Clearance of beta-amyloid is facilitated by apolipoprotein E and circulating high-density lipoproteins in bioengineered human vessels
Figures
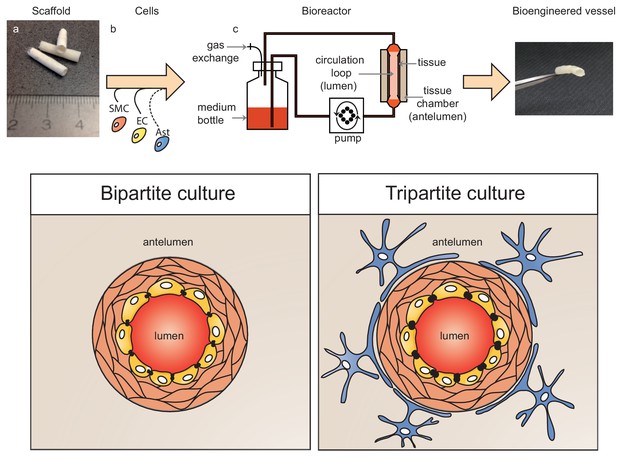
Schematic representation of bioengineered vessels.
Scaffolding material was prepared into a tubular shape approximately 2 mm in diameter and 15 mm long (a). Scaffolds were sequentially seeded with primary human umbilical vein smooth muscle cells (SMC, orange) and endothelial cells (EC, yellow) to form bipartite vessels, or with the addition of primary human astrocytes (Ast, blue) to form tripartite vessels (b). Bioengineered vessels were cultivated for approximately 4–6 weeks in a bioreactor containing a tissue chamber (c) on the anteluminal side to provide extravascular media, and a circulation loop containing endothelial media that flowed through the vessel lumen under pulsatile conditions using a peristaltic pump.
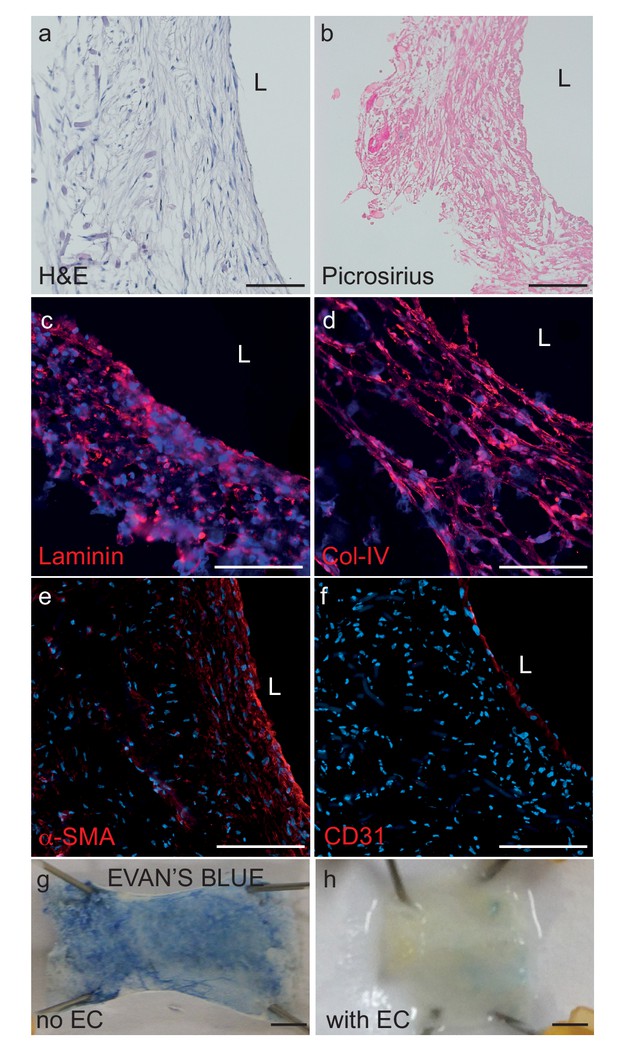
Histological structure of bipartite bioengineered vessels.
