Light reintroduction after dark exposure reactivates plasticity in adults via perisynaptic activation of MMP-9
Figures
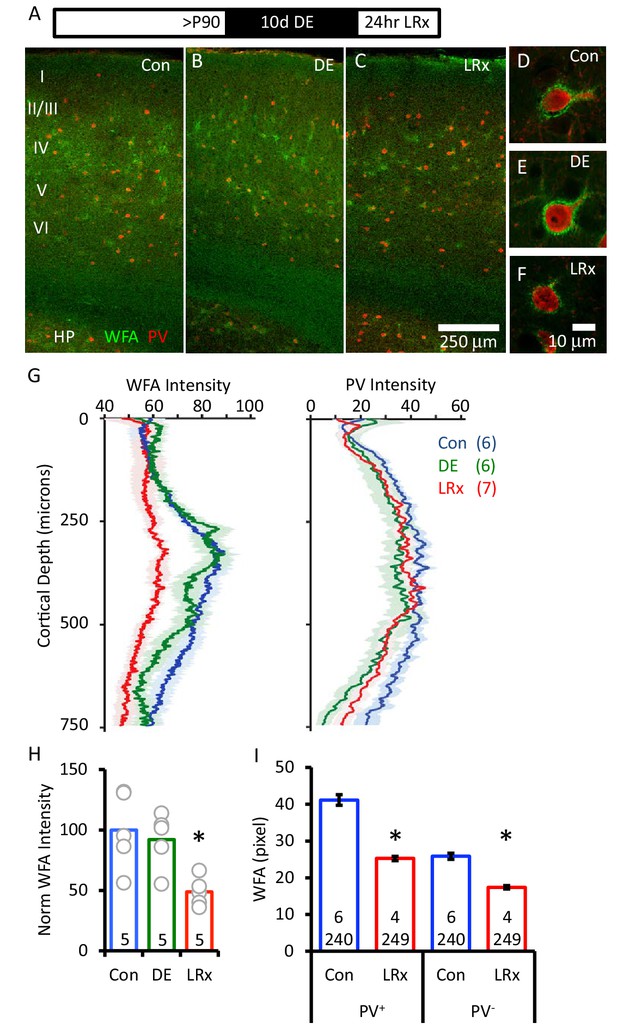
Degradation of ECM in primary visual cortex by light reintroduction (LRx).
Top: Experimental paradigm. C57B/L6J mice raised in 12/12 hr light/dark cycle until postnatal day 90 (P90) received 10 days of dark exposure (DE) with subsequent light reintroduction (1 day; LRx). (A-C) Low magnification (10X) double labeled images of the binocular region of primary visual cortex with FITC-wisteria floribunda agglutinin (WFA; green) and Alexa-546 anti-parvalbumin antibody (PV; red) in control subjects (A), following dark exposure (B) and following dark exposure/light reintroduction (C). Approximate locations of layers I to VI and hippocampus (HP) are shown. (D-F) High magnification images (100X). (G) Quantification of mean fluorescence intensities for WFA (left) and PV (right) in maximal intensity projections (Z-stack 3 × 7.5 μm x of an area 450 μm wide x 750 μm deep; spanning all cortical layers). Mean±SEM; n=6, 6, 7 subjects for Con, DE, LRx, respectively. One way ANOVA, WFA; F=13.57, p=0.0004. PV; F=3.79, p=0.045. (H) Quantification of WFA intensity in region of interest 250 – 400 μm from surface, normalized to average control (n=5 subjects each). One-way ANOVA, F=6.01; p=0.0016. *p<0.05, Tukey-Kramer post hoc. (I) LRx induced a decrease in WFA intensity in PV+ and PV- pixels. (Control 6 subjects, 240 ROIs) versus LRx (4 subjects, 249 ROIs); *p<0.005, Student’s T-test.
