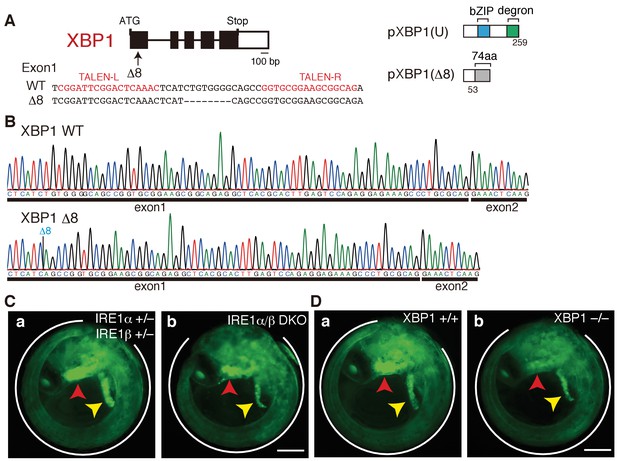
Construction of XBP1-KO medaka and fluorescence microscopic analysis of IRE1α/β-DKO and XBP1-KO medaka.
(A) Schematic representation of the WT and Δ8-mutant XBP1 genes as well as their translational products pXBP1(U) and pXBP1(Δ8). bZIP denotes a basic leucine zipper, whereas the degron denotes a region which targets pXBP1(S) to proteasomal degradation. The grey box of 74 aa in pXBP1(Δ8) indicates foreign aa added to the C-terminus of the truncated XBP1 protein of 53 aa due to the frame shift at aa 54. (B) RT-PCR products corresponding to a part of XBP1 mRNA expressed in embryo at 5 dpf of WT (top) and XBP1-KO (bottom) medaka were sequenced. The position of expected deletion is shown in blue. Note that they do not have an intron. (C) Fluorescence microscopic analysis of IRE1α+/− IRE1β+/− and IRE1α/β-DKO embryos at 5 dpf which were obtained by crossing male IRE1α+/− IRE1β+/− medaka with female IRE1α+/− IRE1β-/- medaka, both carrying PBiP-EGFP. The white outline, red arrowhead and yellow arrowhead point to the tail, hatching gland and liver, respectively; this also applies to (D), 4B, 8A, 8B, 8C, 9B, and 12B. Scale bar: 250 μm. (D) Fluorescence microscopic analysis of XBP1+/+ and XBP1−/− embryos at 5 dpf obtained by incrossing male and female XBP1+/-medaka, both carrying PBiP-EGFP. Scale bar: 250 μm.
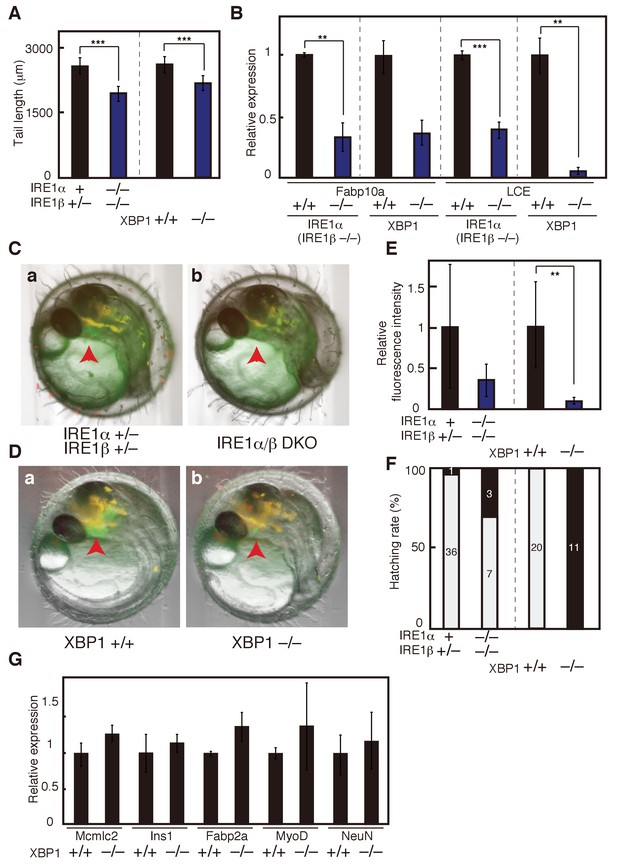
Effect of IRE1α/β-DKO and XBP1-KO on medaka development.