(a) Haematoxylin and Eosin staining revealed dense tissue formation composed of cells and extracellular matrix (ECM). (b) Picrosirius staining confirmed secretion of collagen. Further immunostaining against laminin (c) and collagen IV (d) confirmed ECM secretion by the cells. The expression of α-smooth muscle actin (α-SMA) (e) confirmed the smooth muscle phenotype of the cells in the inner layers, and CD31 positive staining (f) confirmed the presence of an endothelial cell monolayer on the luminal side of the bioengineered vessel. A functional endothelial barrier was confirmed using an Evans blue extravasation assay (g,h). Scale bars represent 200 μm (a–f) or 1 mm (g, h), L: lumen.
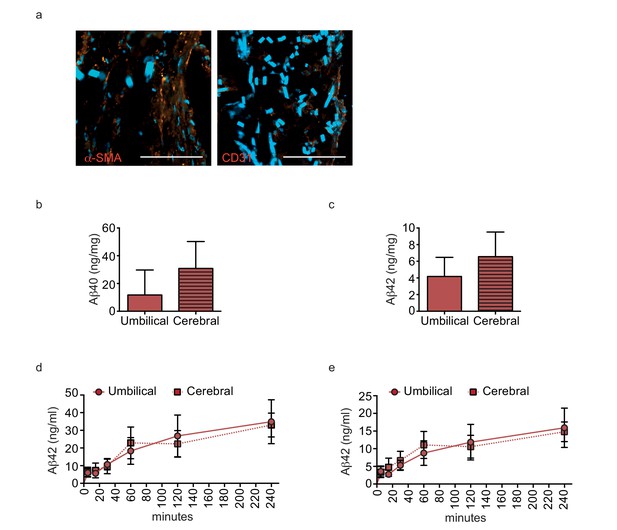
Aβ40 and Aβ42 accumulate similarly in tissues seeded with cells originating from umbilical cord or cortex.
Aβ40 and Aβ42 monomers (1 μM) were injected into the tissue chamber (antelumen) of tissues seeded with SMC from umbilical cords or cerebral arteries and incubated for 24 hr under flow conditions before measuring Aβ deposition within bioengineered vessels with ELISA (a–b). Aβ40 and Aβ42 monomers (1 μM) were injected into the tissue chamber of tissues seeded with HUVEC or cortical microvascular EC (hCMEC). The levels of transported Aβ were measured by ELISA from samples collected from the luminal circulating medium at the indicated times over 4 hr (c–d). Graphs represent mean ± SEM for at least four independent tissues.
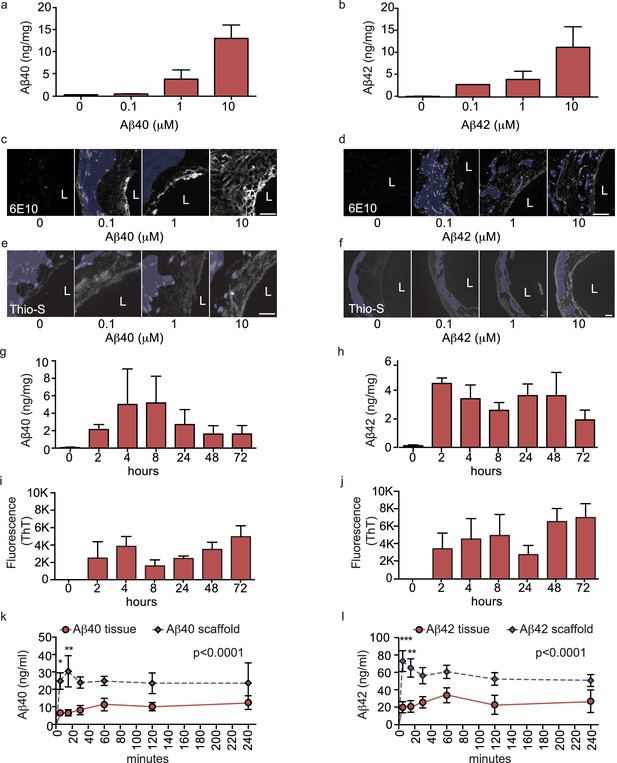
Aβ40 and Aβ42 accumulation within and transport through bipartite vessels.
