Functional dichotomy in spinal- vs prefrontal-projecting locus coeruleus modules splits descending noradrenergic analgesia from ascending aversion and anxiety in rats
Figures
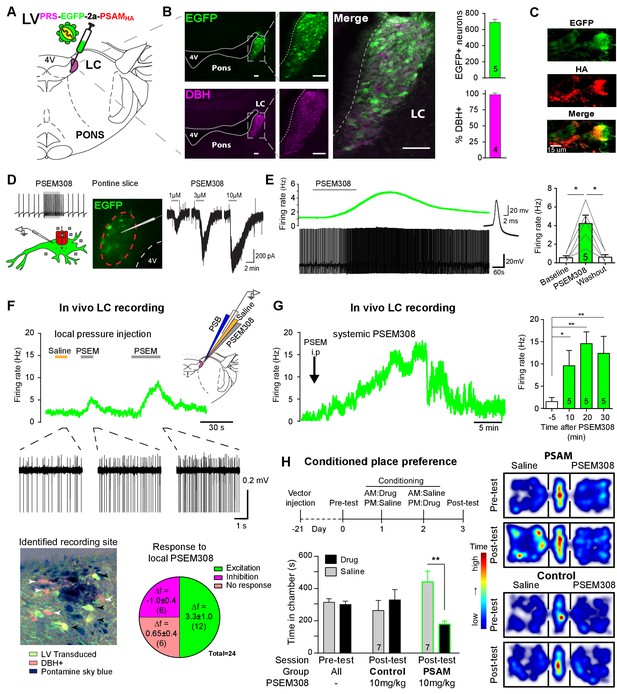
PSAM-mediated chemogenetic activation of LC neurons in vivo.
(A) Strategy using direct stereotaxic injection of lentiviral vector to express the excitatory ionophore PSAM in noradrenergic LC neurons. (B) Selective transduction of LC demonstrated by immunohistochemistry (IHC) for EGFP and dopamine β-hydroxylase (DBH) with 690 EGFP+ neurons per LC of which 98% were DBH+ (scale bar 100 µm). (C) PSAM expression was demonstrated using IHC for the HA tag. (scale bar 15 µm) (D) Schematic of PSEM308-mediated excitation of transduced neurons expressing PSAM. Patch clamp recordings from EGFP+ LC neurons in acute pontine brain slices. Perfusion of PSEM308 evoked concentration-dependent inward currents. (E) PSEM308 (3 µM) increased rate of firing of transduced LC neurons. Inset shows 16 overlaid action potentials. Group data shows increase in firing produced by PSEM308 (3 μM). (F) Extracellular recordings from LC neurons in anaesthetised rats using multi-barrel recording electrodes allowing local pressure ejection of PSEM308/saline/pontamine sky blue (PSB). Traces show graded excitation of an identified LC neuron by PSEM308. Recording sites were subsequently histologically identified by the PSB staining within the LC (transduced cells identified by IHC for EGFP (black arrowheads) and DBH (white arrowheads). The response to local PSEM308 was categorised as excitation or inhibition if it changed firing rate by more than 3 SD from the baseline rate. Application of PSEM308 produced an excitation in 50% of LC neurons. We found a second group of neurons that showed no response, presumably as they were not transduced. A third group showed an inhibition of spontaneous firing in response to local PSEM308 application. (G) Kinetics of the excitatory response to systemic PSEM308 administration (10 mg/kg i.p). (H) Timeline of conditioned place aversion protocol to assess influence of chemogenetic activation of LC neurons on behaviour. In PSAM expressing rats, PSEM308 (10 mg/kg) caused conditioned place aversion but had no effect on control animals. Representative heat maps of rat position in the pre-test and post-test after PSEM308 with bilateral LC transduction with LVPRS-EGFP-2A-PSAM HA or control LVPRS-EGFP All data analysed with repeated measures ANOVA (one or two way as appropriate) with Bonferroni’s post hoc testing (*p<0.05, **p<0.01). (See also Figure 1—figure supplements 1, 2 and 3)
-
Figure 1—source data 1
Figure 1 source data.
