LTP and memory impairment caused by extracellular Aβ and Tau oligomers is APP-dependent
Figures
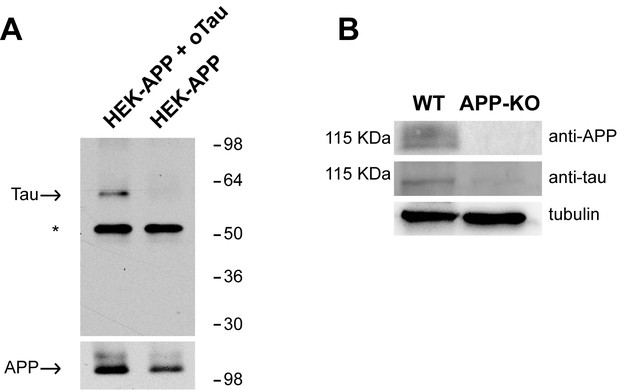
APP binds to oTau.
(A) WB with anti-Tau antibodies Tau5 showing oTau co-IPed with APP in HEK293 cells stably transfected with human APP with the Swedish mutation. * corresponds to the heavy chain of the antibody used for IP. (B) Representative data from fWB experiments performed on hippocampal neurons from WT and APP-KO mice, showing interaction between APP and Tau. Tau binding to APP is demonstrated by the presence of bands recognized by Tau5 antibodies at 110 KDa (the molecular weight of APP). Tubulin was used as loading control. n = 3.
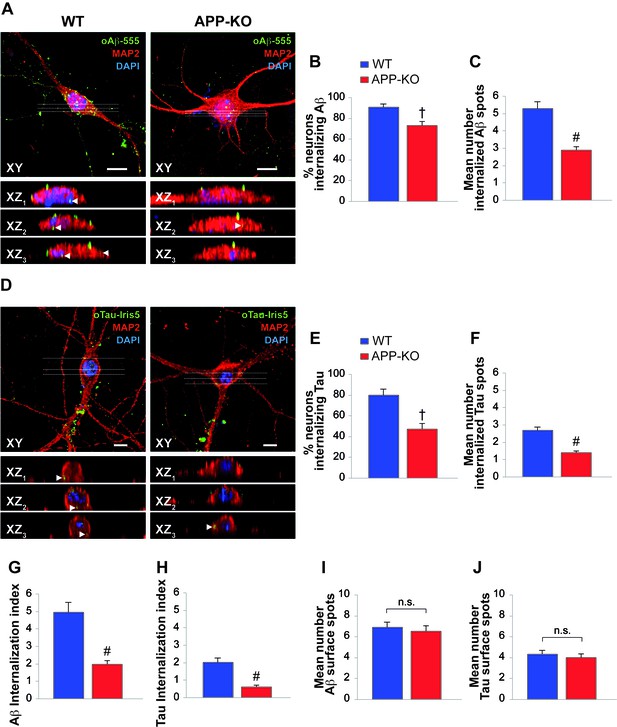
APP suppression reduces internalization of oAβ and oTau into neurons.
(A) Representative images of cultured hippocampal neurons (microtubule associated protein-2 (MAP2) positive cells) obtained from either WT or APP-KO mice and treated with 200 nM human oligomeric Aβ42 labeled with HiLyteTM Fluor 555 (oAβ−555) for 20 min and immunostained for MAP-2. Lower images show different XZ cross-sections from the acquired confocal Z-stack referring to the dotted lines numbered as 1–3 in each panel. Arrowheads indicate internalized proteins. Scale bars: 10 µm. (B–C) After 20 min of extracellular oAβ−555 application, the percentage of WT neurons exhibiting Aβ accumulation was 91 ± 3% of total cells (n = 127) and the mean number of intracellular fluorescent spots/neuron was 5.3 ± 0.4. When the same treatment was applied to APP-KO cultures we found that 73 ± 5% of total cells internalized Aβ (n = 112; t test: t(98) = 2.734; p=0.007 comparing APP-KO vs. WT cells) and a markedly lower mean number of fluorescent spots (2.9 ± 0.2; t(191) = 4.508; p<0.0001 comparing APP-KO vs. WT cells). (D) Representative images of WT and APP-KO cultured hippocampal neurons treated with 100 nM IRIS-5-labeled human recombinant oligomeric Tau (oTau-IRIS5) for 20 min. Lower images show different XZ cross-sections from the acquired confocal Z-stack referring to the dotted lines numbered as 1–3 in each panel. Arrowheads indicate internalized proteins. Scale bars: 10 µm. (E–F) After 20 min of extracellular Tau-IRIS5, the percentage of WT neurons exhibiting Tau was 80 ± 6% of WT cells (n = 88) with 2.7 ± 0.2 fluorescent spots, whereas 47 ± 6% of APP-KO neurons showed Tau internalization (n = 84; t(71) = 3.945; p=0.0002) with a mean number of fluorescent spots equal to 1.4 ± 0.1 (t(92) = 4.331; p<0.0001). (G–H) The ‘internalization index’ shown on the graph was 4.9 ± 0.6 in WT neurons treated with Aβ−555 vs. 1.9 ± 0.2 of APP-KO cells (t(98) = 5.246; p<0.0001), and 2.0 ± 0.3 in WT neurons treated with Tau-IRIS5 vs. 0.6 ± 0.1 of APP-KO cells (t(71) = 5.013; p<0.0001). (I) Fluorescent Aβ spots attached to neuronal surface were 6.9 ± 0.5 and 6.5 ± 0.6 for WT and APP-KO, respectively (t(170) = 0.576; p=0.56). (J) Fluorescent Tau spots attached to neuronal surface were 4.3 ± 0.4 and 4.0 ± 0.4 for WT and APP-KO, respectively (t(93) = 0.363; p=0.72).