(A) Tail lengths of the indicated genotypes shown in Figure 2C and D were measured (n > 3) and are expressed as means with SD (error bars). IRE1α + implies IRE1α+/+ or IRE1α+/-. *p<0.05, **p<0.01, ***p<0.001 for all figures. (B) Total RNA prepared from embryos at 5 dpf of the indicated genotypes shown in Figure 2C and D were subjected to quantitative RT-PCR to determine the levels of Fabp10a mRNA and LCE mRNA, which were normalized with the level of β-actin mRNA (n = 3). The mean value of normal fish is set as 1, and error bars indicate SD. (C) Fluorescence microscopic analysis of IRE1α+/− IRE1β+/− and IRE1α/β-DKO embryos at 3 dpf obtained by crossing male IRE1α+/− IRE1β+/− medaka with female IRE1α+/− IRE1β−/− medaka, both carrying PLCE-EGFP. The red arrowhead indicates the hatching gland; this also applies to (D). (D) Fluorescence microscopic analysis of XBP1+/+ and XBP1−/− embryos at 3 dpf obtained by incrossing male and female XBP1+/- medaka, both carrying PLCE-EGFP. (E) Fluorescence intensities in hatching glands shown in (C) and (D) were quantified. The mean value of normal fish is set as 1, and error bars indicate SD. (F) Hatching rates of embryos of the indicated genotypes were determined. Grey and black boxes indicate hatched and dead embryos, respectively, and the number in each box indicates actual data. (G) Levels of mRNA encoding Mcmlc2, Ins1, Fabp2a, MyoD and NeuN in embryos of the indicated genotypes were determined at 5 dpf and normalized with the level of β-actin mRNA. They are expressed as described in (B) (n = 3).
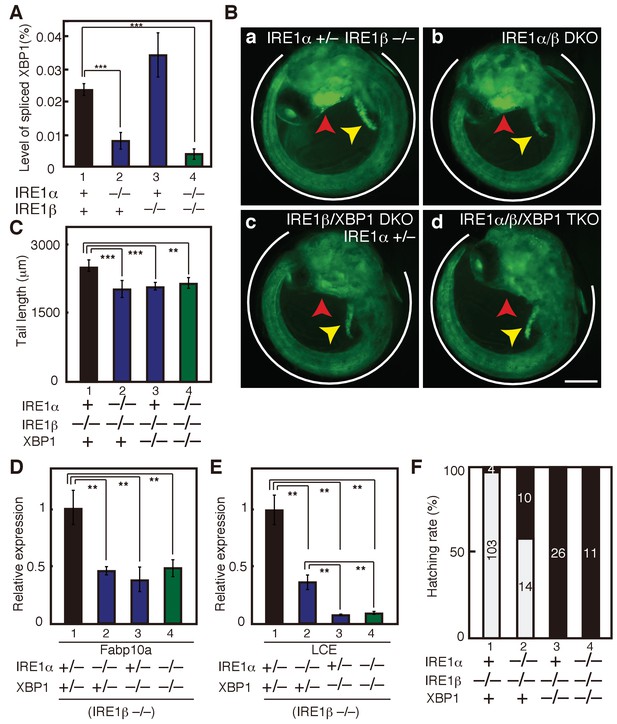
Effect on deleting IRE1α/β on phenotypes of XBP1-KO medaka.