Aβ40 and Aβ42 monomers (0, 0.1, 1.0 and 10 μM) were injected into the tissue chamber (antelumen) and incubated for 48 hr under flow conditions (a–f). Aβ deposition within bioengineered vessels was measured using ELISA (a–b), immunostaining with the anti-Aβ antibody 6E10 Aβ (d–e), and Thioflavin-S staining (e,f). To determine the kinetics of CAA formation, Aβ40 and Aβ42 monomers (1 μM) were injected into the tissue chamber and incubated for the indicated times before measuring Aβ tissue concentrations by ELISA (g,h) or aggregation within the tissue using Thioflavin-T (i,j). Aβ transport was measured after injecting Aβ40 and Aβ42 monomers (1 μM) into the anteluminal chamber and sampling media from the circulation (luminal) chamber at the indicated times (k,l). Graphs represent mean ±SEM for at least four independent tissues **p=0.01 and ***p=0.001. Bars represent 50 μm, L: lumen.
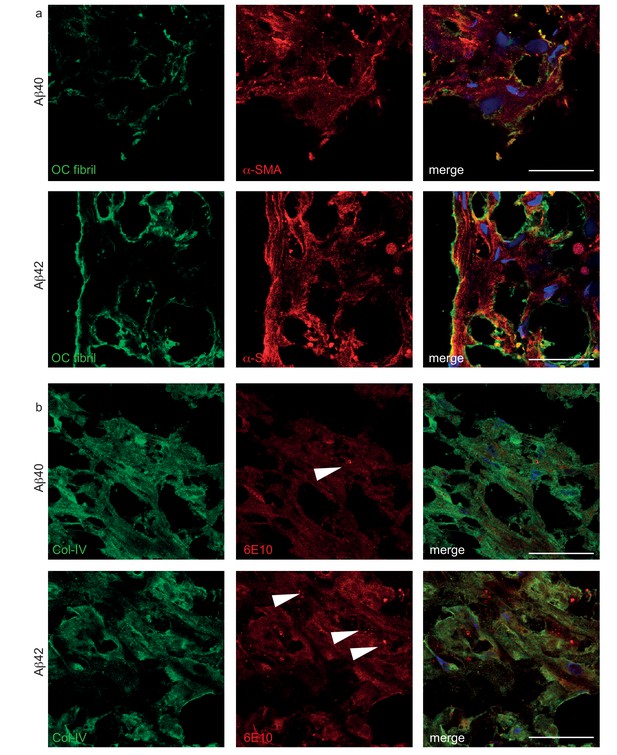
Histological structure of Aβ deposited in bioengineered vessels.
(a) Confocal immunostaining against OC fibrils and α-SMA confirmed the deposition of fibrillized Aβ40 and Aβ42 outside of the cells. (b) Confocal immunostaining against 6E10 and Col-IV confirmed the deposition of Aβ40 and Aβ42 in the extracellular matrix and vesicular (white arrow) localization within the cells. Bars represent 200 μm.
