LRP1 integrates murine macrophage cholesterol homeostasis and inflammatory responses in atherosclerosis
Figures
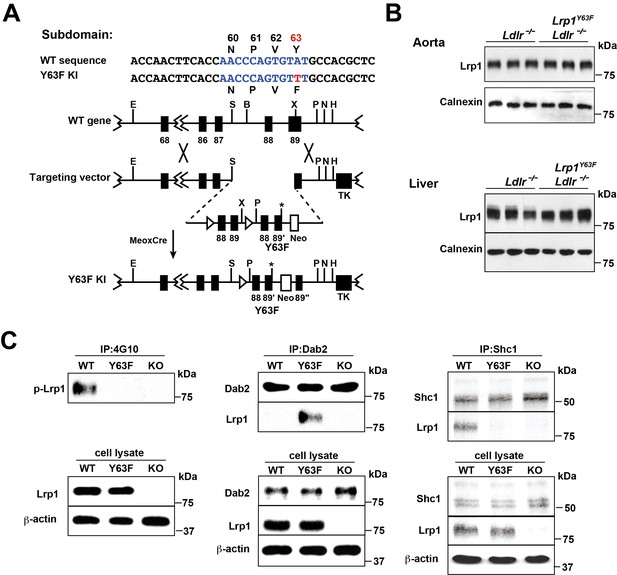
Generation and characterization of Lrp1Y63F knock-in mouse model.
(A) Schematic of the construct used to generate Lrp1Y63F knock-in mice with a point mutation in the tyrosine phosphorylation site (Y63F) as indicated in red and asterisk. Open triangles indicate loxP sites. The neomycin-resistance (Neo) and thymidine kinase (TK) genes were used for selection of the targeted ES cells. Restriction sites: E, EcoRI; S,SpeI; B,BamHI; X,XhoI; P,PmeI; N,NotI; H,HindIII. (B) Western blot analysis of Lrp1 expression levels in aorta and liver from Lrp1Y63F;Ldlr−/− mice (n = 5) and Ldlr−/− controls (n = 5) after 16 week of high cholesterol/high fat (HCHF) diet. (C) Impaired Lrp1 binding to Shc1 in primary smooth muscle cells (SMCs) from Lrp1Y63F mice. Primary SMCs were explanted from the aorta of wild type, Lrp1Y63F, or smLrp1−/− mice. Cells were treated with 10 ng/ml PDGF-BB for 10 min. Representative immunoblots probed for LRP1 (upper) and total protein levels in input cell lysate (lower) from co-immunoprecipitation assays using antibodies against phospho-tyrosine (4G10) (left), Dab2 (middle) or Shc1 (right). p-Lrp1, tyrosine phosphorylated Lrp1. Calnexin and β-actin were used as loading controls.
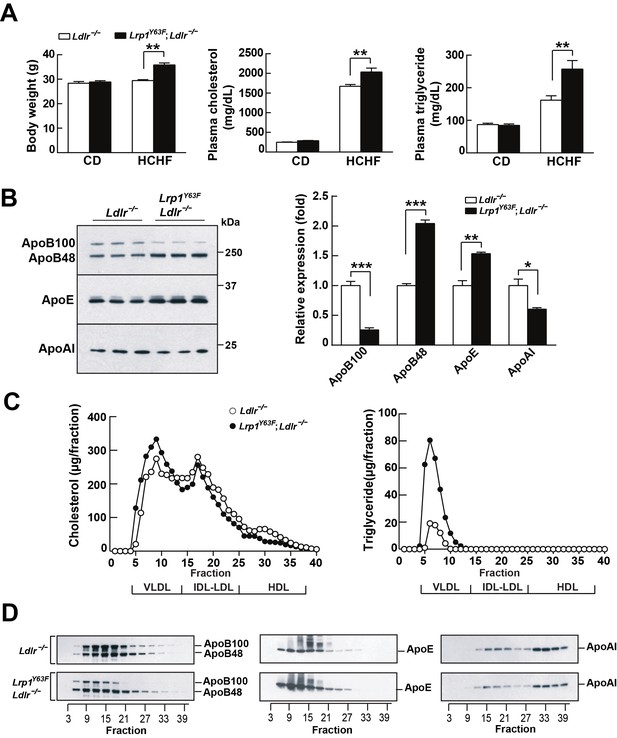
Lrp1Y63F mutation elicits pro-atherogenic lipoprotein profiles on HCHF diet.
(A) Body weight, plasma cholesterol and triglyceride levels of Lrp1Y63F;Ldlr−/− (n = 16) and Ldlr−/− (n = 14) mice after 16 weeks of chow diet (CD) or high cholesterol/high fat (HCHF) diet. (B) Representative immunoblots and quantitative analysis of plasma apolipoproteins in Lrp1Y63F;Ldlr−/− (n = 6) and Ldlr−/− (n = 6) mice after 16 weeks of HCHF diet. (C) FPLC plasma lipid profiles. Cholesterol (left) and triglyceride (right) in fractionated plasma from mice on 16 week HCHF diet. (D) Representative immunoblots of ApoB (left), ApoE (middle) and ApoAI (right) in different fractions. All data are mean ±SEM. *p<0.05, **p<0.01, ***p<0.001.