(A) Total RNA prepared from embryos of the indicated genotypes at 3 dpf were subjected to quantitative RT-PCR to determine absolute expression levels of spliced XBP1 mRNA and β-actin mRNA (n = 3). The level of spliced XBP1 mRNA relative to that of β-actin mRNA in the indicated genotypes is expressed as mean (%) with SD (error bars). (B) Fluorescence microscopic analysis of embryos at 5 dpf of the indicated genotypes which were obtained by incrossing male and female IRE1α+/− IRE1β−/− XBP1+/- medaka, both carrying PBiP-EGFP. Scale bar: 250 μm. (C) Tail lengths of the indicated genotypes were measured and are expressed as in Figure 3A (n > 3). (D) Level of Fabp10a mRNA in embryos of the indicated genotypes at 5 dpf was determined, normalized with the level of β-actin mRNA and expressed as described in Figure 3B (n = 3). (E) Level of LCE mRNA in embryos of the indicated genotypes at 5 dpf was determined, normalized with the level of β-actin mRNA and expressed as described in Figure 3B (n = 3). (F) Hatching rates of embryos of the indicated genotypes were determined and are expressed as described in Figure 3F.
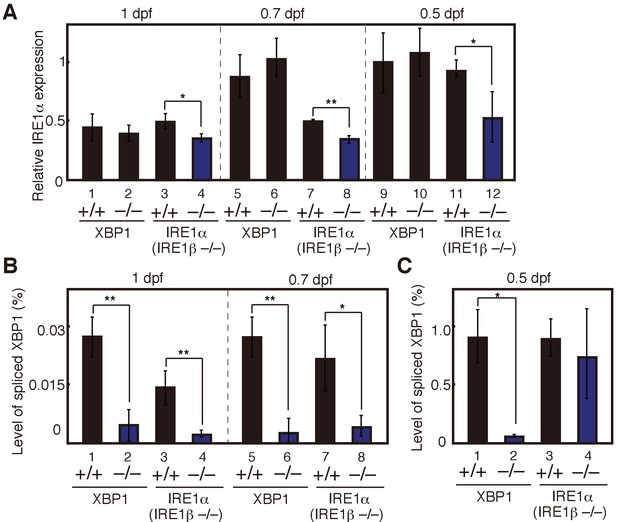
Maternal effect on expression levels of IRE1α mRNA and spliced XBP1 mRNA.
(A) – (C) Embryos were obtained by incrossing male and female XBP1+/− medaka or by incrossing male and female IRE1α+/− IRE1β−/− medaka. (A) Level of IRE1α mRNA in embryos at indicated dpf of the indicated genotypes was determined and normalized with the level of β-actin mRNA as described in Figure 3B (n = 3). The mean value in XBP1+/+ embryos at 0.5 dpf (lane 9) is set as 1, and error bars indicate SD. (B) (C) Level of spliced XBP1 mRNA relative to that of β-actin mRNA at indicated dpf of the indicated genotypes was determined and expressed as described in Figure 4A (n = 3).