-
Figure 1—source data 1
- https://doi.org/10.7554/eLife.27345.003
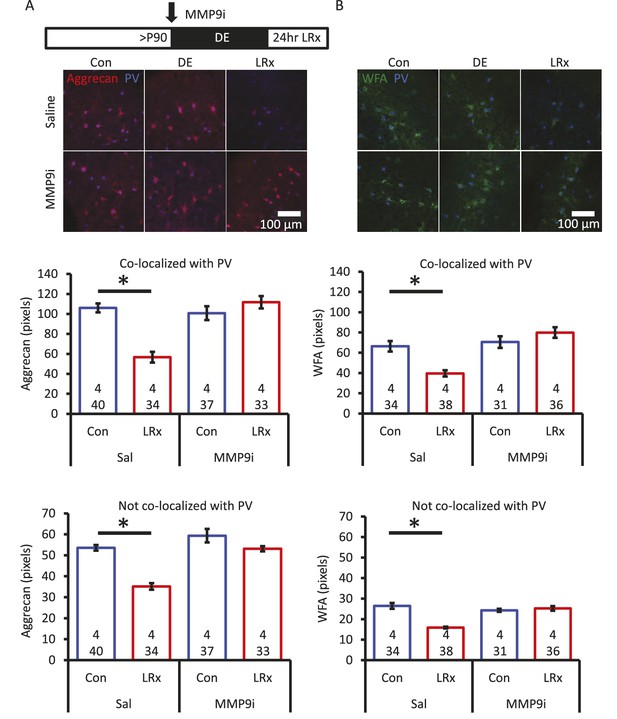
Degradation of ECM by light reintroduction is blocked by MMP-9 inhibitor.
Inset: experimental paradigm. MMP-9 inhibitor (MMP9i; 5 nM) was delivered i.c. via Alzet 1007D micro-osmotic mini pumps 6 days prior to LRx, concurrent with DE. (A) Top: Double label confocal micrographs of anti-aggrecan (red) and anti-parvalbumin immunoreactivity (blue) in each experimental condition. Bottom: Population data. Aggrecan intensity measured in the region 250 – 400 μm from cortical surface. One-way ANOVA, Aggrecan, co-localized with PV: F=18.81, p<0.0001, not co-localized with PV: F=26.32, p<0.0001. *p<0.01, Tukey-Kramer post hoc. (B) Top: Double label confocal micrographs of WFA staining (green) and anti-parvalbumin immunoreactivity (blue) in each experimental condition. Bottom: Population data. WFA intensity measured 250 – 400 μm from cortical surface. One-way