Distributed rhythm generators underlie Caenorhabditis elegans forward locomotion
Figures
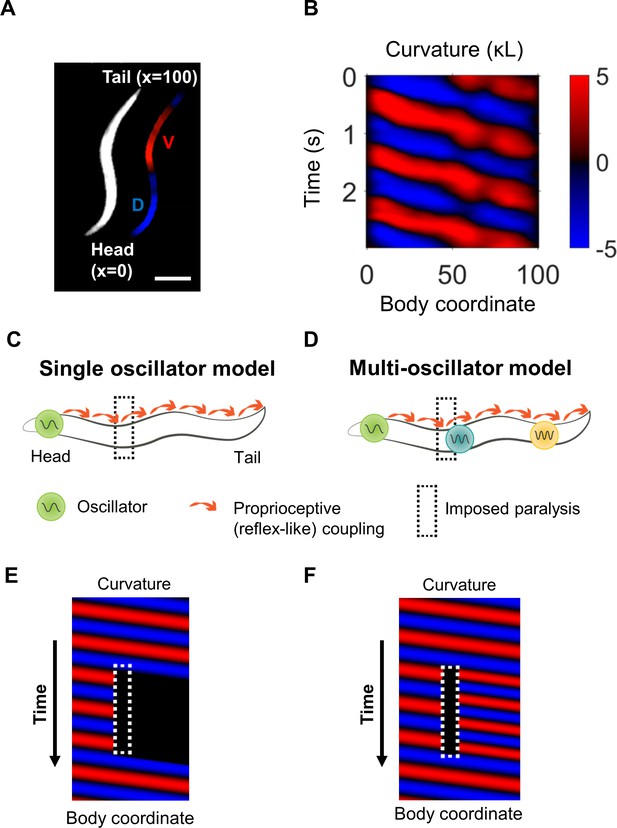
Overview of curvature analysis and models of rhythm generation.
(A) Dark field image of a worm shown with curvature segmentation. Dorsal bending is shown in blue and ventral bending in red. The dorso-ventral orientation is arbitrary unless otherwise specified. The worm’s centerline is used to define a coordinate system in which the head and tail are located at body coordinates 0 and 100, respectively. Scale bar: 200 μm. (B) Curvature map from a normally swimming worm. The curvature at time t = 0 s corresponds to the image shown in (A). (C) In a single-oscillator model of locomotion, an unknown oscillator causes rhythmic head bending, and a reflex-like coupling mechanism mediates propagation of these bends along the rest of the body. (D) A multi-oscillator model (Gjorgjieva et al., 2014) posits the existence of additional circuit units outside the head capable of generating oscillations. (E) Conceptual curvature map showing predicted worm behavior after paralyzing a small region of the body (dotted white box). The single-oscillator model predicts that all regions posterior to the paralyzed region will also become paralyzed. (F) Conceptual curvature map predicting the outcome of the same manipulation applied to a multi-oscillator model. If additional oscillators exist posterior to the paralyzed region, additional tail oscillations may arise, potentially with different amplitude, frequency, and/or phase.
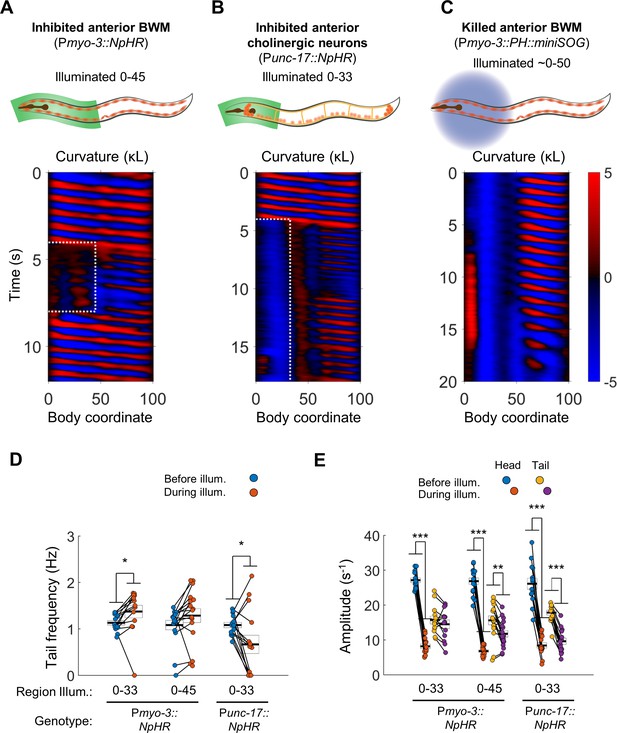
Anterior undulation is not required for posterior undulation.
