Assembly rules for GABAA receptor complexes in the brain
Figures
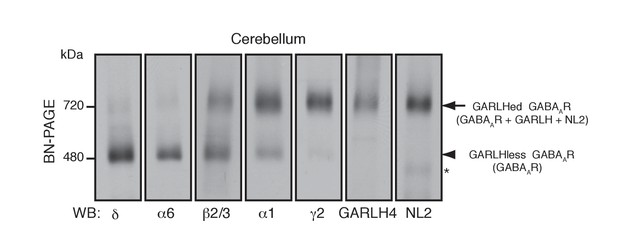
Distinct compositions of GARLHed and GARLHless GABAARs.
Cerebellar membranes solubilized with lauryl maltose-neopentyl glycol (MNG) were subjected to BN-PAGE. The α6 and δ GABAAR subunits preferentially migrated at 480 kDa, while γ2 and α1 as well as GARLH4 and neuroligin-2 (NL2) predominantly migrated at 720 kDa. β2/3 signal was observed equally at 480 and 720 kDa. The arrow and arrowhead indicate the GARLHed and GARLHless GABAAR, respectively, while the asterisk (*) denotes the NL2 band without GABAARs. The images are representative of three independent experiments.
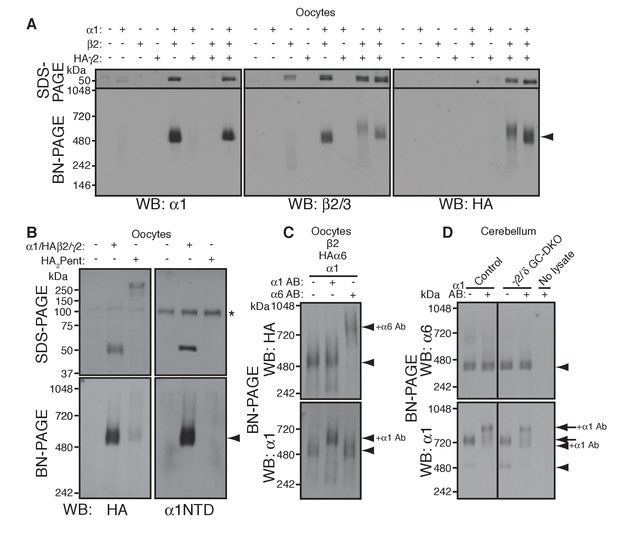
α1 and α6 subunits segregate into distinct pentamers independent of non-α/β subunits.
(A) Reconstitution of GABAAR assembly in Xenopus laevis oocytes. Membranes from oocytes injected with cRNAs encoding the indicated subunits were solubilized with Triton X-100 and subjected to SDS- and BN-PAGE. The GABAAR at 520 kDa was reconstituted by co-expression of α1 and β2 or α1, β2 and HA-tagged γ2 in oocytes injected with the corresponding cRNAs (0.55 ng ea). Co-expressing β2 and HAγ2 produced a weak band at 600 kDa. The images are representative of two independent experiments. (B) Co-migration of a GABAAR oligomer and concatenated pentamer. Membranes from cRNA-injected oocytes were solubilized with Triton X-100 and subjected to SDS- and BN-PAGE. An α1/HAβ2/γ2 GABAAR oligomer and a concatenated pentamer, HAβ2-α1-HAβ2-α1-γ2 (HA2Pent), migrated at 520 kDa. Monomers and HA2Pent were visualized at the expected molecular weights on SDS-PAGE. An anti-α1 antibody that recognizes the N-terminus of mature α1 proteins (α1NTD) detects the monomeric, but not concatenated, α1 subunit. The asterisk (*) denotes a nonspecific band observed on all lanes, indicating that the band is not heterologously expressed GABAAR subunit. The images are representative of three independent experiments. (C) GABAAR complexes from oocytes co-injected with cRNAs of HAα6, α1 and β2 were examined by antibody shift assay. An anti-α6 antibody shifted up HAα6 but not α1 signal, whereas an anti-α1 antibody shifted up α1 but not HAα6 signal. The images are representative of three independent experiments. (D) GABAAR complexes in cerebella from control and γ2/δ GC-DKO mice were examined by antibody shift assay on BN-PAGE. Addition of anti-α1 antibody shifted up α1 signal at 480 and 720 kDa in both genotypes. In contrast, in both genotypes, α6 signal was not shifted by addition of anti-α1 antibody. The images are representative of three independent experiments. The arrow and arrowhead indicate the GARLHed and GARLHless GABAAR, respectively, and antibody bound complexes are indicated.