ANOVA, WFA, co-localized with PV: F=12.92, p<0.0001, not co-localized with PV: F=22.49, p<0.0001. *p<0.01, Tukey-Kramer post hoc. n=(subjects, cells).
-
Figure 2—source data 1
- https://doi.org/10.7554/eLife.27345.007
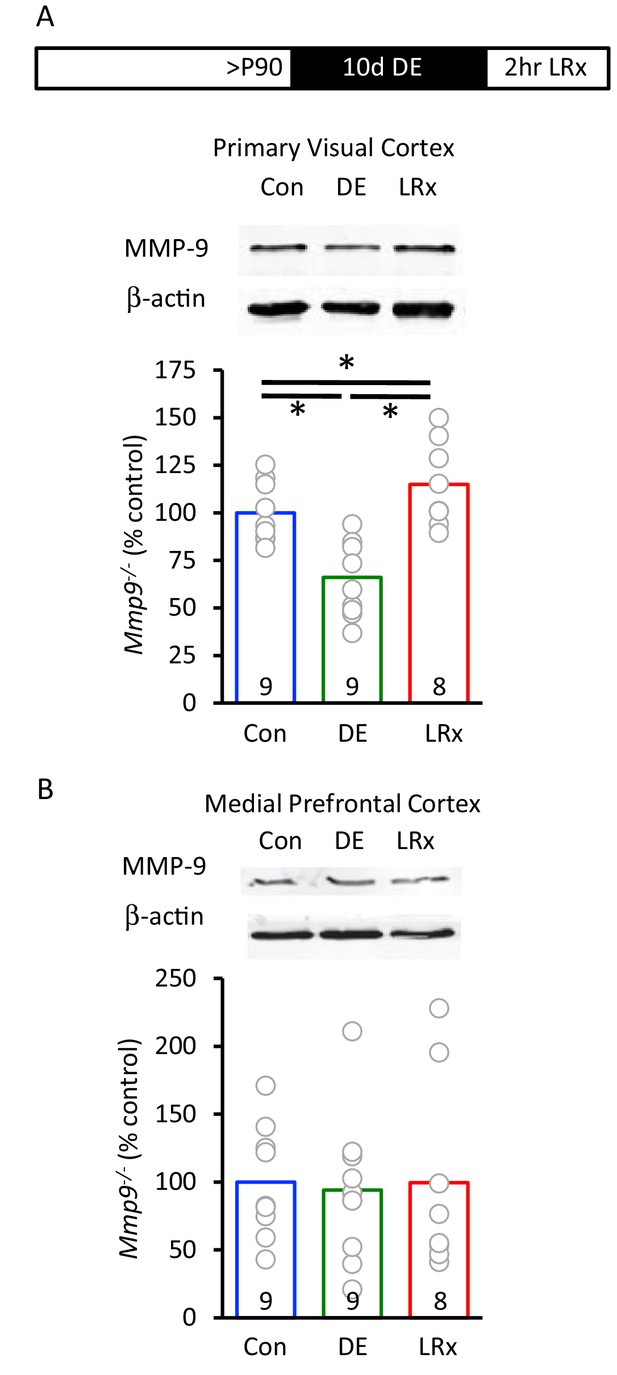
Rapid increase in active MMP-9 in V1 by light reintroduction.
(A) Inset: Experimental paradigm. Dark exposure followed by light reintroduction (2 hr) induced an increase in active MMP-9 (~95 kDa) in V1. MMP-9 was normalized to β-actin as gel loading control (n=9, 9, 8 subjects for Con, DE, LRx, respectively). One-way ANOVA, F=15.99; p<0.001. *p<0.05, Tukey-Kramer post hoc. (B) No change in active MMP-9 in medial prefrontal cortex (n=9, 9, 8 subjects for Con, DE, LRx, respectively). One-way ANOVA, F=0.03; p=0.97.
-
Figure 2—figure supplement 1—source data 1
- https://doi.org/10.7554/eLife.27345.006
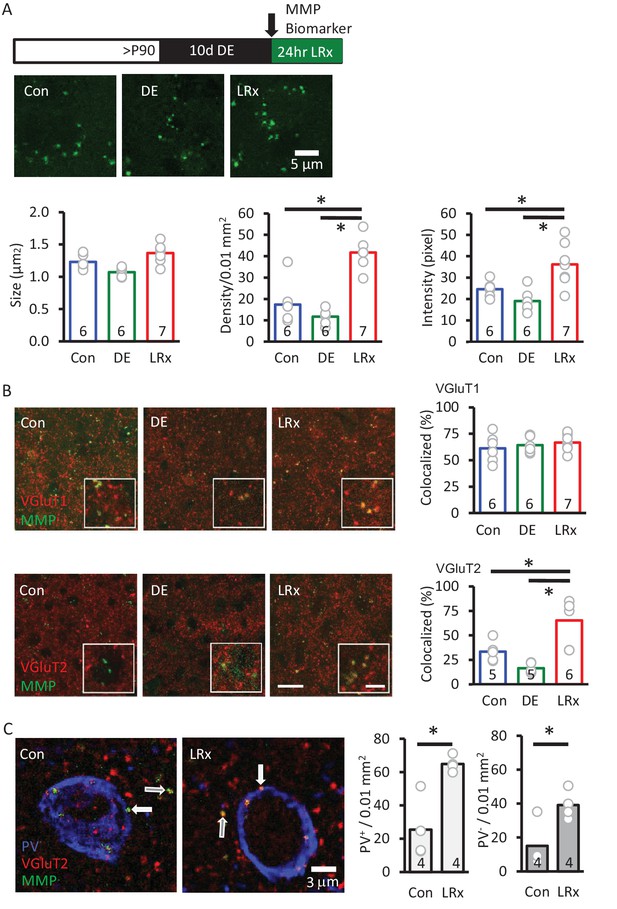
MMP biomarker reports in vivo proteolyis at synapses following LRx.
