Distinct spatial coordinate of visual and vestibular heading signals in macaque FEFsem and MSTd
Figures
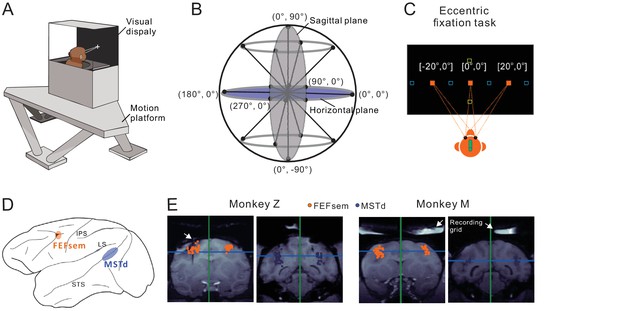
Experimental setup and anatomical locations.
(A) Monkeys were trained to maintain fixation while seated in a virtual-reality setup. The apparatus consists of a 6-DOF motion platform that can translate in any direction. Visual display, monkey chair, and the field coil system are mounted on the motion base. Monkeys are head-fixed within the system. (B) Heading directions are varied in the horizontal and sagittal planes. (C) Eccentric fixation experimental paradigm. The fixation spot is presented at one of three locations: left (20°), center (0°), or right (20°) in the horizontal plane (filled origin square), or up (20°), center (0°), or down (20°) in the vertical plane (open yellow square). The open blue squares are fixation locations sometimes presented at ±30° or ±10° in an additional experiment. (D) Schematic illustration of the locations of the two cortical areas studied: FEFsem at the posterior portion of the arcuate sulcus, and MSTd at the posterior part of the superior temporal sulcus. (E) Coronal sections exhibiting the recording sites from the two monkeys (Monkey Z and M) in FEFsem (orange dots) and MSTd (blue dots). Note that all the recording sites are projected onto one single coronal plane, causing some points artificially appeared outside the region of interest (ROI). The white arrow in the first panel indicates the position of an electrode probe during MRI scanning. White arrows in the last two panels indicate the recording grid used to guide electrode penetrations in the current recording experiments.
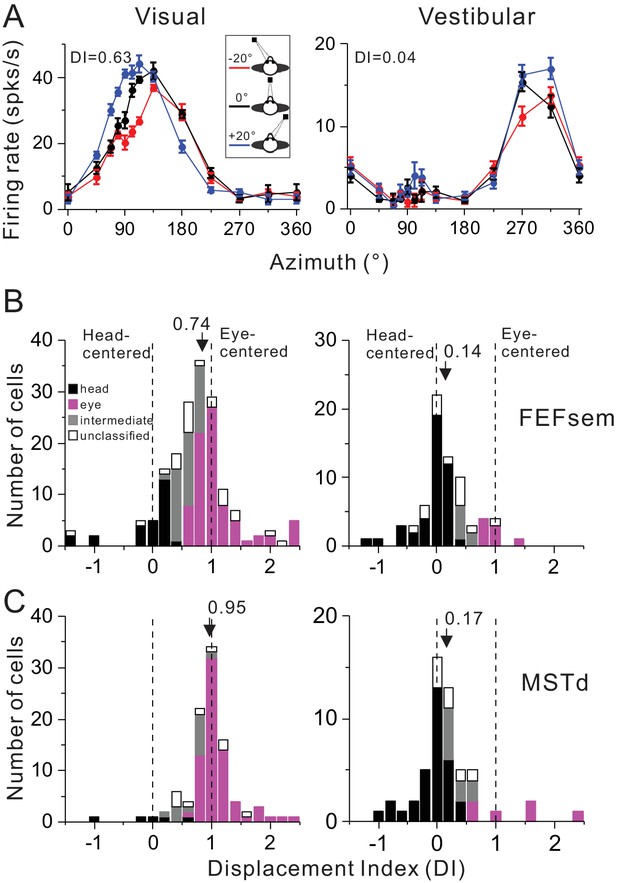
Summary of spatial reference frames as quantified by DI for visual and vestibular heading tuning measured in the eccentric fixation protocol.