- https://doi.org/10.7554/eLife.29808.006

Generation of a traceable version of the PSAM in an expression cassette with enhanced fluorescence.
(A) Schematic of the PSAM (PSAML141F,Y115F:5HT3 HC) and EGFP co-expression plasmid. Top row shows 4 EGFP+ transfected and 10 EGFP- PC12 cells. The bottom row shows [Ca2+]i increases in the transduced cells to the selective agonist PSEM89s. [Ca2+]i measured by Fura2 340:380 fluorescence ratio - F2R. (B) [Ca2+]i responses to PSEM89s in 4 EGFP+ and 5 EGFP- PC12 cells. (C) The maximum Ca2+ transient evoked by PSEM89s is sensitive to the length of C-terminal tag on PSAM. 18 amino acids remain at the C-terminal end of PSAM when the 2A peptide is downstream of PSAM. This C-terminal residue reduced the maximum PSEM89s-evoked response. There was no difference between the original PSAM-IRES-EGFP plasmid and the HA-tagged expression cassette EGFP-2A-PSAMHA (p<0.0001 Kruskal Wallis test with Bonferroni’s multiple comparison NS p>0.05, **p<0.01, ****p<0.0001). (D) The C-terminal HA tag does not interfere with the concentration response relationship (p=0.6384 sum-of-squares F test). EC50: PSAM-IRES-EGFP - 3.37 ± 0.04 vs EGFP-2A-PSAMHA - 3.86 ± 0.09 µM. (E) The upstream EGFP-2A sequence produces a significantly brighter fluorescent signal than the IRES linker (Mann-Whitney test p<0.0001). Calcium transients are shown as mean ± SEM and fluorescence data as median with interquartile intervals. N equals the number of cells analysed from at least three replicated experiments.
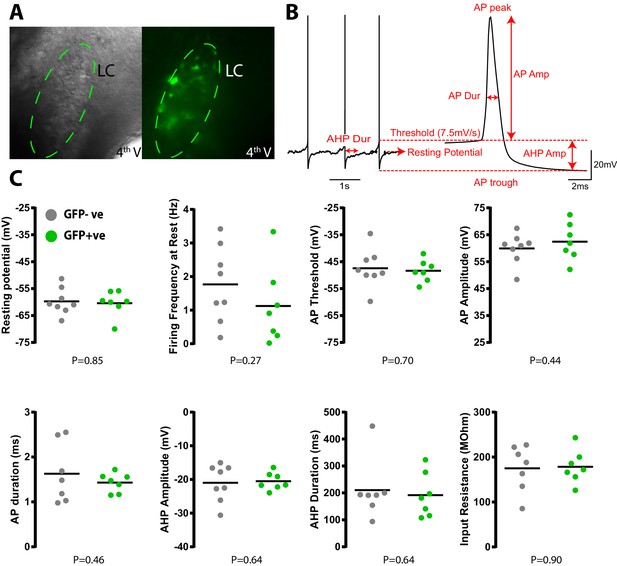
Transduction with PSAM does not alter LC neuronal electrophysiological properties.
(A) Whole cell current clamp recordings from LC neurons in pontine slices 2 weeks after in vivo transduction with LVPRS-EGFP-2A-PSAM. (B) Schematic of action potential analysis. All spike measurements are taken with reference to the AP threshold defined as the point where velocity of depolarisation exceeded 7.5Vs−1. Data from 25 action potentials were averaged per cell. Input resistance was measured in voltage clamp from the average of 10 current responses to a 10 mV membrane hyperpolarization (C) No quantitative difference was observed in the intrinsic electrical properties between transduced (N = 7) and non-transduced (N = 8) LC neurons (unpaired t-test).