Inset: experimental paradigm. The MMP biomarker, dye-quenched gelatin (2 mg/ml), was delivered i.c. via cannula at the initiation of LRx. (A) The biomarker reveals punctate staining, and a significant increase in density and intensity following LRx (n=6, 6, 8 subjects for Con, DE, LRx, respectively). One-way ANOVA, F=9.2; p=0.002 for intensity, F=27.74; p<0.0001 for density, F=10.17, p=0.0014 for size; *p<0.05, Tukey-Kramer post hoc. (B) Double labeled confocal micrographs of control, DE and LRx visual cortex labeled with MMP biomarker (green) and marker for cortical axons (top: VGluT1; red) or thalamic axons (bottom: VGluT2; red). Scale bars: 20 μm (insets: 5 μm). Co-localization with VGluT2, but not VGluT1, is significantly increased by LRx. One-way ANOVA, F=0.95, p=0.41 for VGluT1 (n=6, 6, 7 subjects for Con, DE, LRx, respectively). F=16.16, p=0.0003 for VGluT2 (n=5, 5, 6 subjects for Con, DE, LRx, respectively). *p<0.05, Tukey-Kramer post hoc. (C) Increase in co-localization of MMP biomarker/VGluT2 with PV+ and PV- immunoreactive puncta following LRx (n=4 subjects each for Con and LRx). *p<0.005, Student’s T-test. All quantifications were performed 250 – 400 μm from the cortical surface in V1b.
-
Figure 3—source data 1
- https://doi.org/10.7554/eLife.27345.010
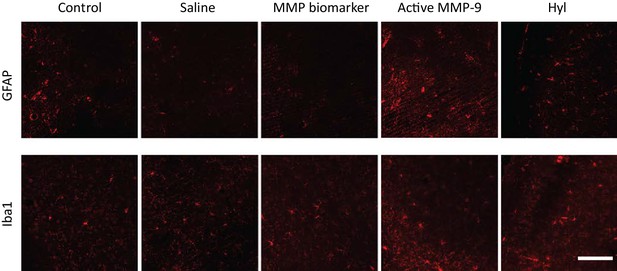
Expression of markers of reactive astrocytes (GFAP) and microglia (Iba1) inV1b following single cortical delivery of saline, MMP-9 biomarker, hyaluronidase and active MMP-9.
4 μl (total volume) was delivered at 100 nl/min with a Hamilton syringe attached to a Microsyringe Pump Controller (World Precision Instruments) through a cannula (2 mm projection, PlasticsOne) implanted 3–4 weeks prior to injection: saline, DQ gelatin (2 mg/ml), active rmMMP-9 (1 μg/ml), Hyl (200 U/ml). Scale bar: 100 μm. Cannula was implanted 500 μm medial and dorsal to V1b (not shown). Analyzed 24 hr after a single injection.

Absence of ECM degradation by light reintroduction in Mmp9−/− mice.
Top Inset: Experimental paradigm. Mmp9−/− mice (P90) raised in 12/12 hr light/dark cycle until postnatal day 90 (P90) received 10 days of dark exposure (DE) with subsequent reintroduction to light (1 day; LRx). (A-C) Low magnification (10X) double labeled images of binocular region of primary visual cortex with FITC-wisteria floribunda agglutinin (WFA; green) and Alexa-546 anti-parvalbumin antibody (PV; red) in normal reared control (A) following dark exposure (B) and following dark exposure/light reintroduction (C). Approximate locations of layer I to VI, and hippocampus (HP) are shown. (D-F) High magnification images (100X). (G) Quantification of mean fluorescence intensities for WFA (left) and PV (right) in maximal intensity projections (Z-stack 3 × 7.5 μm in an area 450 μm wide x 750 μm deep; spanning all cortical layers). Mean±SEM (n=5, 6, 7 subjects for Con, DE, LRx, respectively). One way ANOVA for WFA, F=0.21, p=0.81; for PV F=0.25, p=0.78. (H) Top: experimental paradigm. The MMP biomarker, dye-quenched gelatin (2 mg/ml), was delivered i.c. via cannula at the initiation of LRx. No change in MMP biomarker puncta size (n=4, 6, 6 subjects for Con, DE, LRx, respectively), density (n=5, 6, 7 subjects) or intensity (n=4, 6, 6 subjects) was observed following DE or LRx in Mmp9−/− mice. Dotted lines show values for the wild type controls presented in Figure 3. One-way ANOVA, F=0.02, p=0.98 for size F=1.92; p=0.724 for density; F=0.33; p=0.186 for intensity.