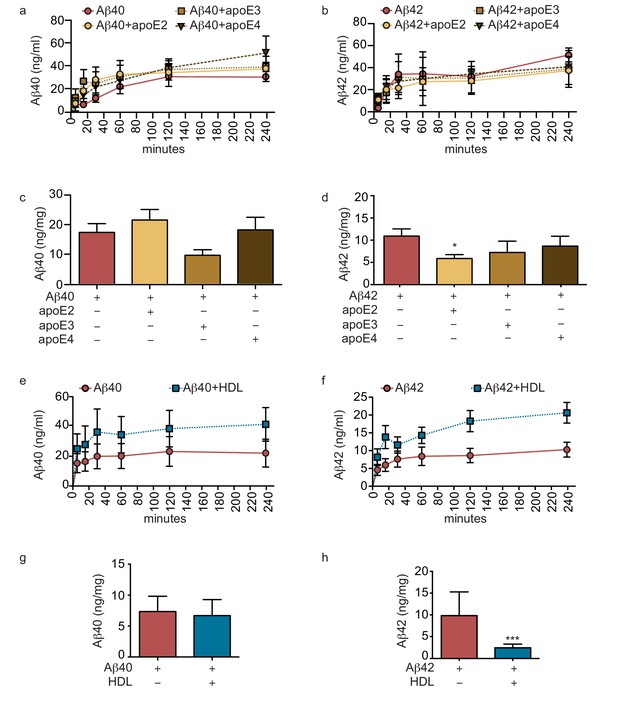
Lipoproteins reduce Aβ42 accumulation within bioengineered bipartite vessels.
Aβ40 and Aβ42 monomers (1 μM) were incubated without or with recombinant apoE (ratio 25:1) for 2 hr at 37°C before injection into the anteluminal tissue chamber. The levels of transported Aβ was measured by ELISA from samples collected from the luminal circulating medium at the indicated times over 4 hr (a–b), and from vascular tissue collected 24 hr after Aβ injection (c–d). Aβ40 and Aβ42 monomers (1 μM) were injected into the anteluminal chamber in the absence or presence of 200 μg/ml of luminal circulating HDL. The levels of transported Ab (e–f) and from vascular tissues (g–h) were measured as above. Graphs represent mean ±SEM for at least four independent tissues. *p=0.05, **p=0.01 and ***p=0.001.
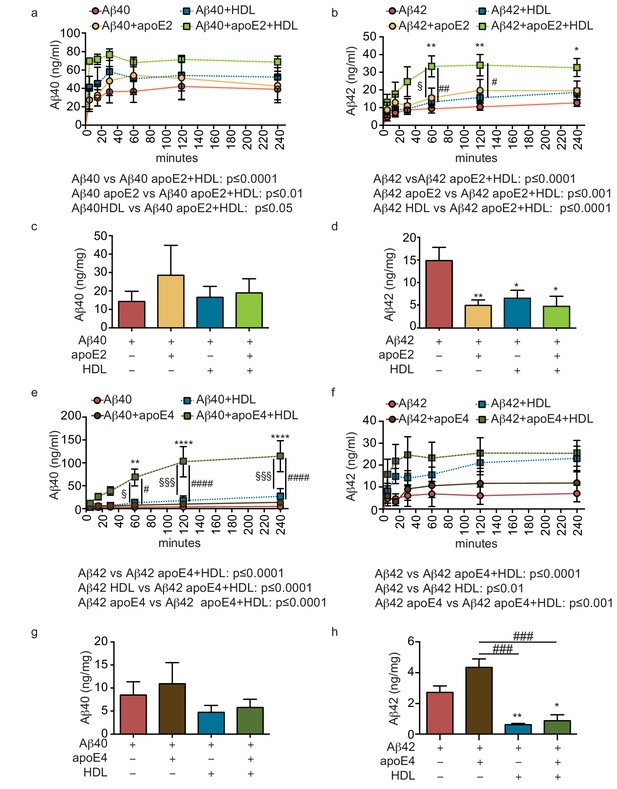
HDL facilitates Aβ42 transport and reduces accumulation in bioengineered bipartite vessels in the presence of recombinant apoE.
Aβ40 and Aβ42 monomers (1 μM) were incubated without or with recombinant apoE2 (a–d) or apoE4 (e–h) (ratio 25:1) for 2 hr at 37°C before injecting into the tissue chamber in the absence or presence of 200 μg/ml of circulating HDL, and evaluating transported (a–b, e–f)) and accumulated (c–d, g–h) by ELISA. Data for Aβ and Aβ +HDL transport presented in Figure 5a–b,e–f represent data generated from specific apoE2 and apoE4 experiments, which were extracted, pooled and graphed in Figure 4e–f to represent total transport data. Graphs represent mean ±SEM for at least four independent tissues. *, § and # p=0.05, ## and **p=0.01 and ***p=0.001.