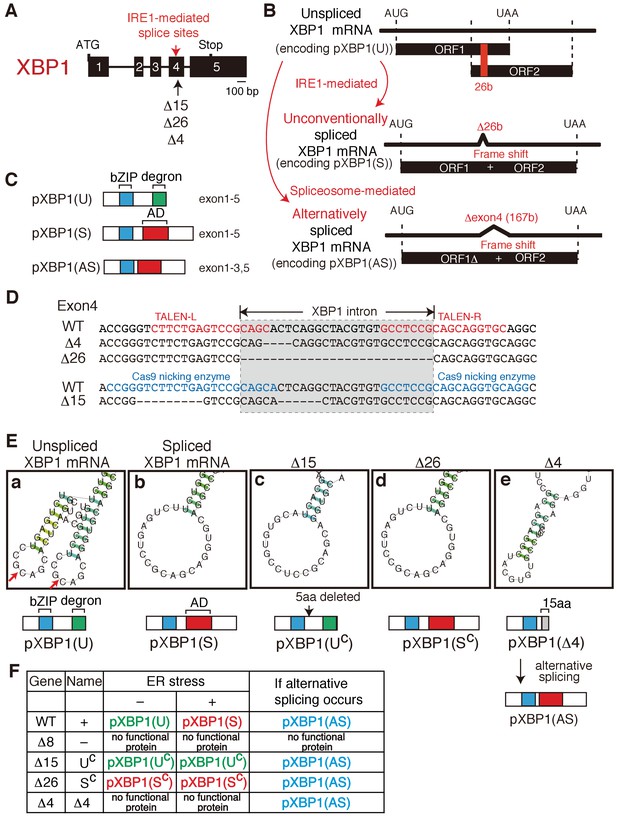
Construction of mutant alleles to express various forms of XBP1.
(A) Schematic representation of the XBP1 gene as well as positions of the IRE1-mediated splice sites and three deletion mutants (Δ15, Δ26, Δ4). (B) Schematic representation of IRE1-mediated, unconventional splicing of XBP1 mRNA which removes the 26 b in exon 4 to produced pXBP1(S) as well as spliceosome-mediated alternative splicing of XBP1 mRNA which removes the entire exon 4 of 167 b to produce pXBP1(AS). pXBP1(U) is produced from unspliced XBP1 mRNA. (C) Schematic representation of pXBP1(U) (unspliced form), pXBP1(S) (spliced form) and pXBP1(AS) (alternatively spliced form). AD denotes transcriptional activation domain. (D) Sequences of a part of exon 4 in which three deletions were introduced by the TALEN method (Δ4 and Δ26) or CRISPR-Cas9 method (Δ15). The position of the XBP1 intron is also shown. (E) CentroidFold-predicted secondary structures of XBP1 mRNA around the IRE1-mediated splice sites (shown by two red arrows in a) produced by three deletion mutants (Δ15, Δ26, and Δ4) in comparison with those of unspliced and spliced XBP1 mRNA. The Δ15 and Δ26 deletion mutants produce constitutively expressed pXBP1(UC) and pXBP1(SC), respectively, which are not modified further after ER stress, as summarized in (F). The Δ4 deletion mutant produces pXBP1(Δ4) containing foreign 15 aa depicted as the grey box after aa165, which is inactive due to the absence of AD, but produces active pXBP1(AS) if spliceosome-mediated alternative splicing occurs, as summarized in (F). (F) Effects of ER stress and alternative splicing on expression of various forms of XBP1.
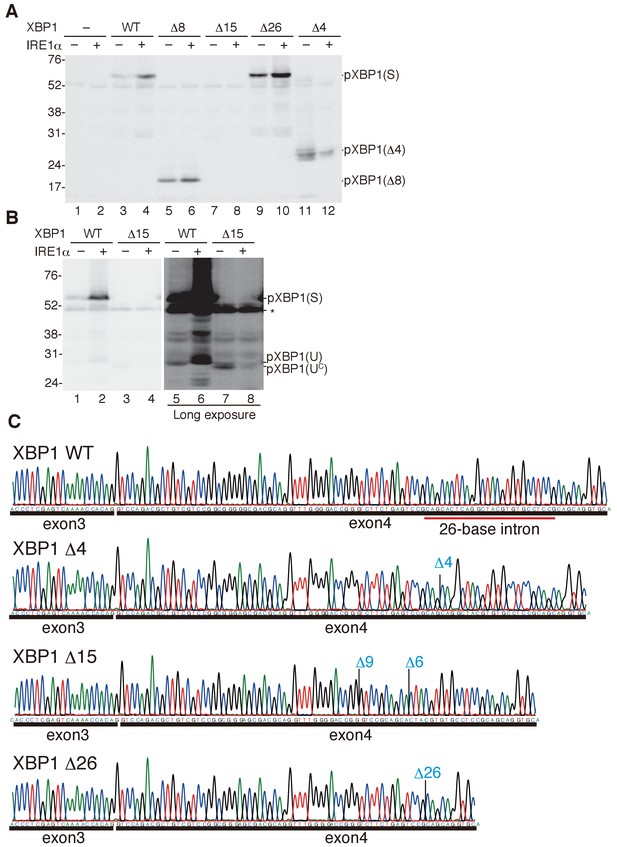
Characterization of various forms of XBP1.