(A) Inhibition of anterior BWMs (via Pmyo-3::NpHR) increases tail frequency. Body coordinates 0–45 were illuminated with green light (532 nm wavelength) to trigger relaxation of the anterior muscles. The spatiotemporal extent of green laser illumination is indicated by the white dotted box. (B) Inhibition of anterior cholinergic neurons (via Punc-17::NpHR; Punc-17::ChR2) does not prevent tail undulation. Body coordinates 0–33 were illuminated with green light to optogenetically inhibit anterior motor activity. (C) Tail undulations persist despite paralysis of the anterior BWMs due to miniSOG-mediated lesion of muscle cells. Animals were subjected to mechanical stimulation to induce locomotion (see Materials and methods). A total of nine animals were illuminated with blue light (472 nm wavelength) in approximately their anterior halves. Of these, five displayed partial-body forward swimming as depicted here, three were immobile, and one was not sufficiently paralyzed in the head. Six control worms, which were mounted identically but not illuminated, all displayed waves propagating normally from head to tail (not shown). (D) Inhibition of some anterior muscles (body coordinate 0–33, N = 10 worms) significantly increases tail frequency. Inhibition of most anterior muscles (0–45, N = 10 worms), or inhibition of anterior cholinergic neurons (N = 14 worms) produces mixed results; some animals generate high frequency tail oscillations while others slow down. Each colored circle represents one trial; worms may have multiple trials. Tail frequency is measured at body coordinate 85. Error boxes represent the mean and SEM. (E) Amplitude of undulation in the head and tail before and during muscle or neuron inhibition. Head frequency is measured at body coordinate 15. Note sharp decreases in head amplitude during all three manipulations. Amplitude here and henceforth is measured as the root mean square of the time derivative of the curvature times worm length and has units of s−1. (*) p<0.05; (**) p<0.01; (***) p<0.001; paired t-test.
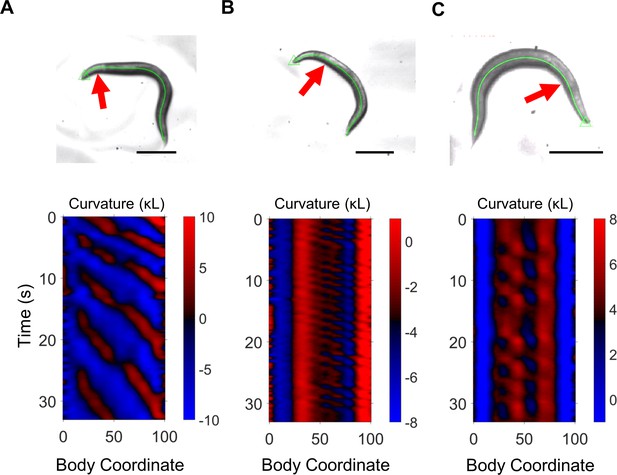
Tail undulation after gross head lesioning.
The approximate location of hot wire lesioning is indicated with a red arrow. Each panel presents data from a different worm. Scale bars: 200 μm. (A) Slow rhythmic undulations are evident posterior to the head. (B) Rhythmic undulations in the mid-body arise after substantial damage is applied to the head. (C) Rhythmic undulations in the neck and mid-body arise after substantial damage is applied to the head.
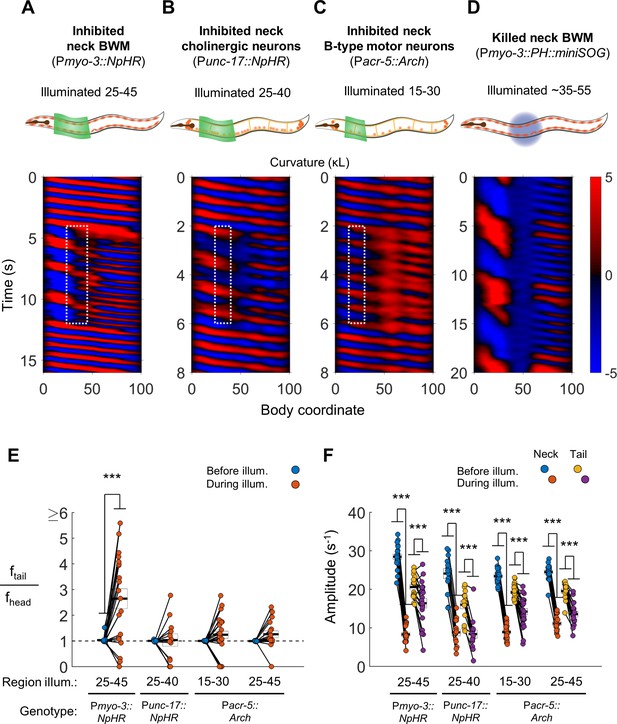
Disruption of motor coupling in the neck de-synchronizes head and tail oscillations.