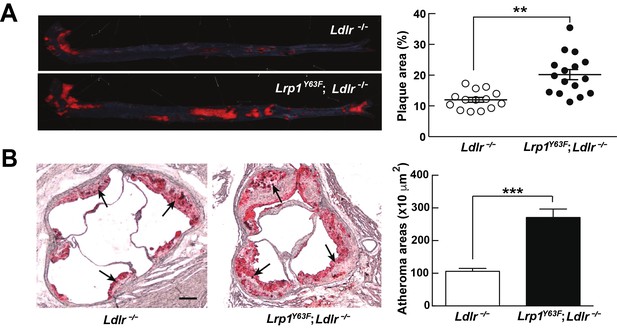
Lrp1Y63F mutation accelerates atherosclerotic development on a HCHF diet.
(A) Representative images of en face assessment of aortic atherosclerotic lesions stained with Oil red O (left) and quantitative analysis of aortic lesion area (right) in Lrp1Y63F;Ldlr−/− (n = 16) and Ldlr−/− (n = 14) mice after 16 weeks of HCHF diet. (B) Analysis of atherosclerotic lesions in aortic roots with Oil red O staining in mice described above. Black arrows indicate atherosclerotic plaque (left, scale bar = 200 μM). Oil red O positive areas in aortic roots were quantified (right). Values are expressed as mean ±SEM. *p<0.05, **p<0.01, ***p<0.001.
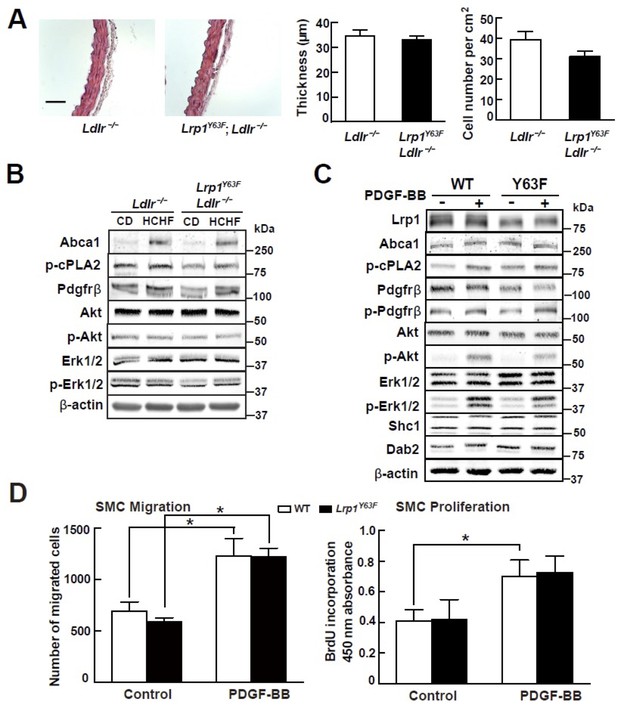
Lrp1Y63F mutation has no effect on atherogenic signaling pathways and morphological phenotype in SMCs.
(A) Left: representative images of H&E stained descending thoracic aortas (scale bar = 50 μm). Right: aortic wall thickness and cell number were quantified for the indicated genotypes. Data are expressed mean ±SEM; n = 3 per group and genotype. (B) HCHF diet in Lrp1Y63F;Ldlr−/− mice has no effect on PDGF and TGFβ-mediated signaling in the aorta. Proteins were extracted from whole aorta in Lrp1Y63F;Ldlr−/− and Ldlr−/− mice fed with indicated diets. Expression of proteins involved in PDGF signaling pathways was determined by Western blot analysis. (C) Lrp1Y63F mutation did not affect atherogenic signaling pathways mediated by PDGF in vitro. Primary SMCs from WT and Lrp1Y63F mice were incubated with or without 10 ng/ml PDGF-BB for 10 min. Expression of proteins involved in PDGF signaling were determined by Western blot analysis. (D) Lrp1Y63F mutation did not alter SMC functions in vitro. Primary cultured SMCs from WT and Lrp1Y63F mice were incubated with or without 10 ng/ml PDGF-BB under indicated conditions, then cell migration (left) and proliferation (right) were determined. Values from three independent experiments are expressed as mean ±SEM. *p<0.05.