(A) HCT116 cells were transfected with pCMV-myc(-), pCMV-myc-medakaXBP1(WT), pCMV-myc-medakaXBP1(Δ8), pCMV-myc-medakaXBP1(Δ15), pCMV-myc-medakaXBP1(Δ26) or pCMV-myc-medakaXBP1 (Δ4) together with (+) or without (-) pcDNA-medakaIRE1α. MG132 was added for 4 hr prior to harvest. 30 hr after transfection, cell lysates were prepared and analyzed by immunoblotting using anti-myc antibody. (B) HCT116 cells were transfected with pCMV-myc-medakaXBP1(WT), pCMV-myc-medakaXBP1(Δ15) together with (+) or without (-) pcDNA-medakaIRE1α. MG132 was added for 2 hr prior to harvest. 24 hr after transfection, cell lysates were prepared and analyzed by immunoblotting using anti-myc antibody. (C) RT-PCR products corresponding to a part of XBP1 mRNA expressed in respective embryo at 5 dpf of WT, Δ4/Δ4, Δ15/Δ8, and Δ26/Δ26 XBP1 medaka were sequenced. The positions of expected deletions are shown in blue. Note that they do not have an intron.

Effect of expression of various forms of XBP1 on medaka development.
(A) Fluorescence microscopic analysis of XBP1+/+ and XBP1Δ4/Δ4 embryos at 5 dpf which were obtained by incrossing male and female XBP1+/Δ4 medaka, both carrying PBiP-EGFP. Scale bar: 250 μm. (B) Fluorescence microscopic analysis of XBP1+/+ and XBP1UC/- embryos at 5 dpf which were obtained by crossing male XBP1UC/+and female XBP1+/- medaka, both carrying PBiP-EGFP. (C) Fluorescence microscopic analysis of XBP1+/+ and XBP1SC/- embryos at 5 dpf which were obtained by crossing male XBP1SC/+ and female XBP1+/− medaka, both carrying PBiP-EGFP. (D) Level of spliced XBP1 mRNA relative to that of β-actin mRNA in embryos of the indicated genotypes at 5 dpf was determined and is expressed as described in Figure 4A (n = 3). (E) Tail lengths of the indicated genotypes were measured and are expressed as in Figure 3A (n > 3). (F) Levels of Fabp10a mRNA and LCE mRNA in embryos of the indicated genotypes at 5 dpf were determined and normalized with the level of β-actin mRNA, and expressed as described in Figure 3B (n = 3). (G) Hatching rates of embryos of the indicated genotypes were determined and are expressed as described in Figure 3F.