(A) Heading tuning functions of an example FEFsem neuron in the visual (left panel) and vestibular condition (right panel). Firing rate is plotted as a function of heading direction. Error bars are standard error of mean (s.e.m.). Different color curves represent tunings measured at different eye-in-orbit positions. (B, C) Distributions of DI measured under 20° eccentricity in FEFsem (B) and MSTd (C). DIs were limited in the range of [−1.5 2.5]. A few cases outside of this range were plotted at the edge of this range for the demo convenience. Black bars: head-centered coordinate; Magenta bars: eye-centered coordinate; Gray bars: intermediate coordinate; Open bars: unclassified coordinate. Arrowheads indicate the mean DI value of all the cases. Vertical dashed lines indicate head-centered (DI = 0) or eye-centered (DI = 1) coordinate.
-
Figure 2—source data 1
Raw data for Figure 2B.
- https://doi.org/10.7554/eLife.29809.007
-
Figure 2—source data 2
Raw data for Figure 2C.
- https://doi.org/10.7554/eLife.29809.008
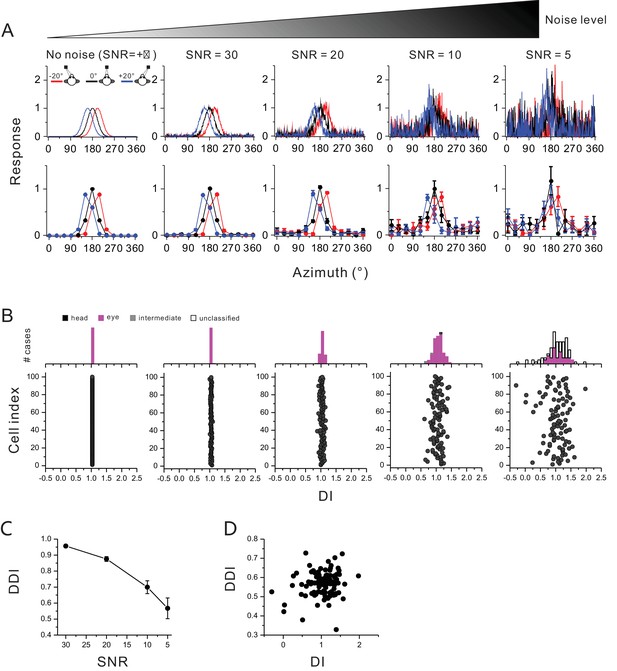
DI under different noise levels in hypothetical neurons.
(A) All neurons were assumed to be completely eye-centered, that is tuning shifted by 20° under eccentric fixation protocol. These tuning curves were injected with different levels of noise by Matlab function ‘awgn’. The smaller the signal to noise ratio (SNR, from left to right), the noisier the tuning curve was. For example, the top panels exhibit one trial of the responses sampled at a resolution of 1°. We repeated this sampling for five times and computed the mean firing rate at each azimuth at a resolution of 12° as the tuning curve (bottom panels), a process of which was similar to the real neural recording experiment. Error bars are standard error of mean. (B) Under each noise level, a population of 100 cells was created, and DI was computed in the exact same way as for the real neurons. It is clear that when noise is getting larger in the tuning curves, the DI values tend to be more broadly distributed, generating some seemingly head-centered units. However, the number of these units is small, and importantly these units are statistically defined as ‘unclassified’ based on the bootstrap test procedure. (C) The strength of directional tuning was quantified using a direction discrimination index (DDI, Takahashi et al., 2007) given by: . DDI not only computes the peak to trough modulation (Rmax-Rmin), but also the response variability (SSE: sum squared error around the mean response; N: total number of trials; M: total number of stimulus directions). DDI ranges within [0 1], corresponding to weak and strong SNR, respectively. (D) DI is not significantly dependent on DDI (R = 0.11, p=0.3, Spearman rank correlation) for hypothetical neurons tested under SRN = 5.