Simultaneous recording of one excited and one inhibited LC neuron in vivo with focal PSEM308 (1 mM) pressure application.
https://doi.org/10.7554/eLife.29808.005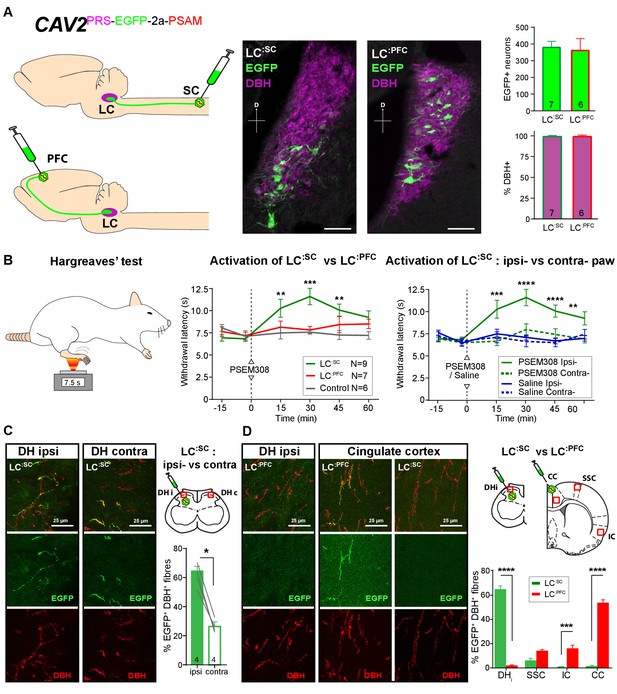
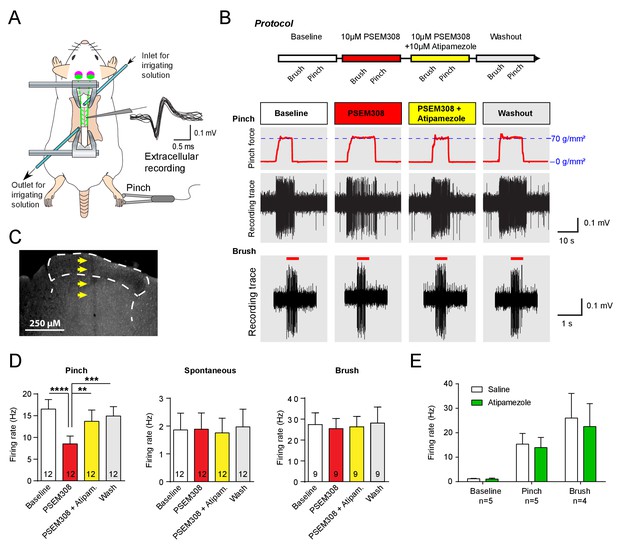
Activation of descending LC:SC but not ascending LC:PFC is anti-nociceptive.
(A) Retrograde transduction strategy with canine adenoviral vectors (CAV2) to target noradrenergic LC neurons with projections to the spinal cord (LC:SC) or prefrontal cortex (LC:PFC). Similar numbers of LC neurons were transduced by injections in lumbar spinal cord (L3-4, 380) and prefrontal cortex (361). In both cases, >99% of neurons were DBH+. (scale bar 100 µm). (B) The Hargreaves’ test (radiant heat) was used to measure hind- paw withdrawal latency. PSEM308 activation of LC:SC but not LC:PFC caused a robust anti-nociception (increase in withdrawal latency). The LC:SC anti-nociceptive effect was only seen in the ipsilateral hind paw (same side as spinal CAV2 injection). (C) Representative images of double immunofluorescence showing DBH-positive fibres in the spinal cord that were anterogradely filled with EGFP after transduction of LC:SC neurons. Quantification of the percentage of DBH+ fibres that were EGFP+ showed a three-fold higher density of EGFP-labelled fibres ipsilateral to vector injection (Mann-Whitney test, *p<0.05) which is consistent with the lateralized analgesic effect seen in (B). (D) Representative images and comparison of the percentage of EGFP labelled fibres in cortical areas and the spinal cord after LC:SC or LC:PFC transduction (N = 3 in each group). Data analysed with two-way repeated measures ANOVA with Bonferroni’s post hoc tests (**p<0.01, ***p<0.001, ****p<0.0001).
-
Figure 2—source data 1
Figure 2 source data.
- https://doi.org/10.7554/eLife.29808.008
Acivation of LC:SC selectively supresses nociceptive excitation of wide dynamic range neurons of the dorsal horn via an alpha2-adrenoceptor in naive rats.