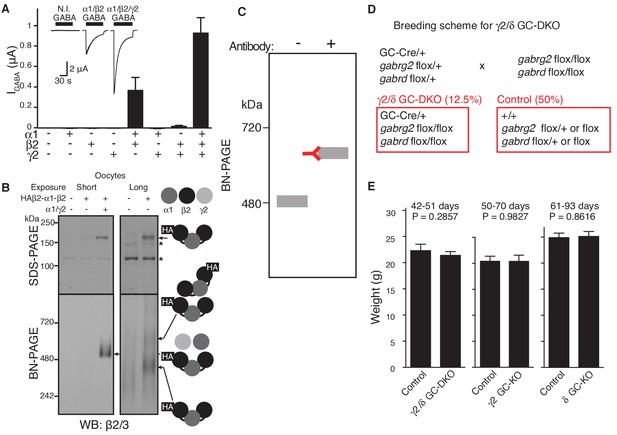
GABAAR assembly in cRNA-injected oocytes and characterization of knockout mice.
(A) Oocytes were injected with the indicated cRNAs (1.65 ng ea) and GABA (100 μM)-evoked currents were measured using two electrode voltage clamp recording (Vh=-40 mV). GABA-evoked currents were detected in oocytes injected with α1/β2/γ2 and α1/β2 cRNAs, and tiny currents were detected in oocytes injected with β2/γ2 cRNAs (n = 5–6 oocytes). Representative traces are shown. N.I., no injection. Data are given as mean ± s.e.m. (B) Oocyte membranes from cRNA-injected oocytes were solubilized with Triton X-100 and subjected to SDS- and BN-PAGE. A tandem trimer, HAβ2-α1-β2, when co-expressed with both α1 and γ2 monomers, migrated at 520 kDa, whereas HAβ2-α1-β2 alone expressed weakly and migrated as 400 and 600 kDa bands, presumably corresponding to the tandem trimer and a dimer of tandem trimers (hexamer), respectively. No degraded products of the trimer were detected on SDS-PAGE. The asterisk (*) denotes a nonspecific band. The images are representative of three independent experiments. (C) A schematic diagram of an antibody shift assay on BN-PAGE. Antibody binding shifts the molecular weight of a protein complex on BN-PAGE. (D) Breeding scheme of γ2 and δ GC-KO mice. Mice that do not carry a transgene of Cre recombinase were used as controls. (E) Weights of granule cell (GC) specific-γ2 or δ knockout (KO) and γ2/δ GC-double KO mice were unchanged relative to control littermates without Cre recombinase (Control) (n = 6–9 animals). Data are given as mean ± s.e.m.; Student’s t-test indicated no significant difference.
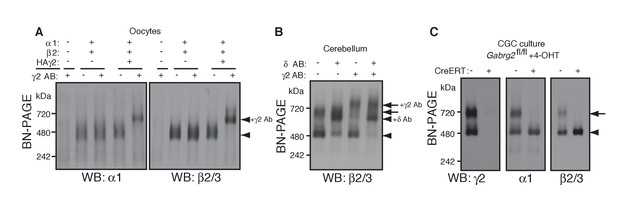
γ2 is required for assembly of the native GARLHed complex.