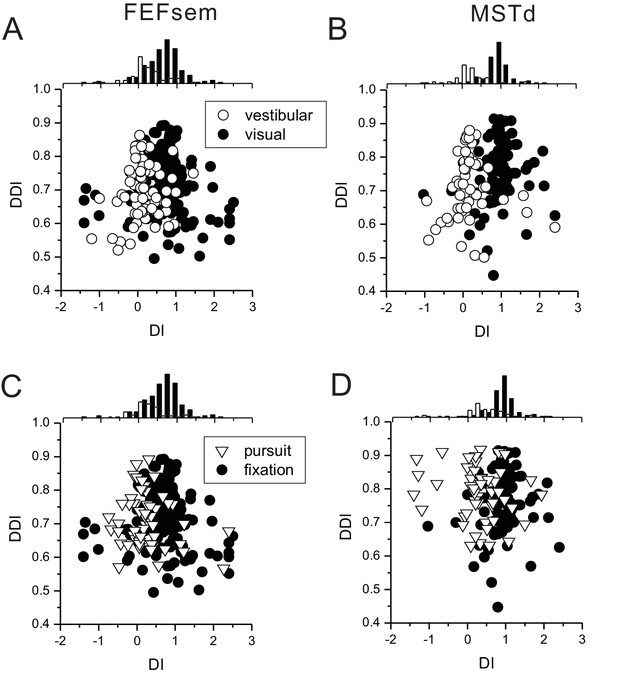
The relationship between DI and DDI in FEFsem (A, C) and MSTd (B, D).
(A, B) Overall DDI is slightly smaller in the vestibular condition than in the visual condition. However, DDIs are largely overlapped in the two conditions (along the vertical axis), which certainly cannot account for the separate spatial coordinate (defined by DI) in the two stimuli conditions. (C, D) Same format as in A, B for eccentric fixation versus smooth pursuit conditions. DDIs overall are similar and even slightly higher in the pursuit condition. Hence, the spatial coordinate defined from DI (together with bootstrap test) cannot be mainly due to signal-to-noise ratio of the tuning curves.
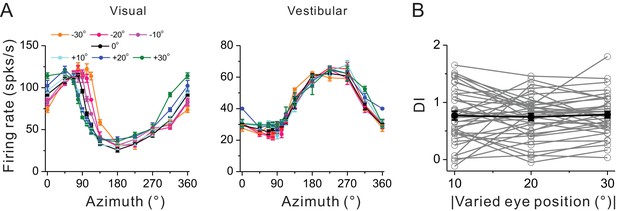
DI measured under a broader range of eccentric fixation task by introducing two extra eccentricities of 10° and 30° in addition to the 20°.
(A) An example neuron with heading tuning curves measured under a broader range of eye-in-orbit positions. (B) DI measured under different eccentric fixation (10°, 20°, 30°) are analogous. Gray curves represent the DI values from each cell. Black symbols represent mean and s.e.m.
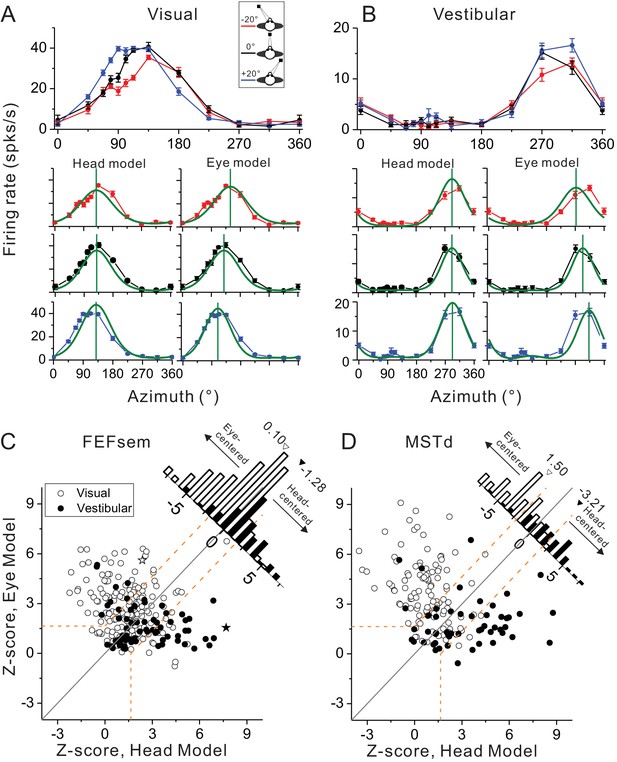
Spatial reference frames as assessed by head- and eye-centered model fittings.
(A, B) Heading tuning functions from an example neuron in the visual (A) and vestibular (B) condition. Red, black and blue curves represent eccentric fixation at −20°, 0° and 20°, respectively. Superimposed green curves depict the fitting of eye-centered or head-centered models. Vertical green lines are the prefer directions of the fitting curves. (C, D) Eye- and head-centered model correlation coefficients (Z-transformed) in FEFsem (C) and MSTd (D). Filled symbols: vestibular; Open symbols: visual. Significance regions are based on the difference between eye- and head-centered Z scores corresponding to p<0.05 (top left, eye centered; bottom right, head centered; central diagonal region, intermediate). The two asterisks, one open and one filled, represent the example cell in (A) and (B), respectively. Diagonal histograms represent distributions of differences between Z-scores for each pair of models (eye- minus head-centered). Arrowheads indicate the mean values of each distribution. Filled bars: vestibular; Open bars: visual.