(A) Spinal extracellular recording from wide dynamic range neurons in the dorsal horn. (B) Experimental protocol for noxious pinch (calibrated forceps) or non-noxious brush applied to the hindpaw during spinal drug application. Original traces of pinch or brush responses from WDR neurons during application of PSEM308 (10 µM) or PSEM308 (10 µM) + atipamezole (10 µM) (C) Posthoc histology of the spinal dorsal horn outlining the electrode track. (D) In LC:SC transduced rats, spinal superfusion of PSEM308 (10 µM) caused a significant attenuation of the response to noxious pinch which was prevented by Atipamezole (10 µM) indicating a spinal α2-adrenoceptor mechanism (two-way repeated measures ANOVA with Bonferroni’s post hoc test, **p<0.01,. ***p<0.001, ****p<0.0001). Application of PSEM308 or atipamezole does not change spontaneous firing or brush evoked discharge of WDR neurons. (E) Quantification of neuronal responses to pinch, brush and spontaneous discharge frequency during saline or atipamezole (10 µM) application (paired t-test p>0.05). Data are presented as mean ±SEM.
-
Figure 3—source data 1
Figure 3 source data.
- https://doi.org/10.7554/eLife.29808.010
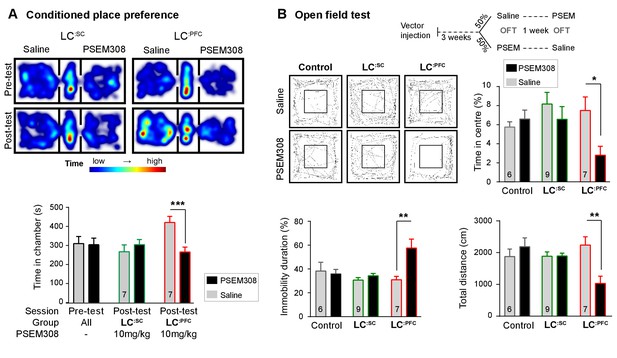
Chemogenetic activation of LC:PFC neurons is aversive and anxiogenic unlike LC:SC.
(A) Representative heat maps showing rat location in the place preference arena before and after conditioning for LC:SC (left) and LC:PFC (right) transduced rats (outer compartments 30 × 30 cm). Activation of LC:PFC with PSEM308 (10 mg/kg) caused conditioned aversion to the paired chamber (two-way repeated measures ANOVA with Bonferroni’s multiple comparison between the conditioned and unconditioned chamber, ***p<0.001) and had no effect on LC:SC transduced rats. Pre-test data pooled for graphical presentation (B) Cross-over design for open-field test (OFT) and tracks showing activity after PSEM308 (10 mg/kg) or saline (inner square 30 × 30 cm). PSEM308 reduced the time LC:PFC rats spent in the centre of the arena and increased the time these rats were immobile. In contrast, PSEM308 had no effect on LC:SC transduced rats (two-way RM- ANOVA with Bonferroni’s multiple comparison test, PSEM308 vs saline, *p<0.05, **p<0.01).
-
Figure 4—source data 1
Figure 4 source data.
- https://doi.org/10.7554/eLife.29808.012
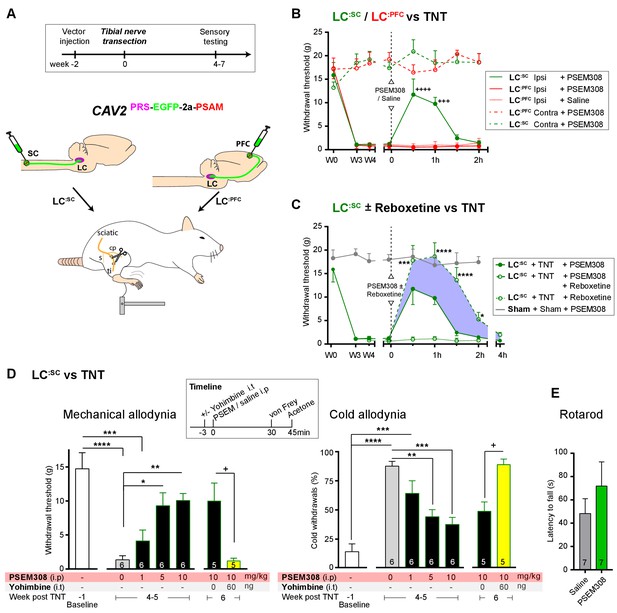
Chemogenetic activation of LC:SC is analgesic via a spinal a2-mechanism (behaviour).