(A) Membranes from cRNA-injected oocytes (0.18 ng ea) were solubilized in Triton X-100 and analyzed by BN-PAGE. α1 and β2 with or without HA-tagged γ2 migrated at 480 kDa. Addition of an anti-γ2 antibody induced an upward shift of GABAARs from α1/β2/HAγ2-injected oocytes but had no effect on GABAARs from α1/β2-injected oocytes, indicating nearly complete incorporation of HAγ2 into GABAAR pentamers. The images are representative of two independent experiments. (B) GABAAR complexes in cerebellum were examined by antibody shift assay. In cerebellum, anti-δ antibody caused most β2/3 signal at 480 kDa to shift up, but did not affect β2/3 signal at 720 kDa. In contrast, anti-γ2 antibody shifted up β2/3 signal at 720 kDa. When anti-δ and anti-γ2 antibodies were combined, both 480 and 720 kDa bands shifted up almost completely. The images are representative of two independent experiments. (C) Primary cultured cerebellar granule cells were prepared from conditional γ2 knockout mice with or without a transgene encoding tamoxifen-inducible Cre recombinase (CreERT), and treated with 4-hydroxytamoxifen (4-OHT) from DIV1.5 to DIV3. At DIV9, cell membranes were solubilized in MNG and examined by BN-PAGE. In neurons expressing CreERT, γ2 was eliminated, and α1 and β2 at 720 kDa collapsed to 480 kDa. The images are representative of three independent experiments. The arrow and arrowhead indicate the GARLHed and GARLHless GABAAR, respectively, and antibody-bound complexes are indicated.
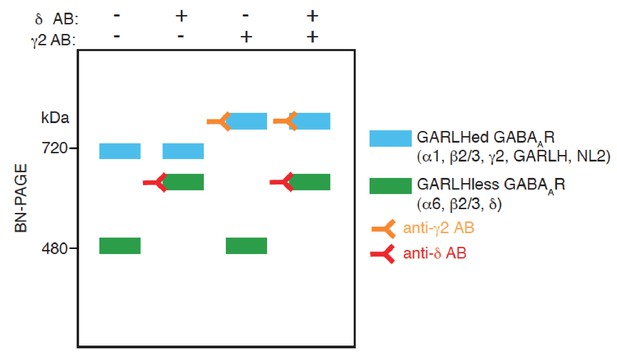
A schematic diagram of an antibody shift assay for distinct GABAAR complexes on BN-PAGE.
GABAARs comprised of α6, β2/3 and δ form a complex at 480 kDa, and the addition of an anti-δ antibody to the complex shifts its mobility on BN-PAGE. On the other hand, GABAARs comprised of α1, β2/3 and γ2 form complexes with GARLH and NL2 at 720 kDa, and the addition of an anti-γ2 antibody to the complex shifts its mobility. When both anti δ and γ2 antibodies are added, mobility of both the complexes at 480 and 720 kDa are shifted.
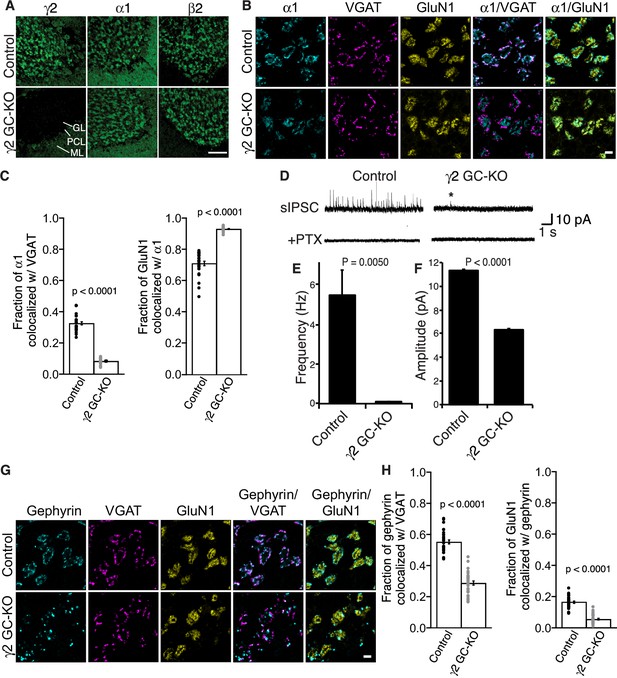
γ2 is essential for GABAAR synaptic localization in the brain.