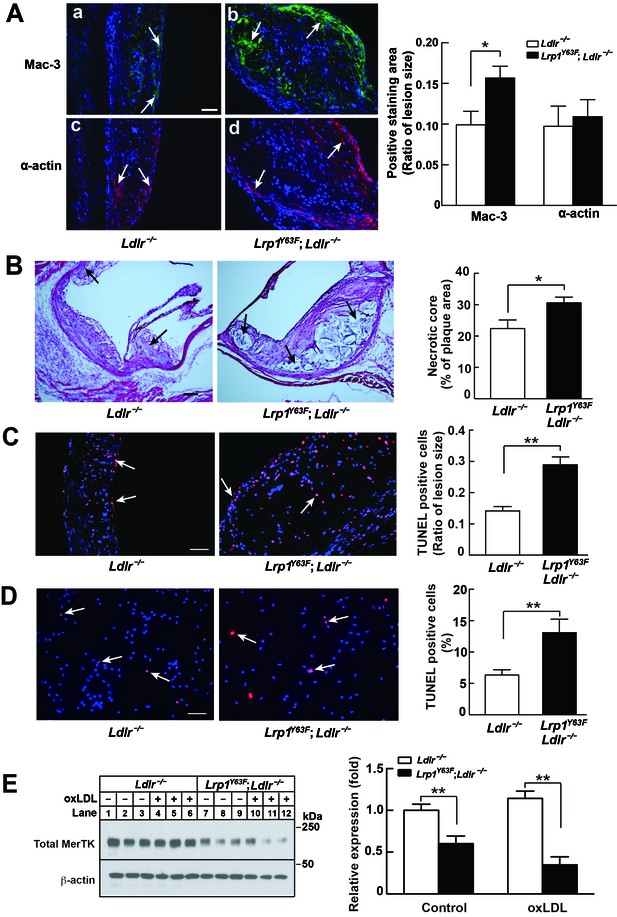
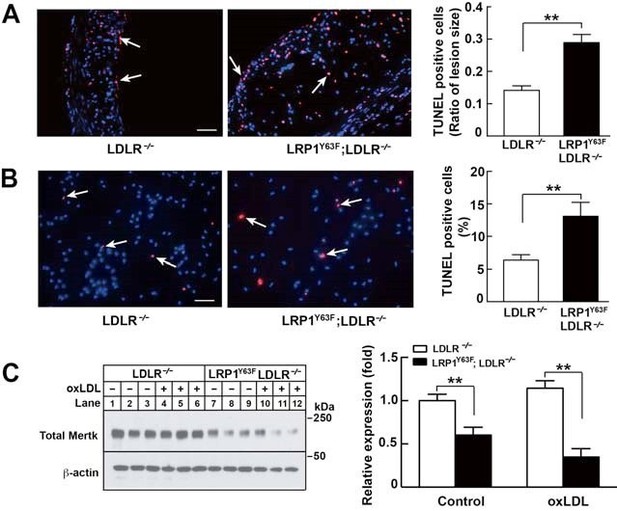
Enhanced necrotic core in atherosclerotic lesions in Lrp1Y63F;Ldlr−/− mice.
(A) Left: Immunohistochemistry of aortic roots sections stained with Mac-3 (a and b, scale bar = 50 μM) and α-actin (c and d, scale bar = 50 μM). White arrows indicate positive immunofluorescent staining. Right: postive staining area was quantified. (B) Representative sections of H&E stained aortic roots from Ldlr−/− (n = 13) and Lrp1Y63F;Ldlr−/− (n = 12) mice after 16 weeks of HCHF diet. Black arrows indicate the necrotic core (left, scale bar = 50 μM) and necrotic core area in the aortic roots was quantified (right). (C) Immunohistochemical analysis of apoptosis in atherosclerotic lesions in mice described above. Representative sections of TUNEL staining of the aortic roots from indicated mice (left: scale bar = 50 μM) and TUNEL-positive cells in the lesions were counted (right). (D) Immunohistochemical analysis of apoptosis in oxLDL-treated peritoneal macrophages in vitro. Representative images of TUNEL-stained macrophages with indicated genotypes (left: scale bar = 50 μM) and TUNEL-positive cells were counted (right). (E) Western blot analysis of MerTK in macrophages in the absence or presence of oxLDL. All data are mean ±SEM. *p<0.05, **p<0.01.