-
Figure 4—source data 1
- https://doi.org/10.7554/eLife.27345.012
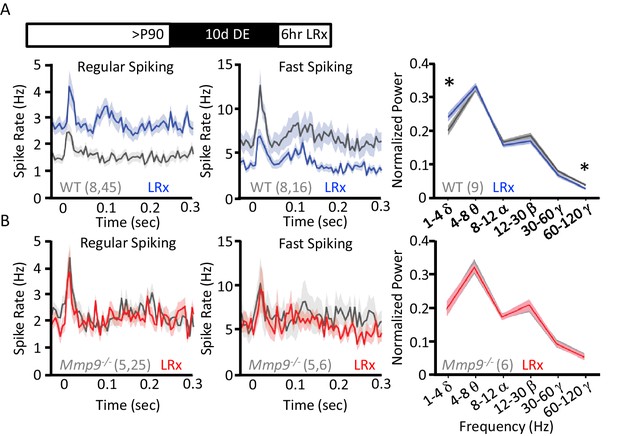
LRx regulates neuronal excitability and synchrony in WT but not Mmp9−/− mice.
(A) Inset: Experimental paradigm. 10 days of dark exposure, followed by LRx (6 hr) induced an increase in visually evoked activity of regular spiking neurons (RS; n=8 subjects, 45 units) and a decrease in evoked activity of fast spiking neurons (FS; n=8 subjects, 16 units) in WT mice. Post-stimulus time histograms (average ±SEM). 200 stimulus presentations of 100% contrast, 0.05 cycle per degree gratings, reversing at 1 Hz, stimulus onset = time 0 s. Right, Fourier transform of spontaneous LFP, normalized to within subject total power, and binned by frequency. In WT mice (n=9 subjects), LRx induces an increase in δ and a decrease in high γ power. (B) No change in regular spiking (n=5 subjects, 25 units) or fast-spiking (n=5 subjects, 6 units) visually evoked activity following LRx in Mmp9−/− mice. Post-stimulus time histograms (average ±SEM). 200 stimulus presentations of 100% contrast, 0.05 cycle per degree gratings, reversing at 1 Hz, stimulus onset = time 0 s). Right, no change in oscillatory activity following LRx in Mmp9−/− mice (n=6 subjects). *p<0.03; paired Student’s T-test.
-
Figure 5—source data 1
- https://doi.org/10.7554/eLife.27345.018
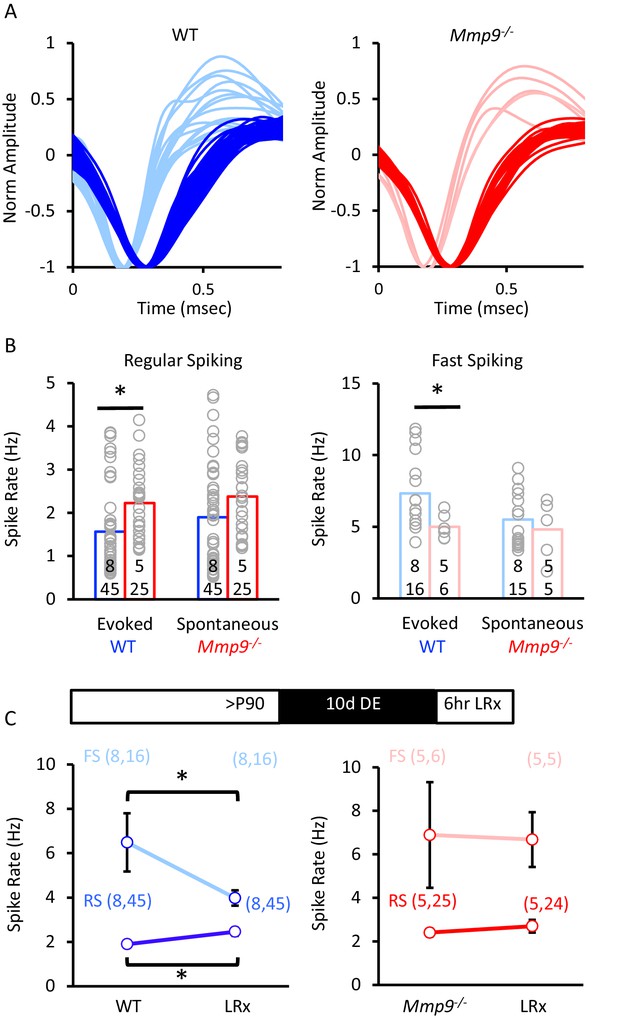
Change in neuronal excitability by LRx in WTs is absent in Mmp9−/− mice.