-
Figure 2—source data 1
Data relating to Figure 2B–C.
- https://doi.org/10.7554/eLife.26991.004
-
Figure 2—source data 2
Data relating to Figure 2E–F.
- https://doi.org/10.7554/eLife.26991.005
-
Figure 2—source data 3
Data relating to Figure 2G–H.
- https://doi.org/10.7554/eLife.26991.006
-
Figure 2—source data 4
Data relating to Figure 2I–J.
- https://doi.org/10.7554/eLife.26991.007
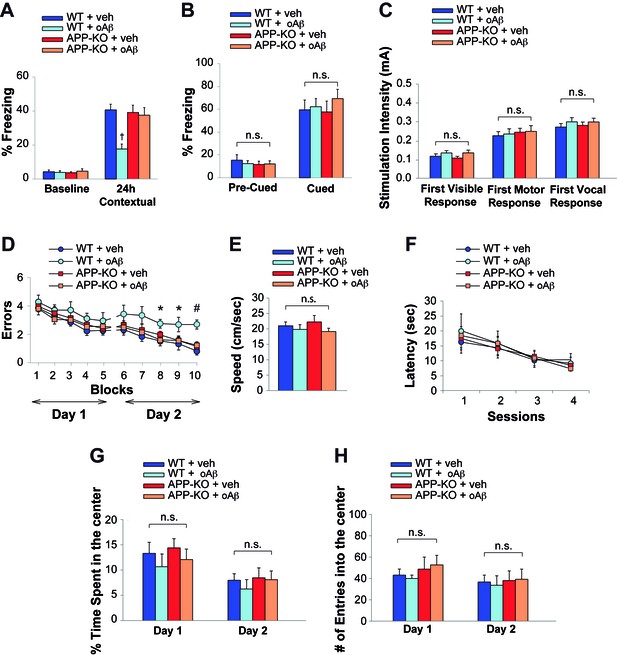
APP is necessary for extracellular oAβ to reduce memory.
(A) oAβ (200 nM) impaired contextual memory in WT mice, whereas it did not impair memory in APP-KO mice. n = 11 per condition in this and the following panels. 24 hr: ANOVA F(3,40) = 8.047, p<0.0001; Bonferroni: WT + vehicle vs. WT + oAβ: † p<0.001. (B) Freezing responses before (Pre) and after (Post) the auditory cue were the same among vehicle- and oAβ-infused APP-KO mice as well as vehicle- and oAβ-infused WT littermates in the cued conditioning test. ANOVA Pre-Cued: F(3,40) = 0.242, p=0.867; Cued: F(3,40) = 0.372, p=0.774. (C) No difference was detected among the four groups during assessment of the sensory threshold. ANOVA for repeated measures F(3,40) = 0.626, p=0.602. (D) oAβ (200 nM) impaired the RAWM performance in WT mice whereas it did not impair the performance in APP-KO mice. ANOVA for repeated measures (day 2) F(3,40) = 5.297, p=0.004. WT + vehicle vs. WT + oAβ: *p<0.05 for block 8 and 9, and # p<0.0001 for block 10. (E–F) Testing with the visible platform task for assessment of visual-motor-motivational deficits did not reveal any difference in average speed [ANOVA: F(3,40) = 0.899, p=0.450] (E), and time to reach the visible platform [ANOVA for repeated measures F(3,40) = 0.05, p=0.985] (F) among the four groups. (G–H) Open field testing showed a similar percentage of time spent in the center compartment [ANOVA for repeated measures F(3,40) = 0.692 p=0.489] (G) and the number of entries into the center compartment [ANOVA for repeated measures F(3,40) = 0.332, p=0.802] (H) in vehicle- and oAβ-infused APP-KO mice as well as vehicle- and oAβ-infused WT littermates, indicating that they had no differences in exploratory behavior.