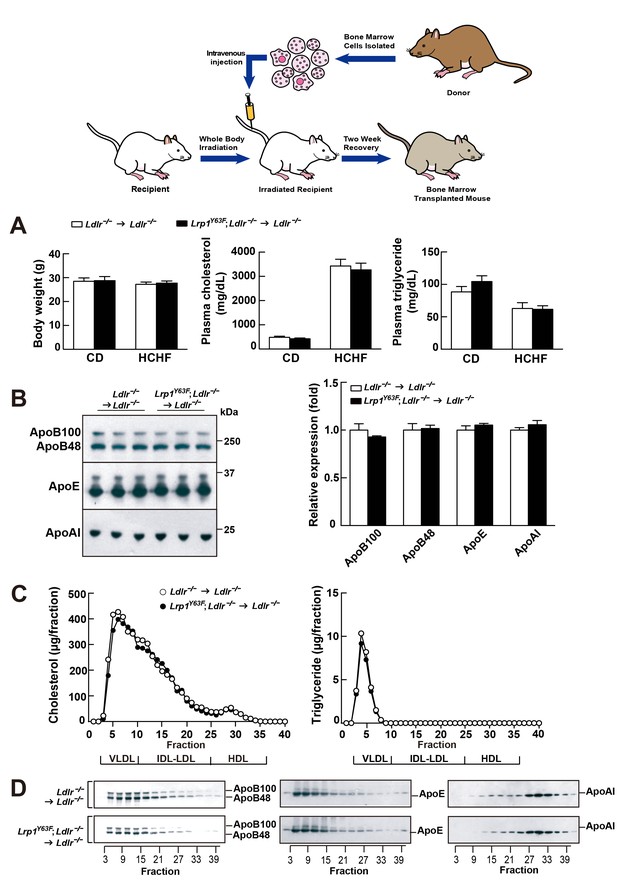
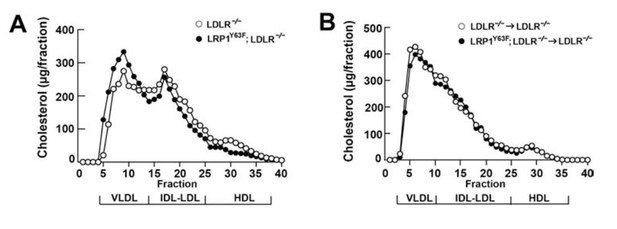
Bone marrow transplantation (BMT) with Lrp1Y63F bone marrow does not affect lipid profiles in Ldlr−/− mice.
Diagram of bone marrow transplant is shown on the top. Bone marrows from Lrp1Y63F;Ldlr−/− mice and Ldlr−/− control mice were transplanted into γ-irradiated-Ldlr−/− mice. After BMT, recipient mice were placed on the indicated diets for 16 weeks. (A) Body weight, plasma cholesterol and triglyceride levels of Ldlr−/−→ Ldlr−/− (n = 9) and Lrp1Y63F;Ldlr−/−→Ldlr−/− (n = 15) mice on CD or HCHF diet. (B) Representative immunoblots and quantitative analysis of plasma apolipoproteins in BMT mice on 16 week HCHF diet (n = 5, each group). (C) FPLC plasma lipid profiles. Cholesterol (left) and triglyceride (right) in fractionated pooled plasma from HCHF-fed mice (n = 8–10, each group). (D) Representative Western blots of ApoB (left), ApoE (middle) and ApoAI (right) in different fractions. All data are mean ±SEM.
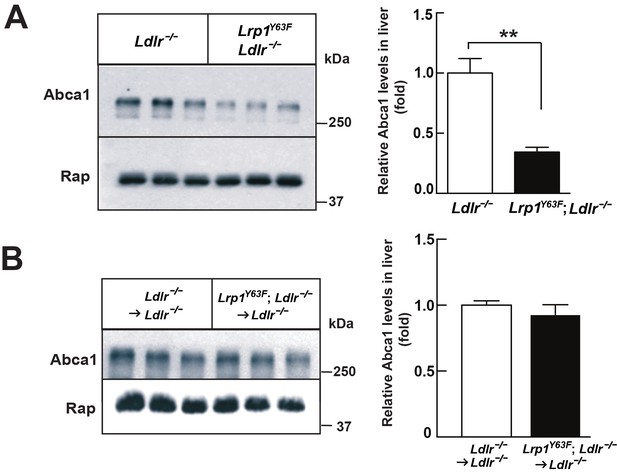
Lrp1Y63F mutation downregulates hepatic Abca1 levels.
(A) Western blot analysis of Abca1 expression in the liver from Lrp1Y63F;Ldlr−/− and Ldlr−/− mice fed with HCHF diet for 16 weeks (n = 5, each group). (B) Western blot analysis of Abca1 expression in the liver from 16 week HCHF-fed Lrp1Y63F;Ldlr−/− and Ldlr−/− mice after BMT (n = 5, each group). All data are mean ±SEM. **p<0.01.
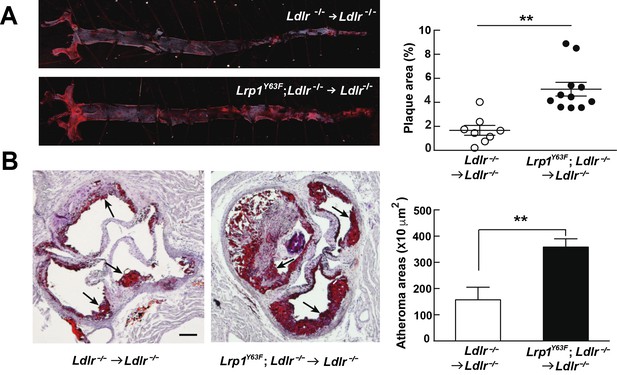
Bone marrow-derived Lrp1Y63F promotes atherosclerosis.