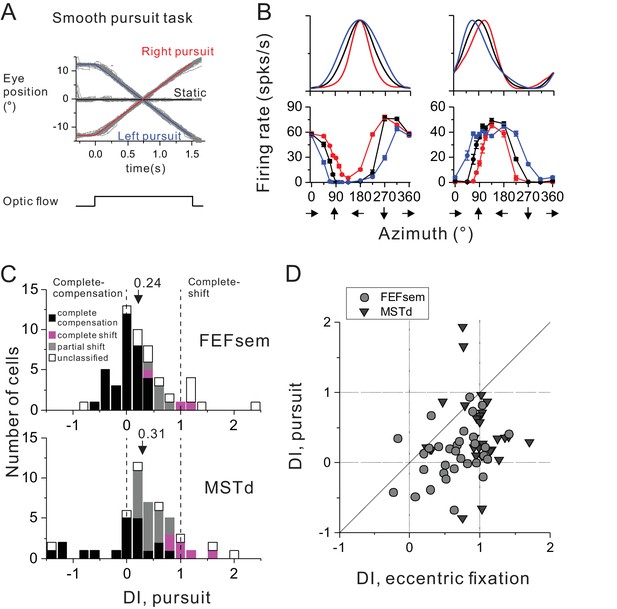
Comparison of spatial reference frames with smooth pursuit eye movement compensation.
(A) Experimental paradigm for smooth pursuit eye movement. Monkeys were required to pursue a smooth moving target crossing the screen either from right to left (blue curve) or from left to right (red curve). These conditions were interleaved with a no-pursuit condition (central fixation, black curve). Gray curves are the raw eye traces from an example block. (B) Heading tuning curves from two hypothetical (top) and two example FEFsem (bottom) neurons under different pursuit conditions. Color is the same as in (A). (C) DI distributions under the pursuit protocol in FEFsem (upper panel) and MSTd (lower panel). Arrowheads indicate mean values. Vertical dashed lines indicate head-centered/complete compensation (DI = 0) or eye-centered/complete shift (DI = 1). Black bars: complete compensation for eye rotation; Magenta bars: complete shift from eye rotation; Gray bars: partial shift; Open bars: unclassified group. (D) Direct comparison of DIs between pursuit and eccentric fixation on a cell by cell basis. Each point represents one neuron. Circle: FEFsem, N = 36; Triangle: MSTd, N = 32.
-
Figure 4—source data 1
Raw data for Figure 4C.
- https://doi.org/10.7554/eLife.29809.012
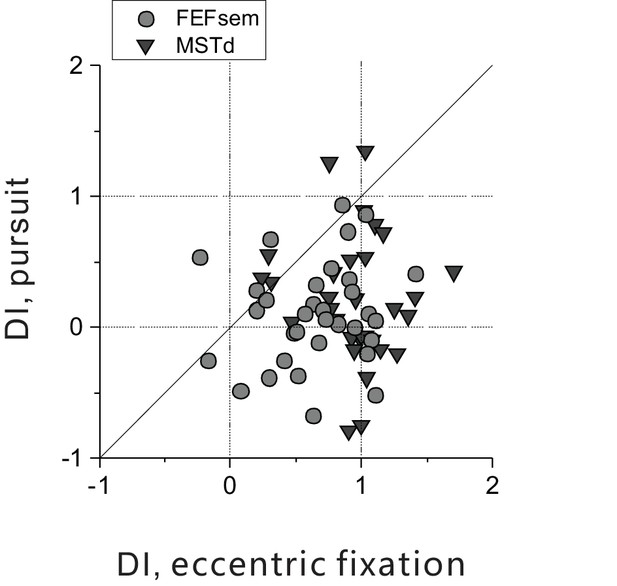
Direct comparison of DI between pursuit and eccentric fixation on a cell by cell basis.