-
Figure 3—source data 1
Data relating to Figure 3A–B–C.
- https://doi.org/10.7554/eLife.26991.009
-
Figure 3—source data 2
Data relating to Figure 3D–E–F.
- https://doi.org/10.7554/eLife.26991.010
-
Figure 3—source data 3
Data relating to Figure 3G–H.
- https://doi.org/10.7554/eLife.26991.011
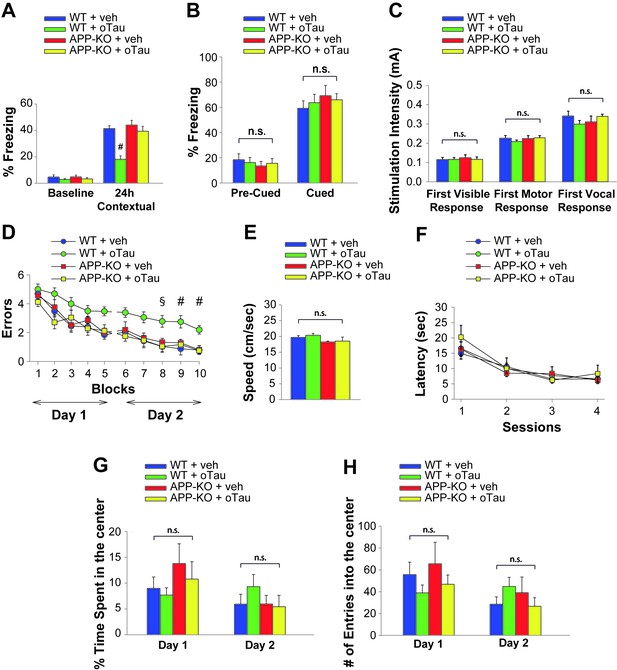
APP is necessary for extracellular oTau to reduce memory.
(A) oTau (500 nM) impaired contextual memory in WT mice, whereas it did not impair contextual memory in APP-KO mice. 24 hr: ANOVA F(3,38) = 18.472, p<0.0001; Bonferroni: WT + vehicle vs. WT + oTau: # p<0.0001. WT + vehicle: n = 11, WT + oTau: n = 12, APP-KO + vehicle: n = 8, APP-KO + oTau: n = 11. (B) Freezing responses before (Pre) and after (Post) the auditory cue were the same among the four groups shown in A in the cued conditioning test. ANOVA Pre-cued: F(3,38) = 0.215, p=0.885; Cued: F(3,38) = 0.410, p=0.747. (C) No difference was detected among the four groups shown in A during assessment of the sensory threshold. ANOVA for repeated measures F(3,38) = 0.643, p=0.592. (D) oTau (500 nM) impaired the RAWM performance in WT mice whereas it did not impair the performance in APP-KO mice. ANOVA for repeated measures (day 2) F(3,34) = 11.309, p<0.0001. WT + vehicle vs. WT + oTau: § p<0.005 for block 8, and # p<0.001 for block 9 and 10. WT + vehicle: n = 11, WT + oTau: n = 12, APP-KO + vehicle: n = 7, APP-KO + oTau: n = 8. (E–F) Testing with the visible platform task for assessment of visual-motor-motivational deficits for animals shown in D did not reveal any difference in average speed [ANOVA: F(3,34) = 1.532, p=0.224] (E) and time to reach the visible platform [ANOVA for repeated measures: F(3,34) = 0.221, p=0.881] (F) among the four groups. (G–H) Open field testing for the same animals as in D showed a similar percentage of time spent in the center compartment [ANOVA for repeated measures F(3,34) = 0.190, p=0.902] (G) and the number of entries into the center compartment [ANOVA for repeated measures F(3,34) = 0.354, p=0.787] (H) in vehicle- and oTau-infused APP-KO mice as well as vehicle- and oTau-infused WT littermates, indicating that they had no differences in exploratory behavior.
-
Figure 4—source data 1
Data relating to Figure 4A–B–C.
- https://doi.org/10.7554/eLife.26991.013
-
Figure 4—source data 2
Data relating to Figure 4D–E–F.
- https://doi.org/10.7554/eLife.26991.014
-
Figure 4—source data 3
Data relating to Figure 4G–H.