(A) Representative images of en face assessment of atherosclerotic lesions in 16 week HCHF-fed recipient mice with indicated bone marrow donor mice (left) and quantification of plaque areas (right). (B) Representative Oil red O-stained sections of aortic roots in each BMT group (left) and quantification of atheroma areas (right). Black arrows indicate atherosclerotic plaque lesions. All data are mean ±SEM. **p<0.01.
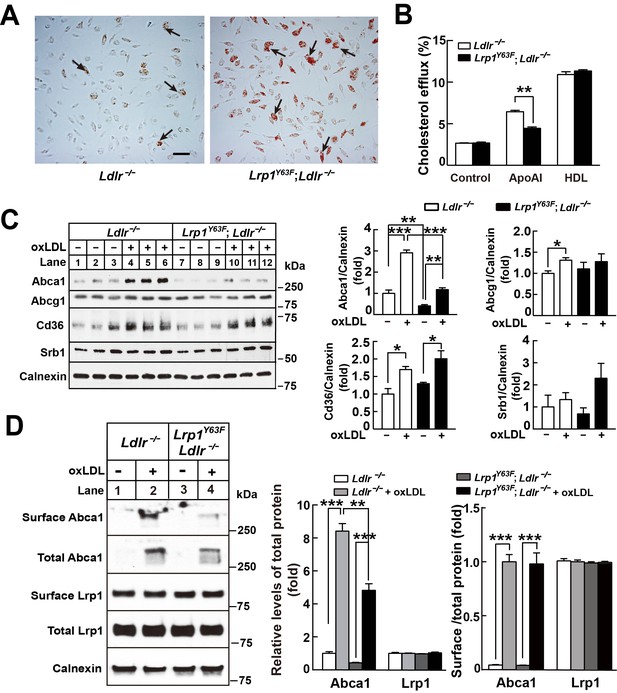
Lrp1Y63F impairs Abca1-mediated cholesterol efflux and increases lipid accumulation in macrophages.
Peritoneal macrophages isolated from Lrp1Y63F;Ldlr−/− and Ldlr−/− mice were starved overnight and treated with or without oxLDL (100 μg/mL) for 24 hr. (A) Representative photomicrographs of lipid accumulation in macrophages stained with Oil red O (left, Ldlr−/−; right, Lrp1Y63F;Ldlr−/−, scale bar = 50 μm). Black arrows indicate Oil red O-stained positive cells. (B) Macrophages isolated from Ldlr−/− and Lrp1Y63F;Ldlr−/− mice were labeled with [3H]-cholesterol in the presence of oxLDL for 24 hr. After equilibration in 0.2% BSA overnight, cells were incubated with either 20 μg/ml apoAI or 30 μg/ml HDL for 4 hr. (C) Western blot analysis of lipid transporters in macrophages treated with or without oxLDL (left) and immunoblot quantitation (right). (D) After treatment with or without oxLDL, surface Abca1 and Lrp1 at plasma membrane in macrophages were biotinylated. The ratio of surface to total protein levels was analyzed by Western blotting (left) and quantification shown (right). All data are mean ±SEM from 3 to 6 independent experiments. *p<0.05, **p<0.01, ***p<0.001.
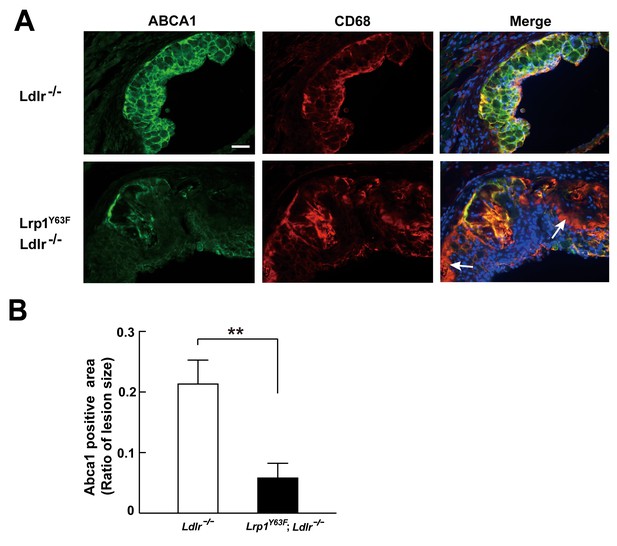
Abca1 expression is reduced in atherosclerotic lesions in Lrp1Y63F;Ldlr−/− mice.