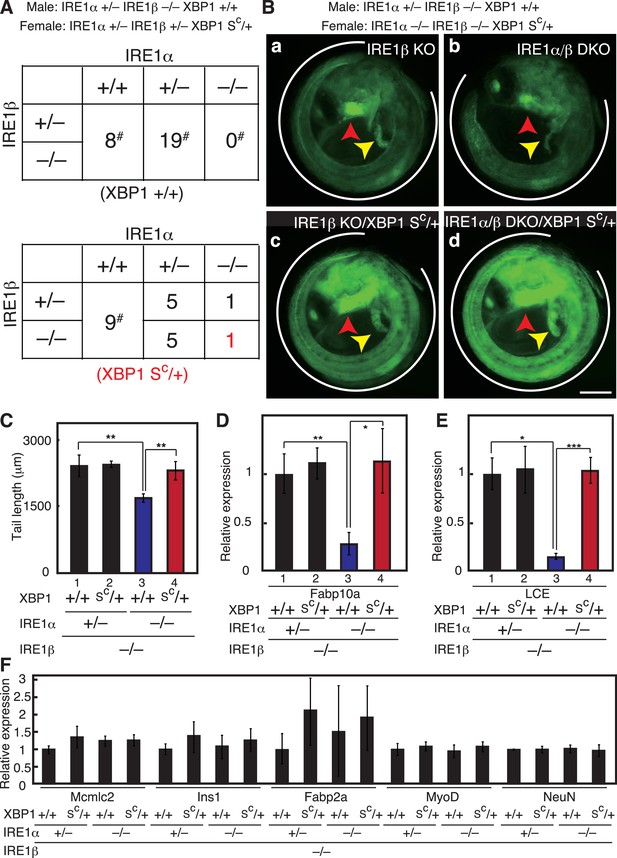
Complete rescue of IRE1α/β-DKO medaka by constitutive expression of pXBP1(S).
(A) Male IRE1α+/− IRE1β−/− XBP1+/+ medaka were crossed with female IRE1α+/− IRE1β+/− XBP1SC/+and the 48 resulting fishes were genotyped 2 months after hatching. The genotype of IRE1β was not determined for fishes marked with #. (B) Fluorescence microscopic analysis of embryos of the indicated genotypes at 5 dpf which were obtained by crossing male IRE1α+/− IRE1β−/− XBP1+/+ medaka with female IRE1α−/− IRE1β−/− XBP1SC/+ medaka, both carrying PBiP-EGFP. Scale bar: 250 μm. (C) Tail lengths of the indicated genotypes were measured and are expressed as in Figure 3A (n > 3). (D) Level of Fabp10a mRNA in embryos of the indicated genotypes at 5 dpf was determined, normalized with that of β-actin mRNA and is expressed as described in Figure 3B (n = 3). (E) Level of LCE mRNA in embryos of the indicated genotypes at 5 dpf was determined, normalized with that of β-actin mRNA and is expressed as described in Figure 3B (n = 3). (F) Levels of mRNA encoding Mcmlc2, Ins1, Fabp2a, MyoD and NeuN in embryos at 5 dpf of the indicated genotypes were determined and normalized with that of β-actin mRNA, and are expressed as described in Figure 3B (n = 3).
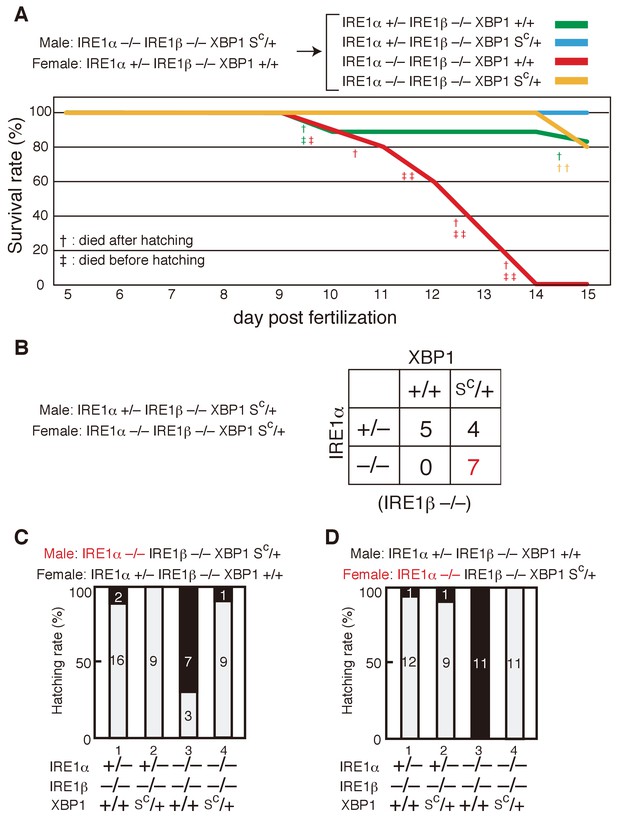
Survival rate and hatching rate of IRE1α/β-DKO medaka with or without the XBP1Sc allele.