(A) Inhibition of neck BWMs (via Pmyo-3::NpHR) increases tail frequency and decreases head frequency. We refer to this effect as two frequency undulation (2FU). Body coordinates 25–45 were illuminated with green light to induce relaxation of neck muscles. The spatiotemporal extent of green laser illumination is indicated by the white dotted box. (B,C) Inhibition of neck cholinergic neurons (Punc-17::NpHR) or neck B-type motor neurons (Pacr-5::Arch) also induces 2FU behavior. (D) Two frequency undulation after miniSOG-induced paralysis of the mid-body BWMs. Animal was subjected to mechanical stimulation to induce locomotion, but also displayed this behavior prior to stimulation. A total of 10 individuals were illuminated with blue light on approximately one-fifth of their body length, centered near the vulva. Of these, seven displayed 2FU as depicted here, one was immobile, and two were not sufficiently paralyzed in the mid-body to disrupt bending waves. Color map data are scaled down by 50% because bends in this animal had higher amplitudes than those shown in A-C. (E) Several optogenetic manipulations produced decoupled head and tail oscillation. 2FU is assayed by dividing tail frequency by head frequency in each worm. Before illumination, the head (body coordinate 15) and tail (body coordinate 85) usually oscillate at the same frequency. During illumination, tail frequency often exceeds head frequency. Each colored circle pair represents one trial; worms may have multiple trials. N = 11, 10, 12, and 10 worms per condition, respectively. Error boxes represent the mean and SEM. (F) Amplitude of undulation in the neck and tail before and during neck muscle or neuron inhibition. Neck amplitude is measured at body coordinate 35. (*) p<0.05; (**) p<0.01; (***) p<0.001; paired t-test.
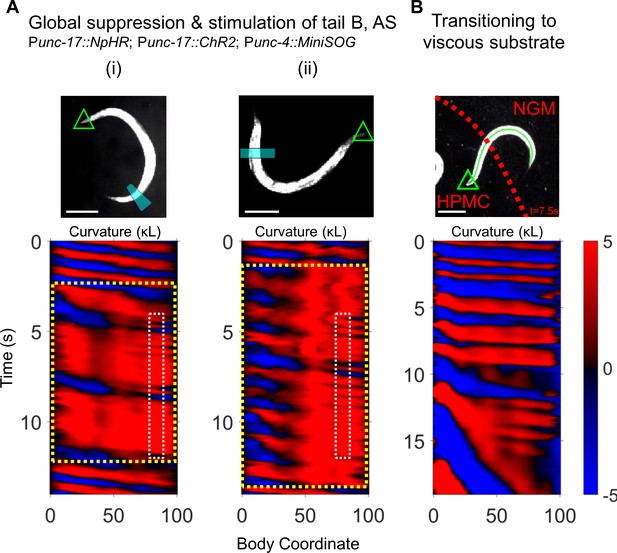
Additional disruptions to motor coupling cause 2FU.
(A) Two examples of worms in which all cholinergic neurons are inhibited (Punc-17::NpHR; global yellow illumination) except for those within a small tail region (Punc-17::ChR2; blue illumination in a small tail region). The A-type motor neurons were killed at the L2 larval stage (Punc-4::MiniSOG). Green triangle: worm head; blue band: blue illumination region. The curvature map (lower pane) indicates the spatiotemporal windows of yellow illumination (yellow dotted box) and blue laser illumination (white dotted box). Scale bars: 200 μm. (B) 2FU induced by an inhomogeneous mechanical environment. Red dotted line indicates the boundary between low-viscosity buffer (NGM) and high-viscosity HPMC. Around t = 11 s, the tail continues oscillating at high frequency even as the head rapidly slows to a crawl inside the HPMC.