(A, B) Localization of GABAAR subunits in the cerebellar granule cell (GC)-γ2 knockout (KO) mice and age matched controls without Cre expression (Control). Inhibitory presynaptic VGAT and excitatory postsynaptic GluN1 were co-stained. (A) Loss of γ2 was observed specifically in the granular layer in γ2 GC-KO mice, whereas α1 and β2 remained. The images are representative of four independent experiments. (B and C) High-magnification representative images showed protein distribution on each glomerulus. Inhibitory inputs project to outer edges of the glomerulus, whereas excitatory inputs project to inner edges of the glomerulus. In the γ2 GC-KO, the fraction of α1 colocalized with VGAT was reduced, whereas the fraction of GluN1 colocalized with α1 was increased (n = 30 areas/2 animal each). (D–F) Spontaneous inhibitory postsynaptic currents (sIPSCs) were recorded from granule cells in acute cerebellar slices, and representative traces are shown (D). In γ2 GC-KO mice, sIPSC frequency (E) and amplitude (F) were dramatically reduced, but not completely eliminated (n = 4 bins (E), n = 69–1740 events (F), see Materials and methods). The asterisk indicates a sIPSC recorded from a γ2 GC-KO mouse. Picrotoxin (100 µM) blocked all sIPSCs. (G and H) Representative images show localization of gephyrin in γ2 GC-KO and control mice. Gephyrin colocalized with VGAT at the glomerular periphery in controls. In the γ2 GC-KO, the fraction of gephyrin colocalized with VGAT was reduced, and at the same time, the fraction of GluN1 colocalized with gephyrin was reduced (n = 30 areas/2 animal each). Scale bars: 60 μm (A), 5 μm (B, G). Data are given as mean ± s.e.m.; p values were determined using student’s t test.
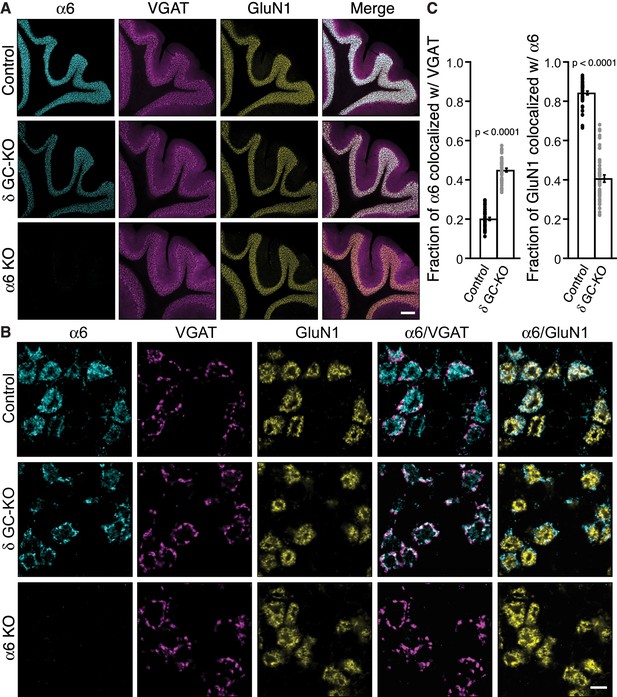
Delta inhibits synaptic localization of α6-containing GABAARs.
(A–C) The distribution of α6 was examined in the cerebellum of δ GC-knockout (KO) and α6 KO mice. Inhibitory presynaptic VGAT and excitatory postsynaptic GluN1 were co-stained. (A) Low magnification images showed specific α6 signal in cerebellar granular layers in wild-type (Control) and δ KO mice, but not in α6 KO mice. The images are representative from three animals for each genotype. (B) High-magnification representative images showed VGAT around the glomeruli and GluN1 inside the glomeruli. In control mice, α6 signal was diffuse over the glomeruli, and overlapped substantially with GluN1. In contrast, in δ KO mice, α6 signal was largely confined to the peripheral glomeruli where it colocalized with VGAT. (C) The fraction of α6 signal co-localized with VGAT was increased in δ KO mice, whereas the fraction of GluN1 signal co-localized with α6 signal was reduced (n = 40–43 areas/3 animal each). Data are given as mean ± s.e.m.; p values were determined with student's t test. Scale bars: 200 μm (A), 5 μm (B).