(A) Male IRE1α-/- IRE1β-/- XBP1SC/+ medaka were crossed with female IRE1α+/− IRE1β-/- XBP1+/+ medaka, and the resulting 47 embryos were grown. Dead fish and fish surviving until 15 dpf were genotyped. (B) Male IRE1α+/− IRE1β-/- XBP1SC/+ medaka were crossed with female IRE1α-/- IRE1β-/- XBP1SC/+ medaka and the 16 resulting fishes were genotyped 2 months after hatching. (C, D) Hatching rates of embryos of the indicated genotypes resulting from the indicated crossing were determined and are expressed as described in Figure 3F.
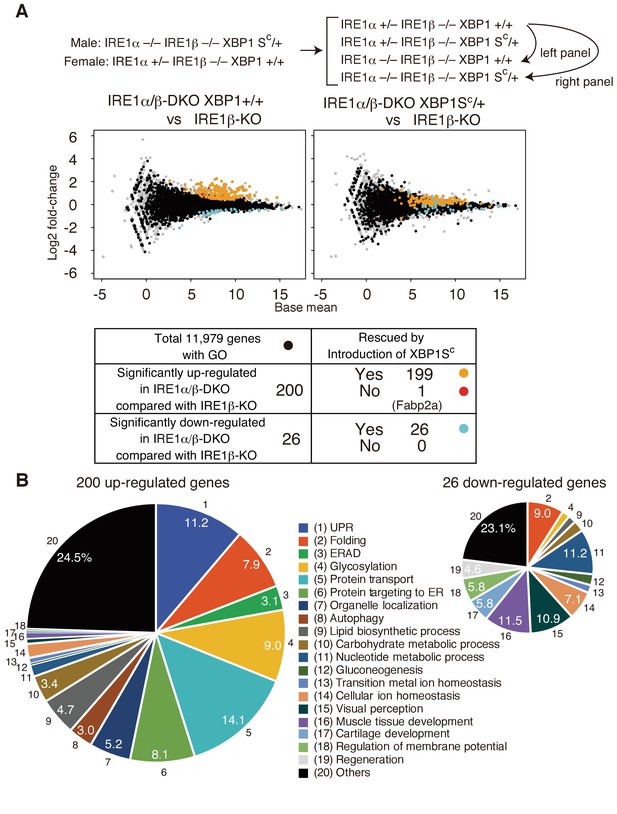
Transcriptomic comparison of IRE1β-KO, IRE1α/β-DKO XBP1+/+ and IRE1α/β-DKO XBP1SC/+ embryos.