(A) Sorting of single units into two populations based on waveform characteristic in normal-reared WT (left, blue) and Mmp9−/− mice (right, red). (B) Single unit recordings of visually evoked and spontaneous regular spiking (left: WT blue, n=8 subjects, 45 evoked and 45 spontaneous units and Mmp9−/− red, n=5 subjects, 25 evoked and 25 spontaneous units) and fast spiking neurons (right: WT blue, n=8 subjects, 16 evoked units, 15 spontaneous units and Mmp9−/− red, n=5 subjects, 6 evoked units, 5 spontaneous units). (C) LRx changes mean spontaneous spike rates in WT (blue, n=45 RS, 16 FS in control; 45 RS, 16 FS LRx) but not Mmp9−/− (red, n=25 RS, 6 FS in control; 24 RS, 5 FS in LRx) mice. *p<0.05 unpaired Student’s T-test.
-
Figure 5—figure supplement 1—source data 1
- https://doi.org/10.7554/eLife.27345.016
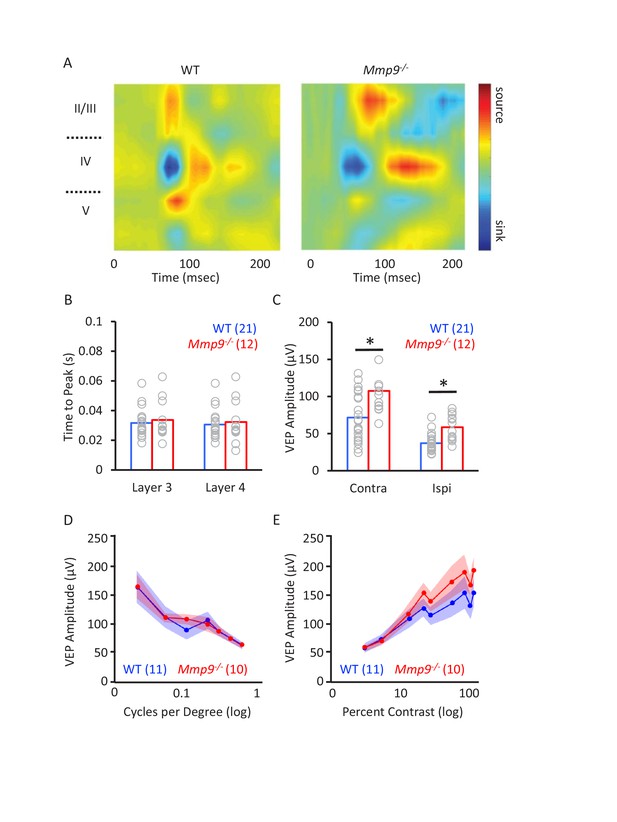
Normal functional organization of Mmp9−/− mouse visual system.
(A) Representative examples of CSD distribution derived from visually-evoked LFP of WT (left) and Mmp9−/− mice (right). The distribution of current sinks/sources is similar across the two genotypes, although the layer 5 current source is stronger in WTs and current sources and sinks are prolonged in Mmp9−/− mice. (B) No difference in the time to peak VEP in WT versus Mmp9−/− mice, n=number of subjects, displayed in histogram. (C) Larger amplitude contralateral and ipsilateral eye VEPs (evoked with 0.05 cycle per degree 100% contrast grating, reversing at 1 Hz; *p<0.03, unpaired Student’s T-test) but no change in the contralateral bias index Mmp9−/− mice (CBI: contra-ipsi/contra + ipsi = WT: 0.28±0.03, Mmp9−/−: 0.29±0.02). (D) Similar variation of VEP amplitudes as a function of visual stimulus spatial frequency (log scale), presented at 50% contrast, in WT and Mmp9−/− mice. (E) Similar variation of VEP amplitudes as a function of visual stimulus contrast (log scale), presented at 0.02 cpd and 100% contrast, in WT and Mmp9−/− mice (WT n=11, Mmp9−/− n=10); n=subjects in parentheses.