(A) Cryo-sections of aortic roots from HCD-fed Ldlr−/− and Lrp1Y63F;Ldlr−/− mice were double-stained with Abca1 (green) and Cd68 (red) antibodies. Nuclei were stained with DAPI (blue). (B) Abca1 immunoreactivity was determined by color and normalized to lesion size. Scale bar = 200 μM. White arrows indicate the areas of macrophages without Abca1 expression. Data are mean ±SEM from five mice for each genotype. **p<0.01.
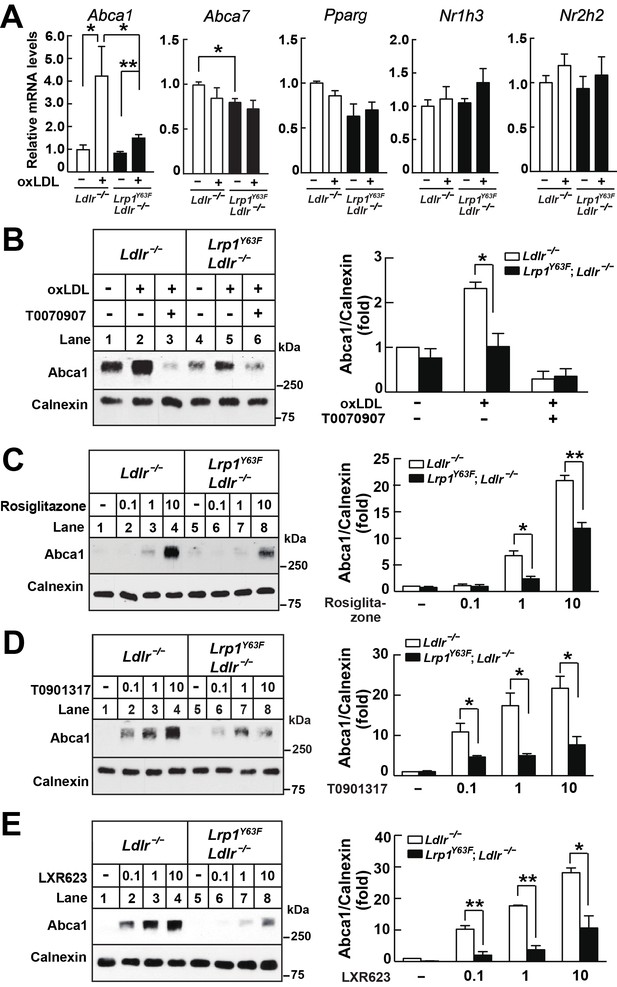
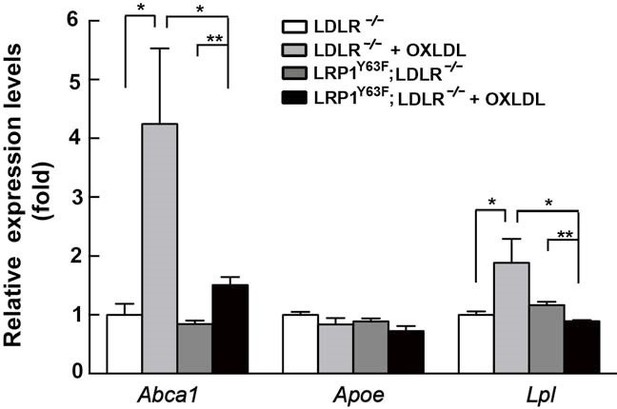
Lrp1Y63F impairs Abca1 induction through inhibiting the PPARγ/LXR pathway.
(A) Real-time PCR analysis of Abca1, Abca7, Pparg, Nr1h3 and Nr2h2 expression in macrophages treated with or without ox-LDL. (B–E) Isolated peritoneal macrophages from Lrp1Y63F;Ldlr−/− and Ldlr−/− were pre-incubated for 1 hr with (B) T0070907 (PPARγ antagonist, 10 μM), (C) Rosiglitizone (PPARγ agonist) at indicated concentrations in μM, (D) T0901317 (nonspecific LXR agonist) at indicated concentrations in μM, (E) LXR623 (LXRβ full agonist and LXRα partial agonist) at indicated concentrations in μM, followed by the treatment with or without oxLDL for an additional 24 hr. Abca1 expression was assessed by immunoblot analysis (left). Protein expression was quantified and analyzed (right). Data are mean ±SEM from three independent experiments. *p<0.05; **p<0.01.