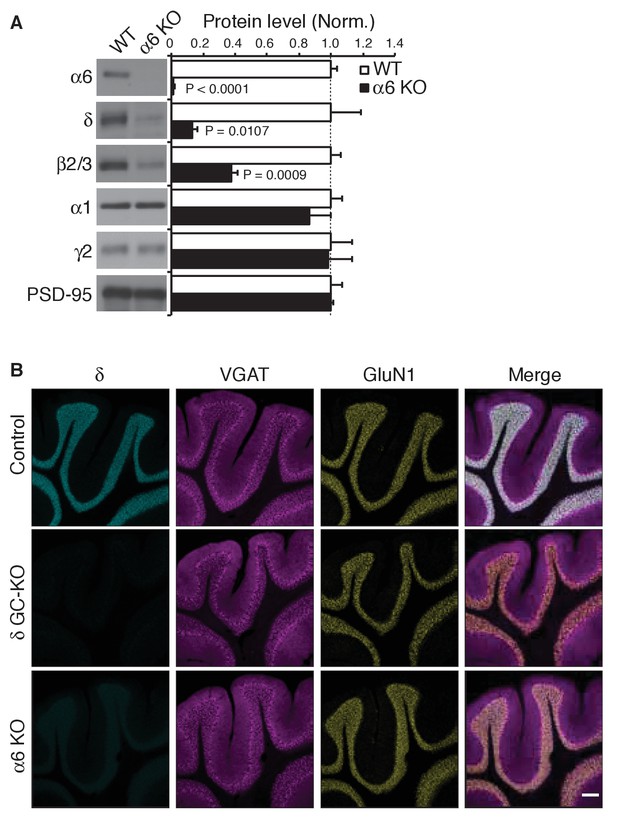
α6 is required for expression of the δ subunit.
(A) Protein levels in cerebella from α6 KO mice were measured. In α6 KO mice, α6 was eliminated, δ was reduced 85% and β2/3 was reduced 60%, whereas α1, γ2 and the excitatory synapse marked PSD-95 were not significantly altered. Protein levels were normalized to those from WT mice (n = 3 animals). Data are given as mean ± s.e.m.; p values were determined with student's t test. (B) δ expression was examined by immunohistochemistry and representative images from two independent experiments were shown. δ signal was observed in cerebellar granular layers in wild-type (Control) mice, but not in δ GC-KO and α6 KO mice. The inhibitory presynaptic marker VGAT and the excitatory postsynaptic marker GluN1 were not altered in these mice. Scale bar: 200 µm.
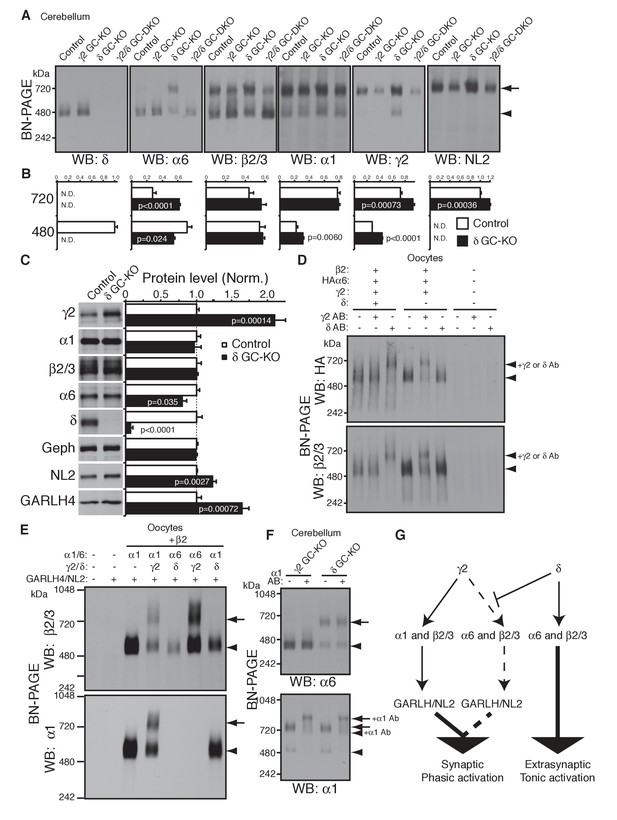
δ suppresses an assembly pathway for α6 with γ2, GARLH and NL2.