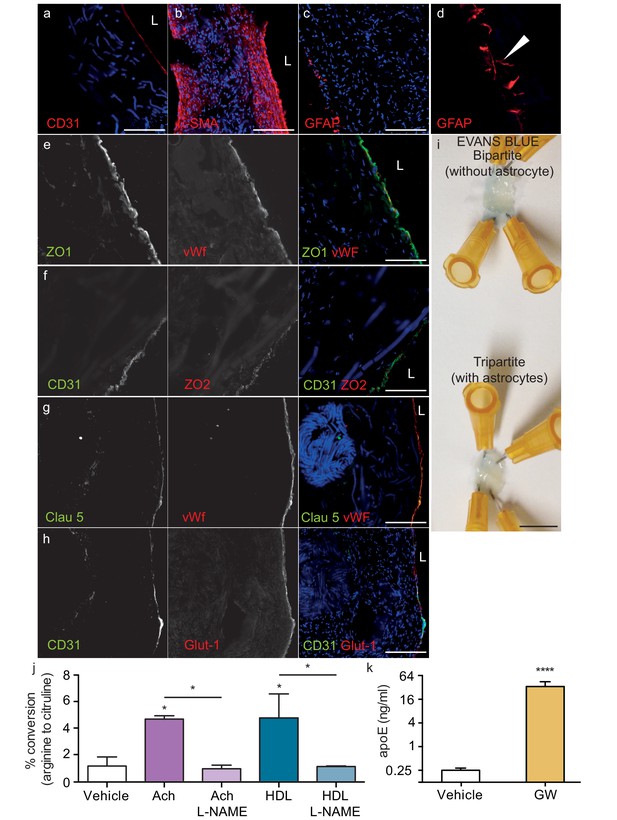
Histological structure of tripartite bioengineered vessels.
Immunostaining against CD31 confirmed the presence of an EC monolayer on the luminal side of tripartite vessels (a), the expression of α-SMA confirmed the smooth muscle phenotype of cells in the inner layers (b), and the expression of GFAP confirmed the presence of astrocytes on the anteluminal layers (c) of bioengineered tripartite vessels. (d) Higher magnification image of a specific GFAP-positive area showing astrocyte end-feet like structures. The EC barrier was further analysed using immunostaining against the tight junction proteins ZO-1 (e), ZO-2 (f) and Claudin 5 (g), as well as the BBB-specific glucose transporter (Glut)−1 (h). Evans blue staining confirmed a tight endothelium (i). EC function was confirmed by measuring NO secretion after either acetylcholine (Ach) or HDL stimulation, in the absence or presence of 1 mM of the eNOS inhibitor L-NAME (j). Astrocyte function was confirmed by treating tissues with 1 μM LXR agonist GW3965 for 72 hr and measuring the levels of astrocyte-derived apoE secreted into the medium (k). Bars represent 200 μm (a–h) or 1 mm (i), L: lumen and graphs represent mean ±SEM for at least four independent tissues. *p=0.05, **p=0.01 and ***p=0.001.
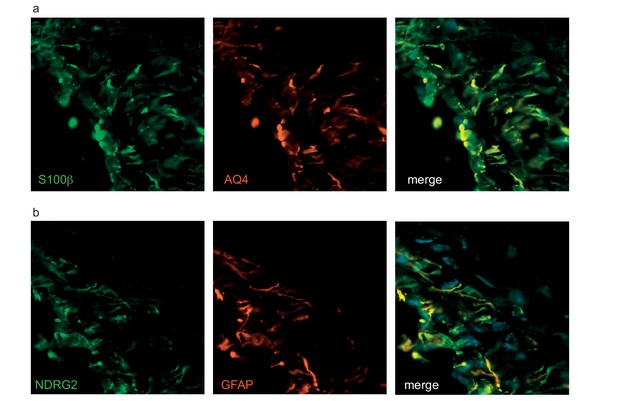
Astrocytes in bioengineered tissues express aquaporin four and NDRG2.