(A) Experimental timeline of vector injection and the tibial nerve transection model of neuropathic pain (s = sural, cp = common peroneal and ti = tibial nerves). (B–C) Quantification of mechanical withdrawal threshold (von Frey, after Chaplan et al., 1994). (B) Nerve injured animals showed a mechanical sensitisation of the ipsilateral limb. Activation of LC:SC neurons (PSEM308 (10 mg/kg)) attenuated the neuropathic sensitisation (comparison vs baseline +++p<0.001, ++++p<0.0001, N = 6 all groups). In contrast, activation of the LC:PFC had no effect on neuropathic sensitisation at any time point (C). Co-application of reboxetine i.t (1 mg/kg) enhanced the analgesic effect of LC:SC activation shown in panel (B). (D) Timeline of sensory testing in weeks 4–6. The analgesic effect of LC:SC activation on mechanical and cold allodynia is dose-dependent and is completely blocked by intrathecal yohimbine the α2-adrenoceptor antagonist (paired t-test, +p<0.05). (E) Activation of LC:SC had no effect on the latency to fall in the rotarod test - a measure of motor impairment (paired t-test, p>0.05). All analysis by repeated measures ANOVA (one- or two-way) with Bonferroni’s multiple comparison, *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 unless otherwise stated (see also Figure 5—figure supplement 1)
-
Figure 5—source data 1
Figure 5 source data.
- https://doi.org/10.7554/eLife.29808.015
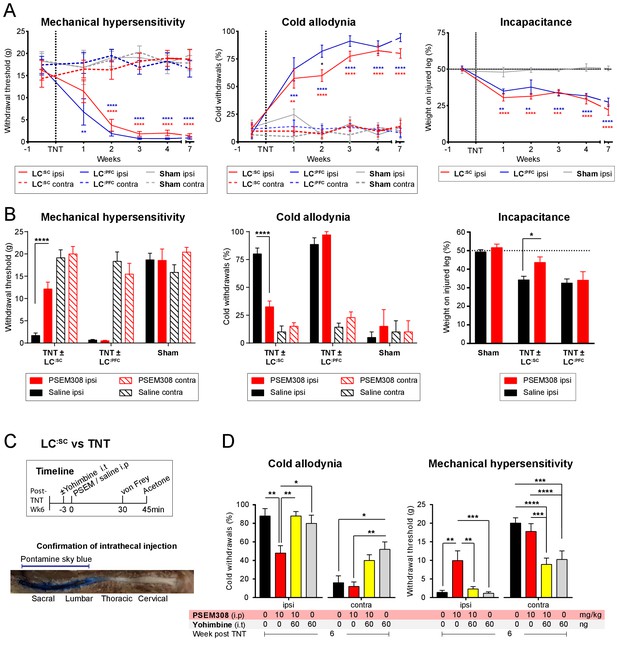
Development of neuropathic pain phenotype after tibial nerve transection and analgesic profile of chemogenetic activation of LC:SC vs LC:PFC.