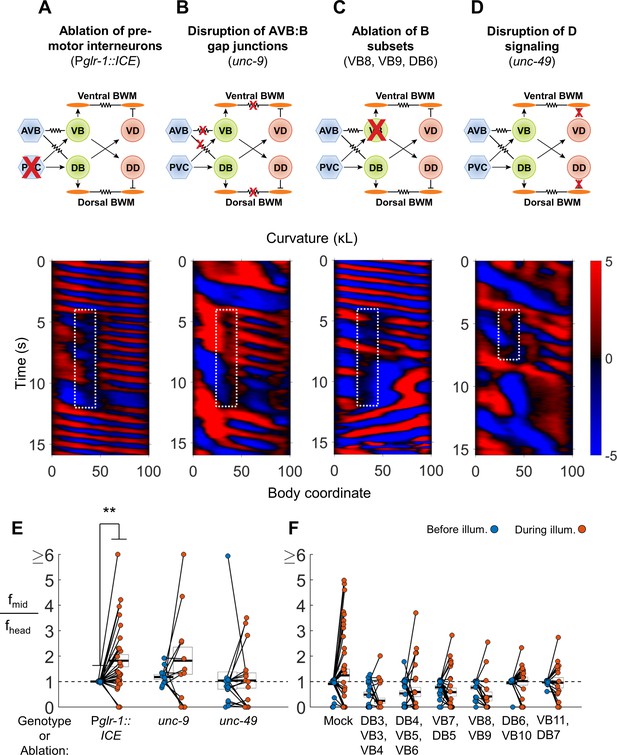
VNC premotor interneurons, D-type motor neuron signaling, and individual B-type motor neurons are not required for 2FU.
(A) 2FU occurs despite ablation of premotor interneurons (Pglr-1::ICE; Pmyo-3::NpHR). The spatiotemporal extent of green laser illumination is indicated by the white dotted box. (B) 2FU occurs despite disruption of AVB:B gap junctions and BWM:BWM gap junctions (unc-9; Pmyo-3::NpHR). (C) 2FU occurs despite ablation of a small subset of the B-type motor neurons (Pmyo-3::NpHR; Pacr-2::wCherry). VB8, VB9, and DB6 were ablated using our pulsed infrared laser system. (D) 2FU occurs despite elimination of GABAergic signaling (unc-49; Pmyo-3::NpHR). (E) When subjected to neck paralysis (Pmyo-3::NpHR; Pacr-2::wCherry), 2FU occurs reliably in Pglr-1::ICE animals and occasionally in unc-9 and unc-49 animals. Each colored circle pair represents one trial; worms may have multiple trials. N = 12, 8, and 10 worms per condition, respectively. Head frequencies are measured at body coordinate 15. Mid-body are frequencies are measured at body coordinate 60. Error boxes represent the mean and SEM. (*) p<0.05; (**) p<0.01; (***) p<0.001; paired t-test. (F) When subjected to neck paralysis (Pmyo-3::NpHR), 2FU occurs at least occasionally despite ablation of subsets of the B-type motor neurons by our pulsed infrared laser system. For each condition, data are only considered from worms that have all specified neurons missing; some worms in each group may have additional B-type or other neurons missing. N = 40, 30, 32, 27, 18, 18, and 16 trials from 10, 10, 10, 8, 7, 7, and 7 worms per condition, respectively. Mean mid-body/head frequency ratios during illumination are significantly lower than mock controls for all ablation conditions except DB6, VB10 and VB11, DB7 (p<0.05 by one-way ANOVA with Bonferroni post-hoc comparisons).