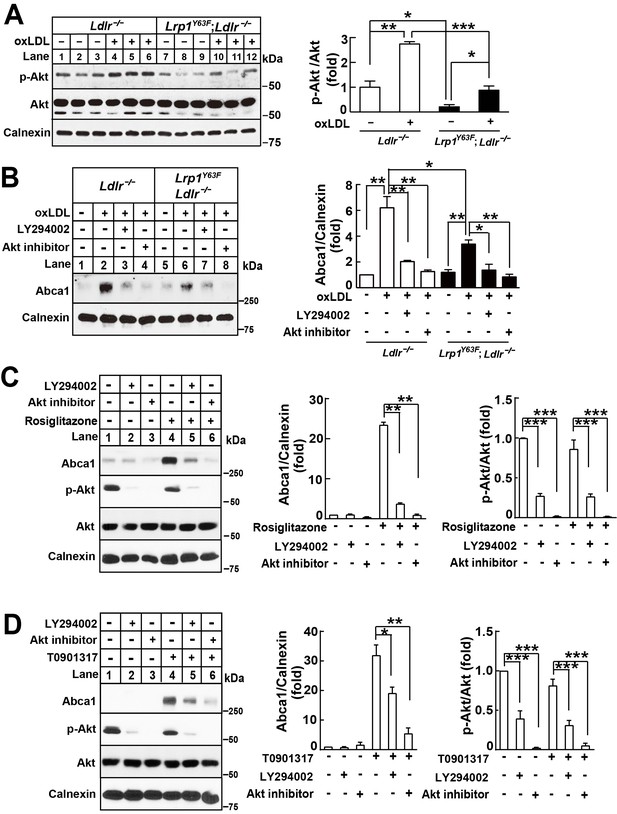
Lrp1Y63F-induced impairment of Abca1 up-regulation is dependent on PI3K/Akt activities.
(A) Western blot analysis of Akt phosphorylation in macrophages treated with or without ox-LDL. (B) Isolated peritoneal macrophages from Lrp1Y63F;Ldlr−/− and Ldlr−/− were pre-incubated with LY294002 (PI3K antagonist, 10 μM) or Akt inhibitor (10 μM) for 1 hr, followed by ox-LDL treatment for an additional 24 hr. Abca1 protein levels were analyzed by Western blot analysis (left) and quantification is shown (right). (C–D) Macrophages were pretreated with LY294002 (10 μM) or Akt inhibitor (10 μM) for 1 hr, followed by the application of 10 μM rosiglitizone (C) or 10 μM T0901317 (D) for an additional 24 hr. Abca1 and pAkt expression were assessed by immunoblot analysis (left) and quantification is shown (right). Data are mean ±SEM from three independent experiments. *p<0.05; **p<0.01; ***p<0.001.
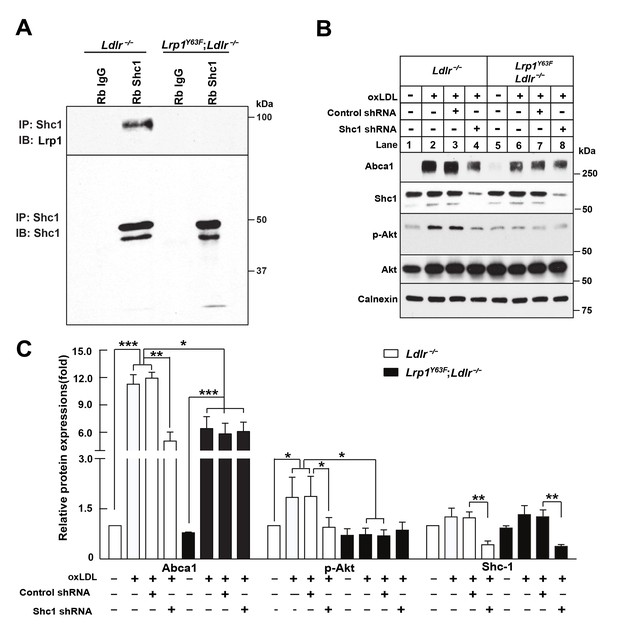
Binding of LRP1 to Shc1 is required for oxLDL-induced Abca1 up-regulation in macrophages.
(A) Representative immunoblots probed for LRP1 (upper) and total immunoprecipitated Shc1 levels in input cell lysate (lower) from co-immunoprecipitation assays using antibodies against Shc1 (Rb Shc1) or rabbit IgG (Rb IgG). Macrophages were treated with oxLDL for 24 hr prior to immunoprecipitation. (B) Peritoneal macrophages were infected with lentivirus expressing scramble shRNA or Shc1 shRNA for 48 hr to knockdown endogenous Shc1 expression, followed by oxLDL treatment for an additional 24 hr. Protein expression was determined by quantitative Western blot analysis. from three independent experiments (C). *p<0.05; **p<0.01; ***p<0.001.