-
Figure 5—figure supplement 2—source data 1
- https://doi.org/10.7554/eLife.27345.017
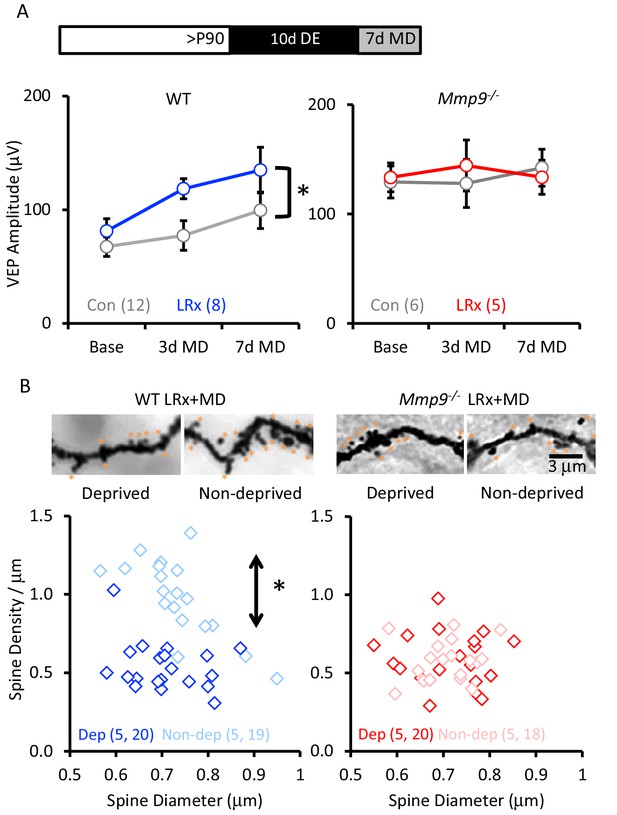
LRx reactivates structural and functional plasticity in visual cortex of adult WT, but not Mmp9−/− mice.
(A) Inset: Experimental paradigm. VEP amplitudes in response to presentation of visual stimuli (100% contrast gratings, 0.05 cycle per degree, reversing at 1 Hz) to non-deprived contralateral eye, prior to monocular dperivation (MD) and following 3 and 7 days of MD. LRx promotes significant enhancement of non-deprived eye VEP following MD in WT adults (left, n=12, 8 subjects for Con and LRx, respectively) but not Mmp9−/− mice (right, n=6, 5 subjects for Con and LRx, respectively). Between subjects repeated measures ANOVA, WT: F=6.33, *p=0.026; Mmp9−/−: F=0.020, p=0.890. (B) Top: Representative images of Golgi stained basolateral dendrites of layer 4 neurons in binocular region of primary visual cortex following 7 days of MD in WT LRx (left) and Mmp9−/− LRx (right) subjects. Significant difference in the distribution of spine densities in deprived versus non-deprived V1b following 7 days of MD in WT LRx but not Mmp9−/− mice. Spine densities and diameters of the basolateral dendrites of layer 4 neurons were measured 75 – 100 μm from soma, spines are labeled with orange asterisks. One-way ANOVA, F=24.9, p<0.0001. KS tests (deprived versus non-deprived) WT LRx+MD Diameter: p=0.37; Density: p<0.0001; Mmp9−/− LRx+MD: Diameter: p=0.62; Density: p=0.95; n=(subjects, neurons).