The astrocytes in tripartite tissue were further analysed with immunostaining against aquaporin 4 (AQ4) (a) or NDRG2 (b) with astrocytic markers (GFAP, S100β). Bars represent 50 μm.
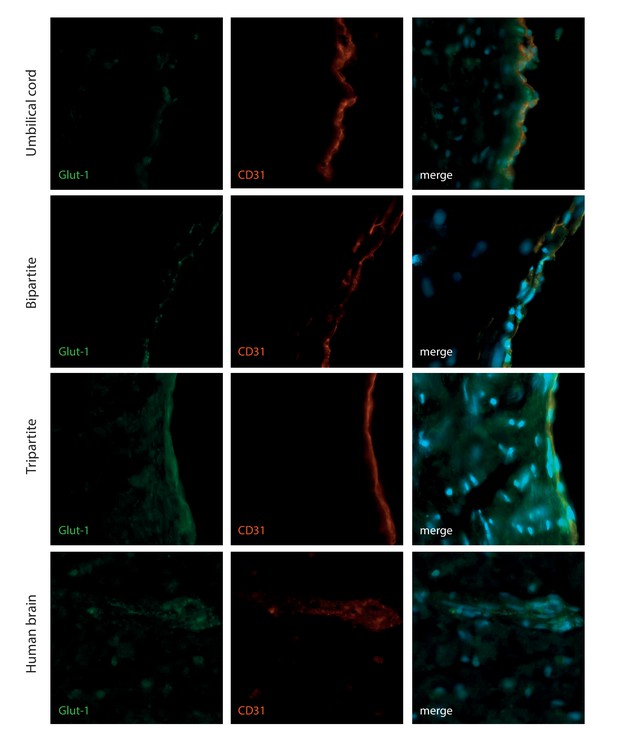
HUVEC express the brain Glut-1 transporter when culture in bioengineered vessels.
The EC bioengineered tissues were further analysed with immunostaining against Glut-1 and the EC marker CD31 in comparison with human brain and umbilical cord. Bars represent 200 μm.
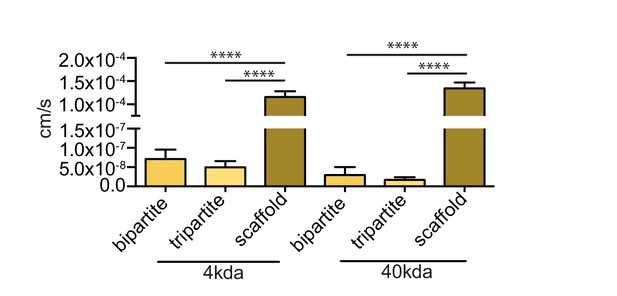
Endothelia of bioengineered vessels are impermeable to FITC-dextran.
Barrier integrity was assessed by measuring permeability of 250 μg/ml of 4 kDa or 40 kDa FITC-dextran circulated through the lumen for 1 hr. Graphs represent mean ±SEM for at least three independent tissues. ****p=0.0001.