(A) RNA prepared from embryos at 5 dpf of the indicated genotypes were subjected to RNA-seq analysis and the data obtained are expressed as MA plot. Genes whose expression was significantly altered in IRE1α/β-DKO compared with IRE1β-KO are colored and summarized in the table below. Grey dots denote genes without GO. n = 4 for IRE1β-KO and n = 3 for IRE1α/β-DKO XBP1+/+ and IRE1α/β-DKO XBP1SC/+. (B) 200 significantly up-regulated and 26 significantly down-regulated genes are categorized based on their assigned GO. GO:0006986, GO:0030968, GO:0034620, GO:0034966, GO:0034976 and GO:0035967 for UPR (1); GO:0006457, GO:0016485 and GO:0051604 for Folding (2); GO:0030433, GO:0030970 and GO:0036503 for ERAD (3); GO:0006486, GO:0006487, GO:0009100, GO:0009101, GO:0018279, GO:0043413 and GO:0070085 for Glycosylation (4); GO:0006888, GO:0006890, GO:0006900, GO:0006901, GO:0006903, GO:0048193, GO:0048199, GO:0048207, GO:0048208 and GO:0090114 for Protein transport (5); GO:0006612, GO:0006613, GO:0006614, GO:0045047, GO:0070972, GO:0072594, GO:0072599, GO:0072657 and GO:0090150 for Protein targeting to ER (6); GO:0051640 and GO:0051656 for Organelle localization (7); GO:0006914 for Autophagy (8); GO:0006644, GO:0008610 and GO:0046486 for Lipid biosynthetic process (9); GO:0005975 for Carbohydrate metabolic process (10); GO:0006164 and GO:0009117 for Nucleotide metabolic process (11); GO:0006094 for Gluconeogenesis (12); GO:0055076 for Transition metal ion homeostasis (13); GO:0006873 for Cellular ion homeostasis (14); GO:0007601 for Visual perception (15); GO:0007517 and GO:0090257 for Muscle tissue development (16); GO:0051216 for Cartilage development (17); GO:0042391 for Regulation of membrane potential (18); GO:0031099 for Regeneration (19).
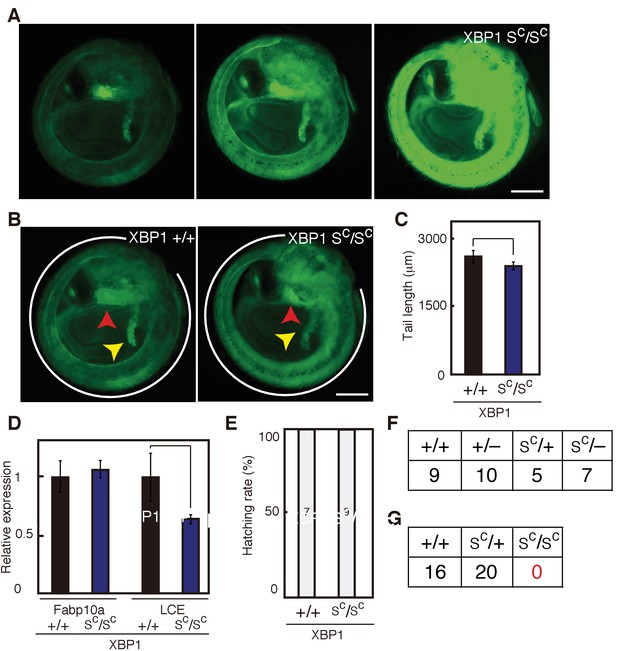
Effect of constitutive expression of pXBP1(S) from both alleles on medaka growth and development.
(A) Fluorescence microscopic analysis of XBP1+/+, XBP1SC/+ and XBP1SC/SC embryos at 5 dpf obtained by incrossing male and female XBP1SC/+ medaka, both carrying PBiP-EGFP. Scale bar: 250 μm. (B) Longer exposure time of A(a) and shorter exposure time of A(c) for better comparison. Scale bar: 250 μm. (C) Tail lengths of the indicated genotypes were measured and are expressed as in Figure 3A (n > 3). (D) Levels of Fabp10a mRNA and LCE mRNA in embryos of the indicated genotypes at 5 dpf were determined and normalized with the level of β-actin mRNA, and are expressed as described in Figure 3B (n = 3). (E) Hatching rates of embryos of the indicated genotypes were determined and are expressed as described in Figure 3F. (F) Male XBP1SC/+ medaka was crossed with female XBP1+/− and the 31 resulting fishes were genotyped 2 months after hatching. (G) Male and female XBP1SC/+ medaka was incrossed and the 36 resulting fishes were genotyped 2 months after hatching.
Additional files
-
Transparent reporting form
- https://doi.org/10.7554/eLife.26845.014