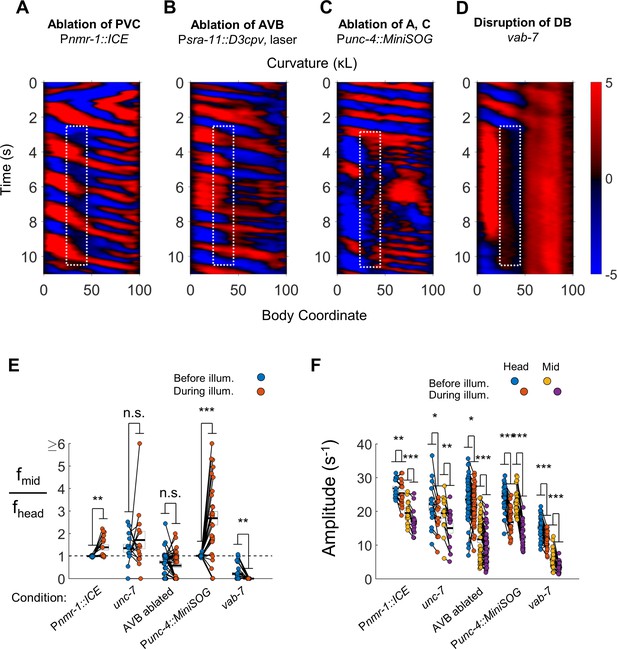
VNC premotor interneurons and several VNC motor neuron classes are not required for de-synchronized oscillations.
(A) 2FU occurs despite ablation of the premotor interneurons AVA, AVD, AVE, PVC, and other neurons (Pnmr-1::ICE; Pmyo-3::NpHR). The spatiotemporal extent of green laser illumination is indicated by the white dotted box. (B) 2FU rarely occurs upon laser ablation of both AVB interneurons (See Figure 4—figure supplement 2). (C) Robust 2FU occurs despite ablation of the A- and C-type motor neurons (Punc-4::MiniSOG; Pmyo-3::NpHR). (D) C. elegans vab-7 mutants, in which DB motor neurons are broadly disrupted, have paralyzed tails and appear incapable of 2FU. (E) When subjected to neck paralysis (Pmyo-3::NpHR), 2FU occurs reliably in Pnmr-1::ICE and Punc-4::MiniSOG animals, occasionally in unc-7 animals, and rarely in animals in which AVB has been ablated. Each colored circle pair represents one trial; worms may have multiple trials (see methods). N = 11, 8, 14, 16, and 19 worms per condition, respectively. Error boxes represent mean and SEM. Head and mid-body data were measured at body coordinates 15 and 60, respectively. (F) Neck paralysis leads to modest decreases in tail bending amplitude in most conditions.
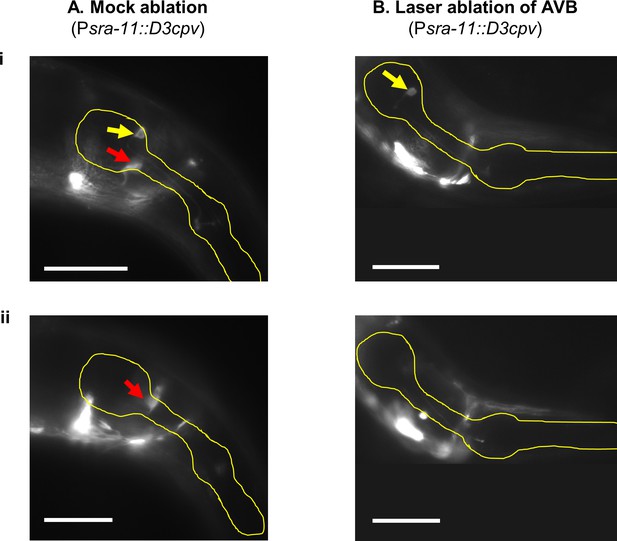
Ablation of the AVB interneurons.
(A) Mock control with both AVB neurons visible (Psra-11::D3cpv). Yellow outline, worm pharynx. Red arrows, AVB L/R in separate focal planes of the same worm. Yellow arrows, a pharyngeal neuron, possibly I4. AVB cell bodies were identified by their placement at the medial anterior edge of the terminal bulb of the pharynx, with processes directed ventrally. Scale bars: 50 μm. (B) Worm in which both AVB interneurons were ablated at the L4 stage. Neither cell bodies nor processes are visible. Behavioral recording of this worm is shown in Figure 4—figure supplement 1.