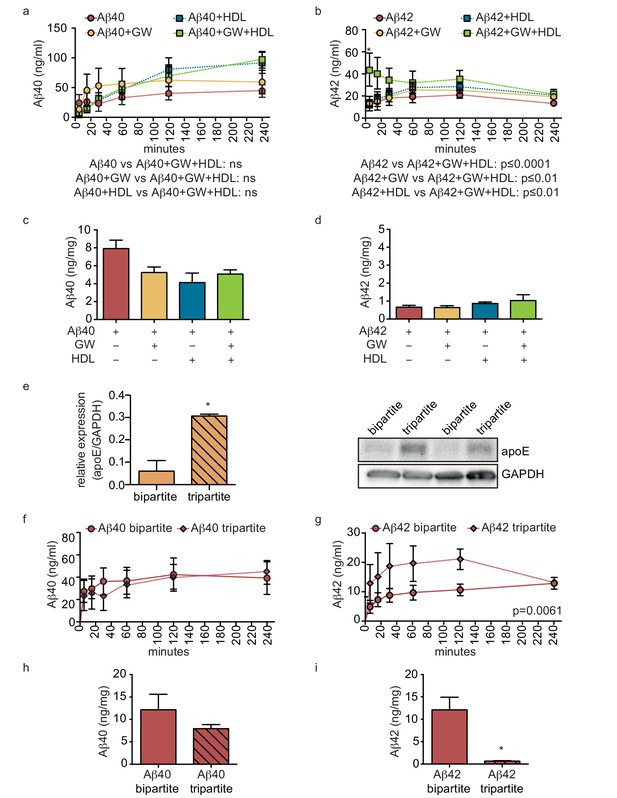
HDL facilitates Aβ transport and reduces accumulation in bioengineered tripartite vessels expressing native astrocyte apoE.
(a–d) Tripartite vessels were treated with the LXR agonist GW3965 (0.8 μM) for 72 hr to stimulate astrocyte apoE3 secretion. Aβ40 and Aβ42 monomers (1 μM) were injected in the tissue chamber in the absence or presence of 200 μg/ml of circulating HDL, with or without GW3965. The levels of transported Aβ was measured by ELISA from samples collected from the luminal circulating medium at the indicated times over 4 hr (a–b), and from vascular tissue collected 24 hr after Aβ injection (c–d). ApoE protein level in bioengineered tissues was quantified by Western blotting (e) with a representative blot. Aβ transport (f–g) and tissue accumulation (h–i) were directly compared between bipartite and tripartite bioengineered vessels as above. Graphs represent mean ±SEM for at least four independent tissues. *p=0.05, **p=0.01 and ***p=0.001.
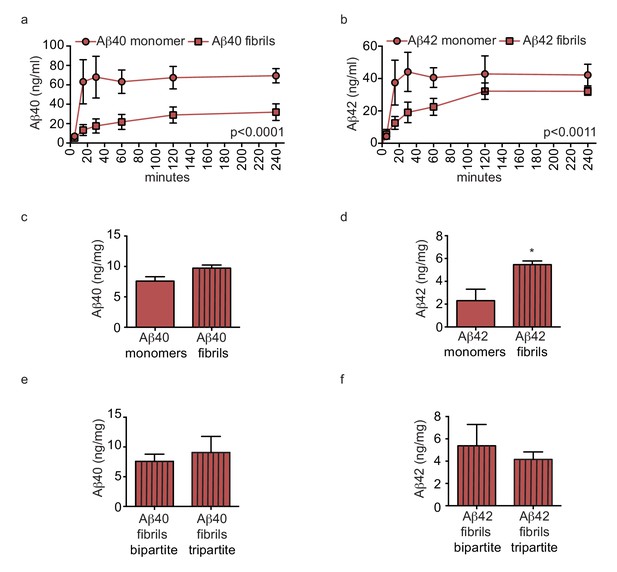
Amyloid fibrils are transported more slowly but accumulate similarly between bipartite and tripartite tissues.
(a–d) Pre-aggregated or monomeric Aβ40 and Aβ42 (1 μM) were injected in the tissue chamber. The levels of transported Aβ were measured by ELISA from samples collected from the luminal circulating medium at the indicated times over 4 hr (a–b), and from vascular tissue collected 24 hr after Aβ injection (c–d). Aβ tissue accumulation (e–f) was directly compared between bipartite and tripartite bioengineered vessels as above. Graphs represent mean ±SEM for at least four independent tissues. *p=0.05, **p=0.01 and ***p=0.001.
Additional files
-
Transparent reporting form
- https://doi.org/10.7554/eLife.29595.016