-
Figure 6—source data 1
- https://doi.org/10.7554/eLife.27345.020
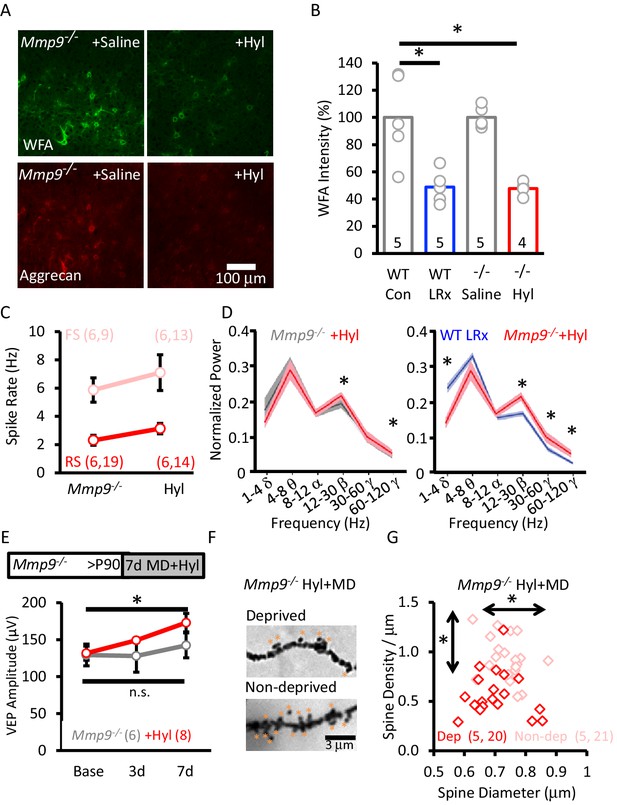
Rescue of structural and functional plasticity in Mmp9−/− mouse by hyaluronidase.
(A) Hyaluronidase (Hyl, 4 μl of 200 U/ml at 100 nl/min) or vehicle was delivered i.c. via cannula at the initiation of LRx (1X/2 days for 7 days). Top: Reduction in WFA-FITC labeling following hyaluronidase treatment. Bottom: Reduction in aggrecan immunoreactivity following hyaluronidase treatment. (B) Reduction in WFA labelling following hyaluronidase treatment in Mmp9−/− mice compared to LRx in WT mice. WFA intensities was quantified 250 – 400 μm from cortical surface (WT Con: 100±14.3%, WT LRx: 48.8±5.3%, Mmp9−/− saline: 100.1±3.5%, Mmp9−/− Hyl: 47.8±2.6%, One-way ANOVA, F=12.7; p=0.0002, *p<0.01) n=subjects. (C) No change in the excitability of FS neurons from Mmp9−/− mice following hyaluronidase treatment (light red, n=6 subjects, 9 units; +Hyl n=6 subjects, 13 units) or RS (red, n=6 subjects, 19 units; +Hyl n=6 subjects, 14 units). (D) Hyluronidase-induced change in neuronal synchrony does not mimic the response to LRx in WTs (n=6 subjects, *p<0.03, paired Student’s T-test). Comparison of oscillatory activity in two cohorts of subjects that exhibit robust adult ocular dominance plasticity: WT+LRx (n=9 subjects) and Mmp9−/−+Hyl; *p<0.03, unpaired Student’s T-test. (E) Inset: Experimental paradigm. Hyaluronidase treatment (n=8 subjects; red) promotes significant enhancement of non-deprived eye VEP following monocular deprivation in adult Mmp9−/− mice (n=6 subjects; gray). Repeated measures ANOVA, F=16.668, p=0.001, Bonferroni post-hoc *p=0.001, Baseline versus 7d. MD alone, repeated measures ANOVA, F=0.446, p=0.655 (n.s.=not significant). (F) Representative images of Golgi stained basolateral dendrites of layer 4 neurons in V1bfollowing 7 days of MD in Mmp9−/−+Hyl subject. (G) Significant difference in distribution of spine densities and diameters in deprived versus non-deprived visual cortex following 7 days of MD in Mmp9−/−+Hyl mice, quantified 75 – 100 μm from soma). One-way ANOVA, F=6.6, p<0.0001. KS for diameter: p=0.0149; density: p<0.0001. n=(subjects, neurons).
-
Figure 7—source data 1
- https://doi.org/10.7554/eLife.27345.022
Additional files
-
Transparent reporting form
- https://doi.org/10.7554/eLife.27345.023