(A) Progression of mechanical sensitivity, cold sensitivity and incapacitance before and for 7 weeks after nerve injury (LC:SC N = 8, LC:PFC N = 7, sham N = 6, 2-way repeated measures ANOVA with Bonferroni’s multiple comparison, *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001, red stars: LC:SC vs Sham, blue stars: LC:PFC vs Sham, black star LC:SC vs LC:PFC). (B) PSEM308 (10 mg/kg) alleviates ipsilateral hypersensitivity and improves incapacitance only in the LC:SC group 4 weeks after nerve injury (two-way repeated measures ANOVA with Bonferroni’s multiple comparison PSEM308 vs saline, *p<0.05, ****p<0.0001, LC:SC N = 8, LC:PFC N = 7, sham N = 6). (C) Timeline of sensory testing and representative image of an intrathecal injection of pontamine sky blue 5 min before trans-cardiac perfusion (N = 3). Dye was restricted to the caudal region of the spinal cord. (D) Ipsilateral chemogenetic analgesia was blocked by pre-treatment with yohimbine (repeated measures ANOVA with Bonferroni’s multiple comparison, *p<0.05, **p<0.01, ***p<0.001). Note also that Intrathecal yohimbine (60 ng i.t) lowered the mechanical and cold thresholds contralateral to nerve injury in the presence or absence of PSEM308 (10 mg/kg i.p) (two-way repeated measures ANOVA with Bonferroni’s multiple comparison, *p<0.05, **p<0.01). Unmasking of contralateral sensitization was used as a positive control for successful intrathecal delivery of yohimbine (Hughes et al., 2013). Data are presented as mean ± SEM.
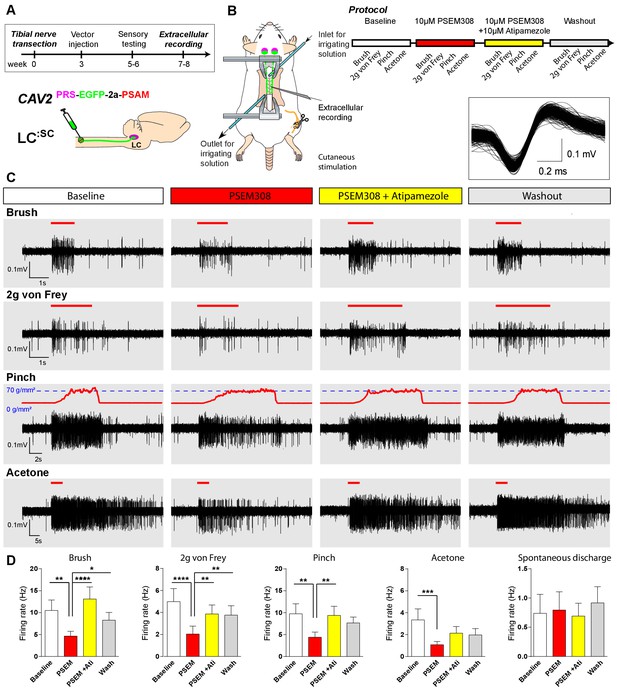
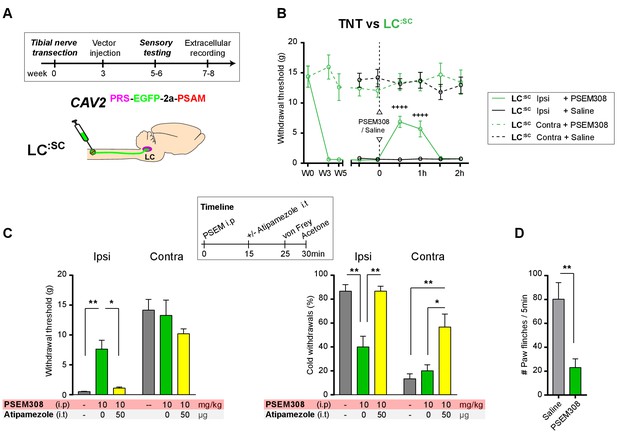
Chemogenetic activation of LC:SC is analgesic via a spinal α2-mechanism (cell recording).
(A) Experimental timeline of vector injection and the tibial nerve transection. CAV2PRS-EGFP-2A-PSAM was injected to the spinal cord after the neuropathic phenotype was fully developed in week 3. (B) Spinal extracellular recording and experimental protocol. The insert shows the waveform of 500 action potentials from one discriminated unit from the recording traces below. (C) Representative recording traces (containing two discriminated WDR units) from the spinal dorsal horn with responses to mechanical and cold stimuli during spinal drug application. (D) Spinal superfusion of PSEM308 (10 µM) caused a significant attenuation of the response to all stimuli which was prevented by Atipamezole (20 µM) indicating a spinal a2-adrenoceptor mechanism. All data analysed with two-way repeated measures ANOVA with Bonferroni’s post hoc testing (**p<0.01, ***p<0.001, ****p<0.0001).