Each point represents one neuron. DI for the pursuit protocol was computed in the same way, that is from the whole tuning curve at once as for the eccentric fixation data. Circle: FEFsem, N = 36; Triangle: MSTd, N = 32. DI under pursuit is significantly smaller compared to that under the eccentric fixation task (FEFsem: p=1.9E-4; MSTd: p=6.4E-8, paired t-test), and they are not significantly correlated with each other (p>0.5, Spearman rank correlation). The same data set as Figure 4D.
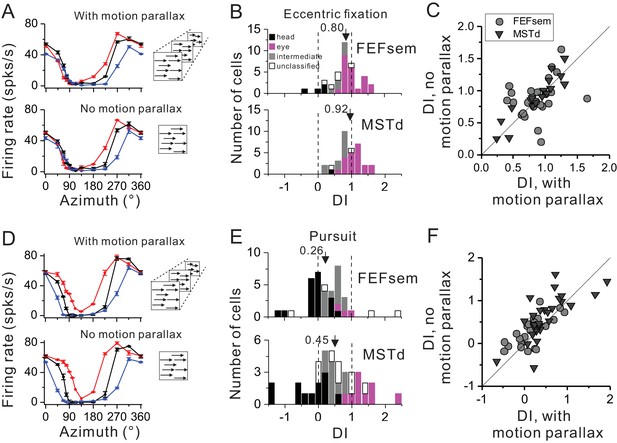
Influence of motion parallax on spatial reference frame (A–C) and pursuit compensation (D–F) measurements.
(A) Visual tuning functions of an example FEFsem neuron with and without motion parallax in the optic flow. Red, black and blue curves represent eccentric fixation at −20°, 0° and 20°, respectively. (B) Distribution of DI without motion parallax cue in FEFsem (upper panel, N = 38) and MSTd (bottom panel, N = 34). Black bars: head-centered coordinate; Magenta bars: eye-centered coordinate; Gray bars: intermediate coordinate; Open bars: unclassified coordinate. Arrowheads indicate the mean value. (C) Direct comparison of DIs between with and without motion parallax cues on a cell by cell basis. Each symbol represents a neuron. Circle: FEFsem, N = 27; Triangle: MSTd, N = 17. (D–F) Same format as in (A–C) but for the pursuit protocol. Red, black and blue curves represent rightward, fixation only (no-pursuit) and leftward pursuit, respectively. In (E), for FEFsem, N = 39; for MSTd, N = 38; In (F), for FEFsem, N = 24; for MSTd, N = 29.
-
Figure 5—source data 1
Raw data for Figure 5B.
- https://doi.org/10.7554/eLife.29809.014
-
Figure 5—source data 2
Raw data for Figure 5E.
- https://doi.org/10.7554/eLife.29809.015
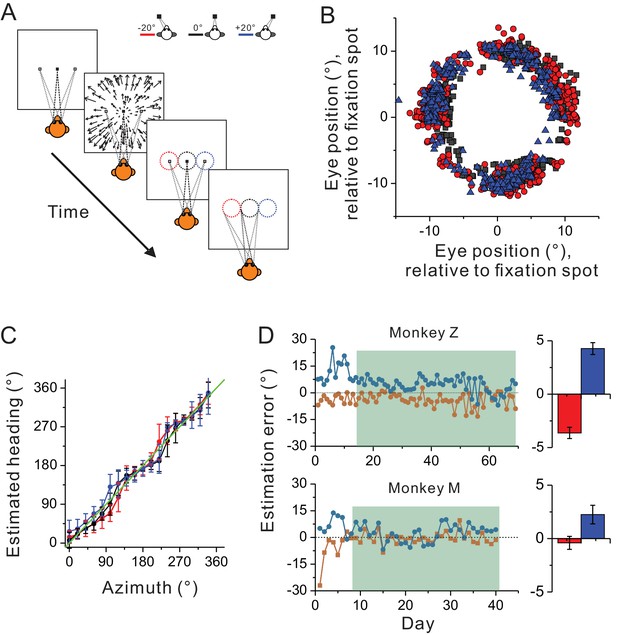
Heading estimation task in the visual condition.