- https://doi.org/10.7554/eLife.26991.015
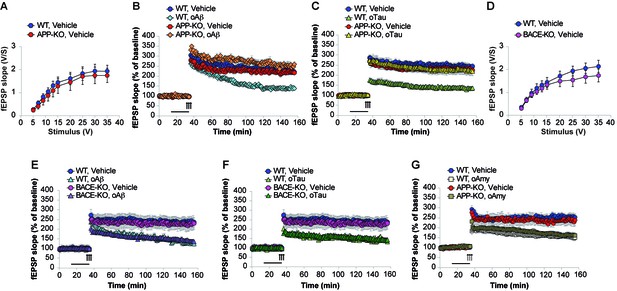
APP is necessary for extracellular oAβ and oTau to reduce LTP.
(A) Basal synaptic transmission (BST) at the CA3-CA1 connection in slices from 3- to 4-month-old APP-KO mice was similar to WT littermates (n = 18 slices from WT vs. 18 slices from APP-KO; ANOVA for repeated measures F(1,34) = 0.416, p=0.524). (B) LTP was impaired in hippocampal slices from WT mice perfused with oAβ (200 nM), whereas there was no impairment in slices from APP-KO littermates. ANOVA for repeated measures F(3,30) = 19.738, p<0.0001. WT + vehicle vs. WT + oAβ: F(1,16) = 29.393, p<0.0001. WT + vehicle vs. APP-KO + oAβ: F(1,13) = 3.297, p=0.093. WT + vehicle: n = 9, WT + oAβ: n = 9, APP-KO + vehicle: n = 10, APP-KO + oAβ: n = 6. (C) LTP was impaired in hippocampal slices from WT mice perfused with oTau (100 nM), whereas there was no impairment in slices from APP-KO littermates. ANOVA for repeated measures F(3,35) = 11.033, p<0.0001. WT + vehicle vs. WT + oTau: F(1,16) = 50.543, p<0.0001. WT + vehicle vs. APP-KO + oTau: F(1,16) = 0.382, p=0.575. WT + vehicle: n = 8, WT + oTau: n = 10, APP-KO + vehicle: n = 11, APP-KO + oTau: n = 10. (D) CA3-CA1 BST in slices from 3- to 4-month-old BACE1-KO mice was similar to WT littermates (n = 24 slices from WT vs. 26 slices from BACE-KO; ANOVA for repeated measures F(1,48) = 0.714, p=0.402). (E) LTP was impaired in hippocampal slices from both WT and BACE-KO mice perfused with oAβ (200 nM). ANOVA for repeated measures F(3,29) = 5.738, p=0.003. WT + vehicle vs. WT + oAβ: F(1,14) = 23.663, p<0.0001. WT + vehicle vs. BACE-KO + oAβ: F(1,14) = 38.295, p<0.0001. WT + vehicle: n = 8, WT + oAβ: n = 8, BACE-KO + vehicle: n = 9, BACE-KO + oAβ: n = 8. F) LTP was impaired in hippocampal slices from both WT and BACE-KO mice perfused with oTau (100 nM). ANOVA for repeated measures F(3,30) = 6.919, p=0.001. WT + vehicle vs. WT + oTau: F(1,14) = 33.230, p<0.0001. WT + vehicle vs. BACE-KO + oTau: F(1,15) = 36.9961, p<0.0001. WT + oTau: n = 8, BACE-KO + oTau: n = 9. G) LTP was impaired in hippocampal slices from both WT and APP-KO mice perfused with oAmy (200 nM). ANOVA for repeated measures F(3,38) = 8.900, p<0.0001. WT + vehicle vs. WT + oAmy: F(1,21) = 34.694, p<0.0001. WT + vehicle vs. APP-KO + oAmy: F(1,19) = 19.277, p<0.0001. WT + vehicle: n = 11, WT + oAmy: n = 12, APP-KO + vehicle: n = 9, APP-KO + oAmy: n = 10.
-
Figure 5—source data 1
Data relating to Figure 5A.
- https://doi.org/10.7554/eLife.26991.017
-
Figure 5—source data 2
Data relating to Figure 5B.
- https://doi.org/10.7554/eLife.26991.018
-
Figure 5—source data 3
Data relating to Figure 5C.
- https://doi.org/10.7554/eLife.26991.019
-
Figure 5—source data 4
Data relating to Figure 5D.
- https://doi.org/10.7554/eLife.26991.020
-
Figure 5—source data 5
Data relating to Figure 5E.
- https://doi.org/10.7554/eLife.26991.021
-
Figure 5—source data 6
Data relating to Figure 5F.
- https://doi.org/10.7554/eLife.26991.022
-
Figure 5—source data 7
Data relating to Figure 5G.
- https://doi.org/10.7554/eLife.26991.023