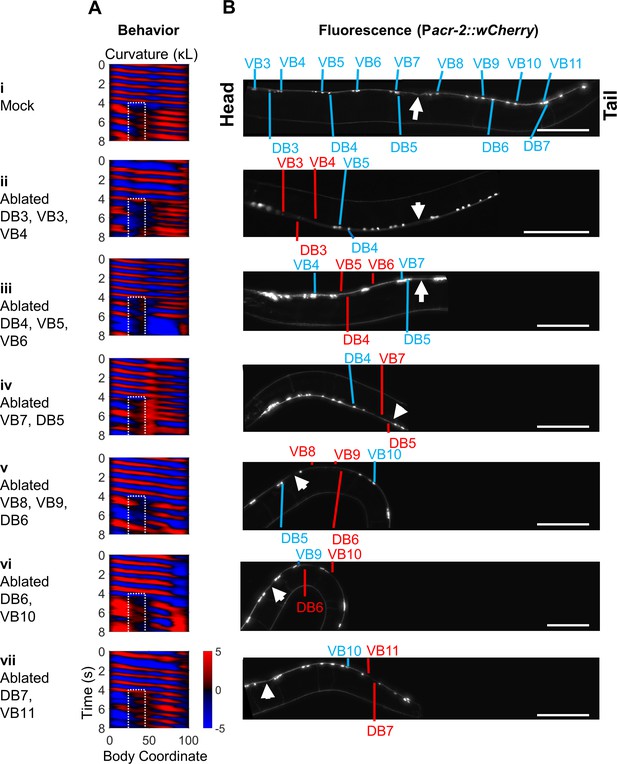
Subsets of B-type motor neurons are not required for 2FU.
Groups of 2–3 B-type motor neurons at a time were ablated using a pulsed infrared laser in worms expressing Pmyo-3::NpHR; Pacr-2::wCherry. See also Figure 4F. (A) Curvature maps of worms that exhibited 2FU despite ablation of the indicated B-type motor neurons and potentially some other neurons. (B) wCherry fluorescence images of each worm to confirm cell death. Blue labels indicate some or all B-type motor neurons. Red labels indicate missing B-type neurons. White arrows indicate the vulva. Scale bars: 100 μm.
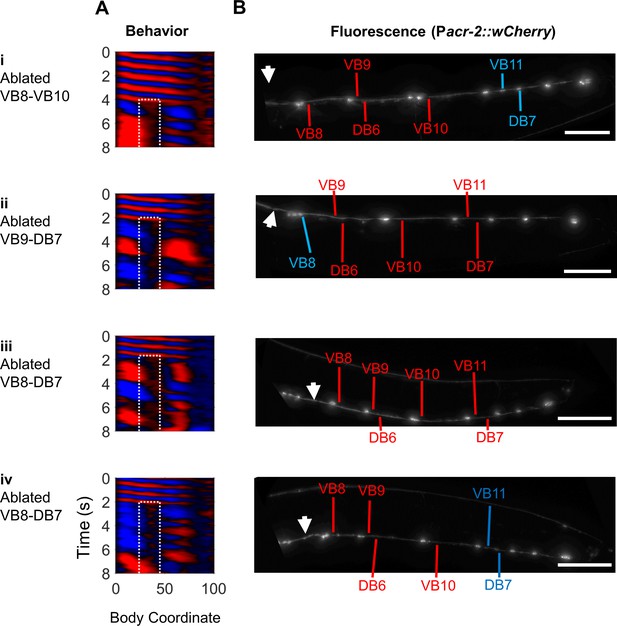
B-type motor neurons posterior to the vulva are not required for 2FU.
We attempted to ablate all B-type motor neurons posterior to the vulva in worms expressing Pmyo-3::NpHR; Pacr-2::wCherry. (A) Curvature maps of worms that exhibited 2FU despite ablation of the indicated B-type motor neurons and potentially other neurons. (B) wCherry fluorescence images of each worm to confirm cell death. Blue labels indicate some or all B-type motor neurons. Red labels indicate missing B-type neurons. White arrows indicate the vulva. Scale bars: 100 μm.
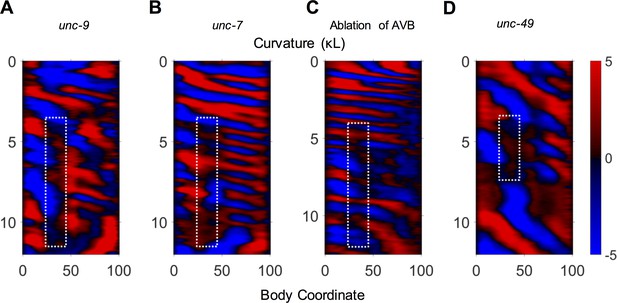
Examples of 2FU occurrence prior to optogenetic inhibition of neck muscles.