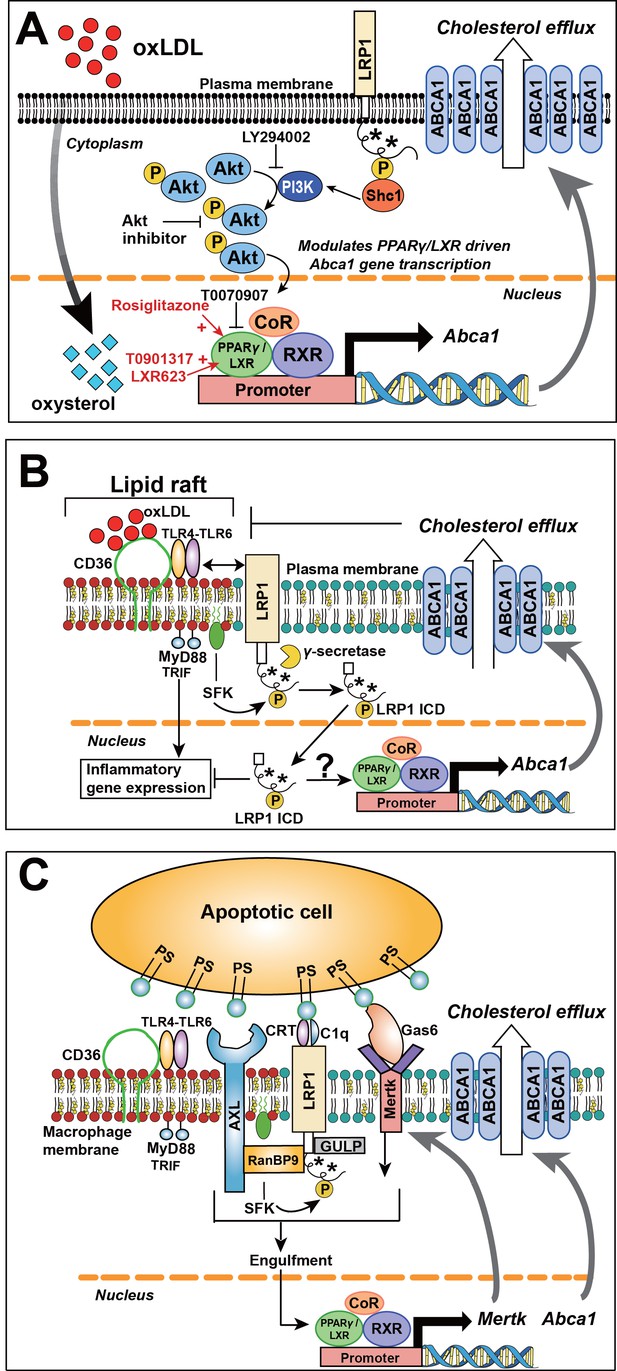
Role of LRP1 NPxY phosphorylation in macrophage lipid accumulation and atherogenesis.
(A) Tyrosine phosphorylation of LRP1 NPxY motifs (as indicated by asterisks), specifically the distal NPxY motif, is necessary for interaction with Shc1, which in turn activates PI3K/Akt signaling. Phospho-Akt modulates PPARγ/LXR driven Abca1 gene transcription and is necessary for the full effect of ox-LDL and PPARγ/LXR induced expression of Abca1 to mediate cholesterol efflux. Inhibition of PI3K/Akt (using LY294002 or Akt inhibitor) results in only partial induction of Abca1 in response to ox-LDL and PPARγ/LXR agonists (rosiglitazone, T0901317, LXR623). (B) LRP1 integrates inflammatory signals and cholesterol homeostasis in macrophages. Inflammatory signals are initiated by the interaction between oxLDL and CD36/TLR4-6 in lipid rafts. In the setting of inflammation or cholesterol loading, increased proximity of LRP1 with activated SFKs in lipid rafts favors LRP1 tyrosine phosphorylation. This, in turn, activates a Shc1/PI3K/Akt/Pparγ/Lxrα axis that promotes Abca1 expression and cellular cholesterol export, which then leads to reduction of lipid raft cholesterol content and dissociation of LRP1 from the lipid raft, thus creating a negative feedback loop. LRP1 can also be cleaved by γ–secretase, thereby releasing the intracellular domain (ICD) which translocates to the nucleus where it suppresses inflammatory gene expression (Zurhove et al., 2008). Whether the LRP1 ICD, in a phosphorylated or unphosphorylated state, can also alter ABCA1 expression is currently unknown. CD36, cluster of differentiation 36; TLR, toll like receptor; MyD88, myeloid differentiation primary response protein 88; TRIF, TIR domain-containing adaptor protein inducing IFNβ; SFK, SRC family kinase. (C) LRP1 regulates clearance of apoptotic cells (AC) through efferocytosis. LRP1 coordinates with other receptors, such AXL and MerTK, and adaptors such as RanBP9 and GULP, to recognize and engulf apoptotic cells. Engulfment of apoptotic cells is a critical step in the activation of LXR-responsive genes, including Abca1 and Mertk. PS, protein S; CRT, cell surface calreticulin; GAS6, growth arrest-specific 6; AXL, receptor tyrosine kinase; MerTK, Proto-oncogene tyrosine-protein kinase; RANBP9, RAN binding protein 9; GULP, engulfment adaptor PTB domain containing one.
Additional files
-
Transparent reporting form
- https://doi.org/10.7554/eLife.29292.017