-
Figure 6—source data 1
Figure 6 source data.
- https://doi.org/10.7554/eLife.29808.017
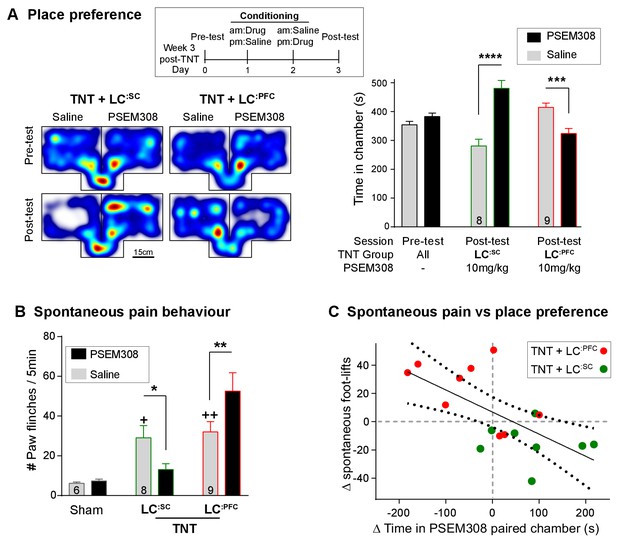
Chemogenetic activation of LC:SC is rewarding in neuropathic pain model whilst activation of LC:PFC neurons exacerbates spontaneous pain behaviour.
(A) Timeline and representative location heat maps for a rat in CPP arena. In TNT animals, activation of LC:SC now induces a robust place preference, whereas activation of LC:PFC continues to produce aversion (two-way RM-ANOVA with Bonferroni’s multiple comparison between the conditioned and unconditioned chamber, ***p<0.001, ****p<0.0001). (B) TNT animals showed an increased frequency of paw flinches - a measure of spontaneous pain (saline treatment, RM-ANOVA with Bonferroni‘s multiple comparison vs sham, +p<0.05, ++p<0.01). Activation of LC:SC reduced and LC:PFC increased the frequency of flinches (two-way RM-ANOVA with Bonferroni’s multiple comparison PSEM308 (10 mg/kg) vs saline, *p<0.05, **p<0.01). (C) Across both LC:SC and LC:PFC groups there was a correlation between the degree of modulation of spontaneous pain and the chamber preference (r = −0.65, p<0.01). (See also Video 1).
-
Figure 7—source data 1
Figure 7 source data.
- https://doi.org/10.7554/eLife.29808.020
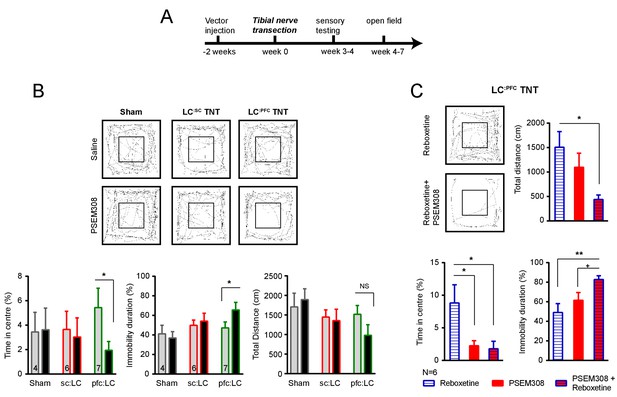
Chemogenetic stimulation of the LC:PFC module is anxiogenic in chronic pain.
(A) Timeline of nerve injury and open-field testing (OFT). (B) Representative heat maps showing activity in the OFT after saline or PSEM308 (10 mg/kg) injection for sham, LC:SC and LC:PFC groups. Chemogenetic activation of LC:PFC reduced the time spent in the centre of the arena and increased immobility but PSEM308 had no effect on LC:SC transduced rats or sham rats (two-way repeated measures ANOVA with Bonferroni’s multiple comparison PSEM308 vs saline, *p<0.05). (C) Co-application of reboxetine (1 mg/kg) and PSEM308 (10 mg/kg) in LC:PFC rats increased immobility time, decreased the distance travelled and decreased the time spent in the centre of the arena as compared to reboxetine alone. Co-application of reboxetine and PSEM308 (10 mg/kg) also increased immobility as compared to PSEM308 alone (paired t-test PSEM308 vs PSEM308 + Reboxetine, +p<0.05).