(A) Schematic illustration of the heading estimation task. The four panels depict the event sequence in one trial. Each trial begins with one of the three fixation locations (−20°, 0°, or 20°). After capturing fixation, visual optic flow is provided simulating heading in the horizontal plane. Headings are varied with a resolution of 20°, spanning a full 360° range. At the end of the trial, a target ring appears for ocular motor response. The ring is made of 36 dots apart by 10°. It is 20° in diameter, and its center is aligned with the fixation location in each trial (−20°, 0°, or 20°). Saccade endpoints within a window of 5 × 5° are taken as correct choice and the animals will be rewarded. Red, black and blue symbols represent eccentric fixation at −20°, 0° and 20°, respectively, and are the same for the rest of the figure. (B) Behavioral data from one experimental session with 30 repetitions for each stimulus condition. Note that saccade endpoints are plotted relative to the center of the target ring instead of the visual screen, thus data from the three fixation locations are roughly overlapped in this plot. (C) Mean and circular SD of the monkey’s heading estimates are plotted as a function of the real heading direction. The green diagonal line represents perfect performance. (D) Performance of the two monkeys in the training (unfilled areas) and recording sessions (shaded areas). Heading estimation errors are evaluated by computing the difference in the heading estimate between the eccentric and central fixation conditions. Right histograms represent the mean ± s.e.m. of the data in the recording sessions (shaded area).
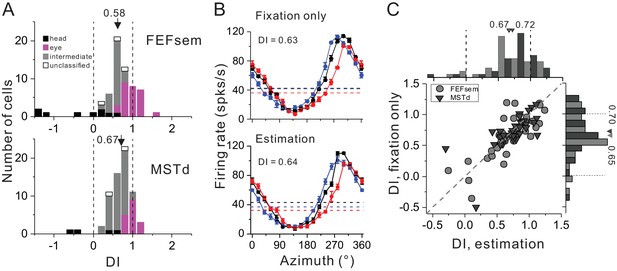
Spatial reference frame of cortical neurons during heading estimation task.
(A) DI distributions post training of heading estimation task in FEFsem (upper panel: N = 65) and MSTd (bottom panel: N = 68). Black bars: head-centered coordinate; Magenta bars: eye-centered coordinate; Gray bars: intermediate coordinate; Open bars: unclassified coordinate. Arrowheads indicate mean values. Vertical dashed lines indicate head- (DI = 0) or eye-centered (DI = 1) coordinate. (B) Tuning functions of an example FEFsem neuron during the fixation only (post training) and active estimation conditions. (C) Comparison of DIs between fixation only and active estimation conditions on a cell by cell basis. Circle: FEFsem, N = 36; Triangle: MSTd, N = 35.
-
Figure 7—source data 1
Raw data for Figure 7A.
- https://doi.org/10.7554/eLife.29809.018
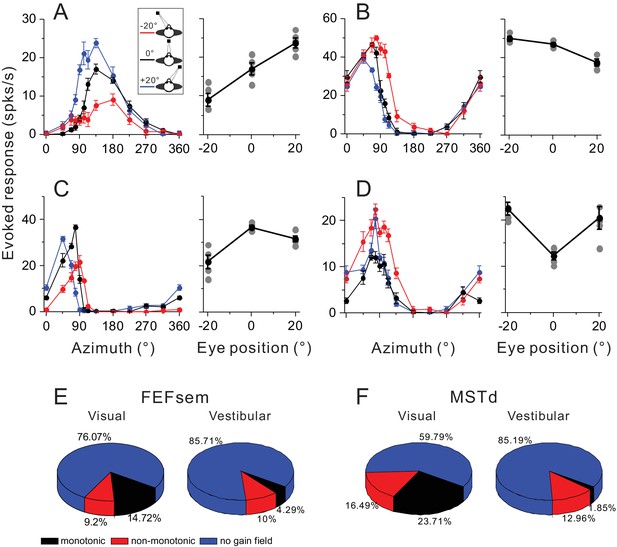
Gain modulations in FEFsem and MSTd.
(A–D) Four example units with significant gain modulations across fixation locations. A and B show two cases with monotonic gain effect. In C and D, the effect is not monotonic, but rather that the responses are either strongest or weakest during central fixation. Tuning curves are the evoked responses with the minimum mean firing rate subtracted. Red, black and blue symbols represent eccentric fixation at −20°, 0° and 20°, respectively, and are the same for the rest of the figure. (E, F) Proportion of different category of neurons in the visual and vestibular conditions in FEFsem (E) and MSTd (F). Red: monotonic group; Black: non-monotonic group; Blue: no-gain modulations.
Additional files
-
Transparent reporting form
- https://doi.org/10.7554/eLife.29809.020