(A and B) δ suppresses incorporation of α6 into GABAAR/GARLH/NL2 complexes. (A) GABAAR complexes in cerebella from mice of various genotypes were examined by BN-PAGE. In control, γ2 GC-KO and γ2/δ GC-double KO cerebella, α6 expressed predominantly at 480 kDa. In contrast, in δ KO cerebellum, α6 expressed predominantly at 720 kDa, and an increase in signals of β2/3, γ2 and NL2, but not α1, at 720 kDa was also observed. As expected, in γ2 GC-KO cerebellum, γ2 signal was reduced but not eliminated. The residual γ2 signal originates from other cell types in the cerebellum that still expressed γ2. The images are representative of three independent experiments. (B) Relative ratios of the 720 and 500 kDa complex in cerebella from control and δ GC-KO (n = 5 animals). Signal intensity of each band was measured. Relative ratios of bands at 720 and 480 kDa were calculated in control mice, and relative changes in each band intensity in δ GC-KO were estimated. Data are given as mean ± s.e.m.; p values were determined with student's t test. (C) Total protein expression in cerebella from δ KO mice. Results are shown relative to control littermates (n = 5 animals each). Elimination of δ expression was confirmed and α6 was modestly reduced. A substantial increase in γ2, GARLH4 and NL2 was observed without changes in other GABAAR subunits (α1 and β2/3) or inhibitory synaptic marker protein Gephyrin (Geph). Data are given as mean ± s.e.m.; p values were determined with student's t test. (D) δ inhibits α6 assembly with γ2. GABAAR complexes from cRNA-injected oocytes were examined by BN-PAGE. In oocytes expressing HAα6, β2 and γ2 with or without δ, HAα6 and β2/3 migrated at 520 kDa. Addition of anti-δ antibody, but not addition of anti-γ2 antibody, to membranes from HAα6/β2/γ2/δ-expressing oocytes shifted HAα6 and β2 signal upward, indicating preferential assembly of α6 with δ relative to γ2. On the other hand, when δ was not present, addition of anti-γ2 antibody shifted HAα6 and β2 signal upward. The images are representative of four independent experiments. (E) Membranes from oocytes injected with the indicated cRNAs (0.2 ng ea for α1, β2, γ2 and GARLH4; 0.5 ng for δ; 1.0 ng for α6 and NL2) were analyzed using BN-PAGE. Upon co-expression with γ2, but not δ, both α1 and α6 assembled with β2 and formed complexes with GARLH4 and NL2 at 720 kDa. The images are representative of two independent experiments. (F) α1 and α6 segregate into distinct complexes, even when both associate with GARLH/NL2. GABAAR complexes in cerebella from γ2 GC-KO and δ GC-KO mice were examined by antibody shift assay on BN-PAGE. Addition of anti-α1 antibody shifted up α1 signal at 480 and 720 kDa in both genotypes. In contrast, in both genotypes, α6 signal was not shifted by addition of anti-α1 antibody. The images are representative of three independent experiments. The arrow and arrowhead indicate the GARLHed and GARLHless GABAAR, respectively, and antibody-bound complexes are indicated. (G) δ suppresses an assembly pathway for α6-containing GARLHed GABAARs. γ2 assembles with α1, β2/3 and GARLH/NL2 to mediate synaptic localization and phasic activation. Normally, δ sequesters α6, thereby suppressing γ2 interaction with α6. α6/δ-containing receptors do not interact with GARLH and neuroligin-2 (NL2), which are required for synaptic localization and phasic activation, and thus α6/δ-containing receptors localize at extrasynaptic sites and mediate tonic activation. In the absence of δ, α6 assembles with γ2, β2/3 and GARLH/NL2 to mediate synaptic localization and phasic activation.