Chemogenetic activation of LC:SC as an intervention for established neuropathic pain.
(A) Experimental timeline of vector injection and the tibial nerve transection. CAV2PRS-EGFP-2A-PSAM was injected after the neuropathic phenotype was fully developed in week 3. (B) Quantification of mechanical withdrawal threshold (von Frey, after Chaplan et al., 1994). Activation of LC:SC neurons (PSEM308 (10 mg/kg)) in week 5 attenuated the neuropathic sensitisation (repeated measures two-way ANOVA with Bonferroni’s multiple comparison, ++++p<0.0001, N = 7). (C) Timeline of sensory testing in week 6. The analgesic effect of LC:SC activation on mechanical and cold allodynia is completely blocked by intrathecal atipamezole the a2-adrenoceptor antagonist (repeated measures two-way ANOVA with Bonferroni’s multiple comparison, *p<0.05, **p<0.01, N = 7). (D) Activation of LC:SC reduced the frequency of spontaneous flinches (paired t-test, **p<0.01).
-
Figure 8—source data 1
Figure 8 source data.
- https://doi.org/10.7554/eLife.29808.023
Videos
Chemogenetic activation of LC:SC module suppresses spontaneous pain behaviour in neuropathic pain model.
https://doi.org/10.7554/eLife.29808.021Tables
| Reagent or Resource | Source | Identifier |
|---|---|---|
| Antibodies | ||
| Chicken polyclonal anti-EGFP | Abcam | #AB13970 |
| Mouse monoclonal anti Dopamine-β-hydroxylase (DBH) | Chemicon | #MAB308 RRID:AB_2245740 |
| Rabbit monoclonal anti-HA tag | Cell Signalling | #C29F4 RRID:AB_10693385 |
| Chemicals, Peptides and Recombinant Proteins | ||
| (Z)−4-Hydroxytamoxifen | Sigma | H7904 |
| urethane (ethyl carbamate) | Sigma | #U2500 |
| PSEM308/89S | Initially: Peter Lee from Sternson group Subsequently: Custom synthesis by Apex Scientific Inc | N/A |
| Yohimbine hydrochloride | Tocris | # 1127 |
| Reboxetine mesylate | Tocris | # 1982 |
| Atipamezole hydrochloride | Pfizer, Antisedan | |
| Recombinant DNA | ||
| rAAV-syn::FLEX- rev::PSAML141F, Y115F:5HT3HC- IRES-GFP | Addgene | 32477 |
| pCAVΔE3Sce | Kremer lab | N/A |
| pCMV-EGFP-2A-PSAM HA | GeneArt AG | N/A |
| Cell lines | ||
| Lenti-X293T cells | Clonetech | # 632180, identity confirmed by manufacturer and certified as mycoplasma free |
| BJ5183 cells | Agilent Technologies | # 50-125-019, # 632180, identity confirmed by manufacturer and certified as mycoplasma free |
| DK-SceI | Kremer lab | Mycoplasma free. |
| Software and Algorithms | ||
| Ethovision ET | Noldus | N/A |
| Photoshop CS5 | Adobe | N/A |
| Illustrator CS5 | Adobe | N/A |
| Prism7 | GraphPad | N/A |
| Excel | Microsoft | N/A |
| A plasmid Editor (ApE v2.0.51) | M. Wayne Davis | http://biologylabs.utah.edu/jorgensen/wayned/ape/ |
| Spike2 v7 | Cambridge Electronic Design | N/A |
| Fiji | ImageJ | https://fiji.sc |
Additional files
-
Supplementary file 1
List of animals used in electrophysiological and behavioural studies.
- https://doi.org/10.7554/eLife.29808.024
-
Transparent reporting form
- https://doi.org/10.7554/eLife.29808.025