In some disruptions to the motor circuit, 2FU was observed occurring without inhibition of neck muscles. Examples are shown here from an unc-9 mutant (A), an unc-7 mutant (B), an AVB-ablated worm (C), and an unc-49 mutant (D).
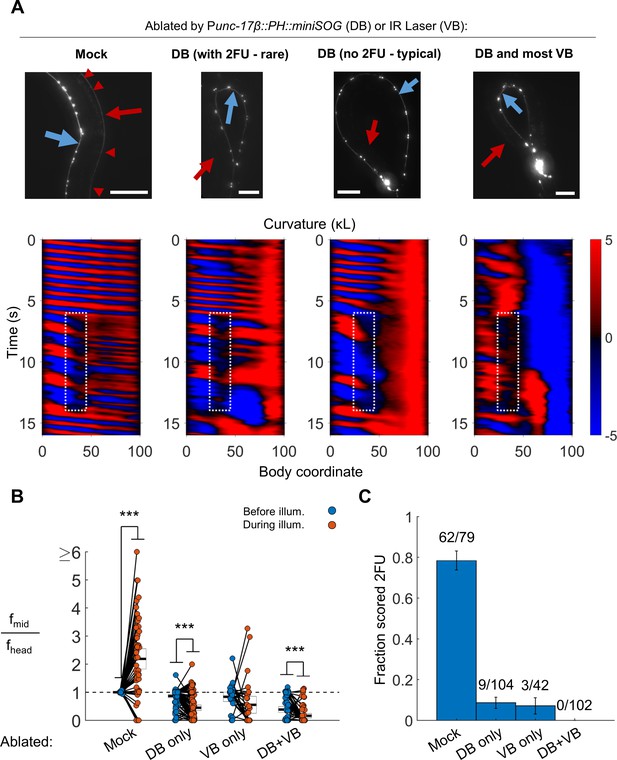
B motor neurons are required for 2FU.
(A) Top panels: assessment of ablations using a Pacr-2::mCherry label. After removal of dorsal B motor neurons (Punc-17β::PH::miniSOG), pairs of motor neurons – each corresponding to one VA and one VB type neuron – are visible along the VNC (blue arrows). Neither the DNC (red arrows) nor the DB or DA commissures (red arrowheads in mock) are visible. Scale bars: 100 μm. Bottom panels: Corresponding examples of worm locomotion after removal of DB (via miniSOG) and VB (via IR laser) motor neurons. Removal of DB always resulted in tail paralysis in a coiled position, but a minority of worms were able to generate a rhythmic midbody wave. Additional removal of VB motor neurons by laser ablation completely prevented 2FU and resulted in severe tail paralysis. (B,C) 2FU, as assayed by frequency measurements (B) or blinded, randomized scoring (C), is sharply reduced or eliminated by removal of DB, VB, or both. Head and mid-body frequencies were measured at body coordinates 15 and 60, respectively. Error boxes in (B) are the mean and 95% confidence interval of the mean. Error bars in (C) are standard error of the sample proportion. Numbers above each bar in (C) indicate the number of trials scored 2FU over the total number of trials for the condition; each individual worm contributed between one and five trials (3.6 on average). (***) p<0.001; paired t-test.
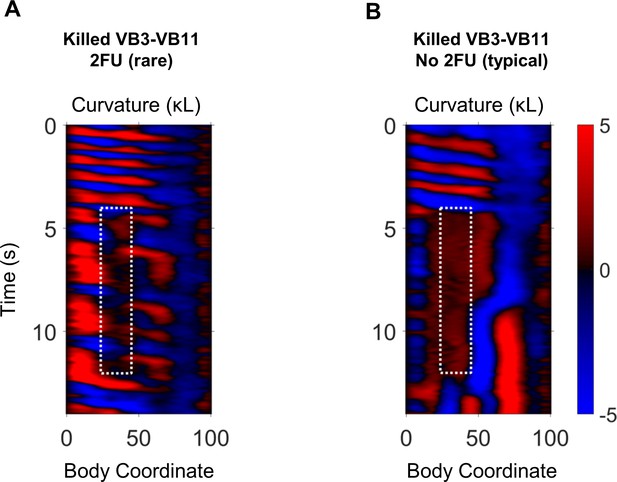
Behavior after ablation of VB motor neurons.
Example curvature maps from trials in which 2FU was (A) and was not (B) observed after IR laser ablation of VB motor neurons. VB3-VB11 were removed in both worms.
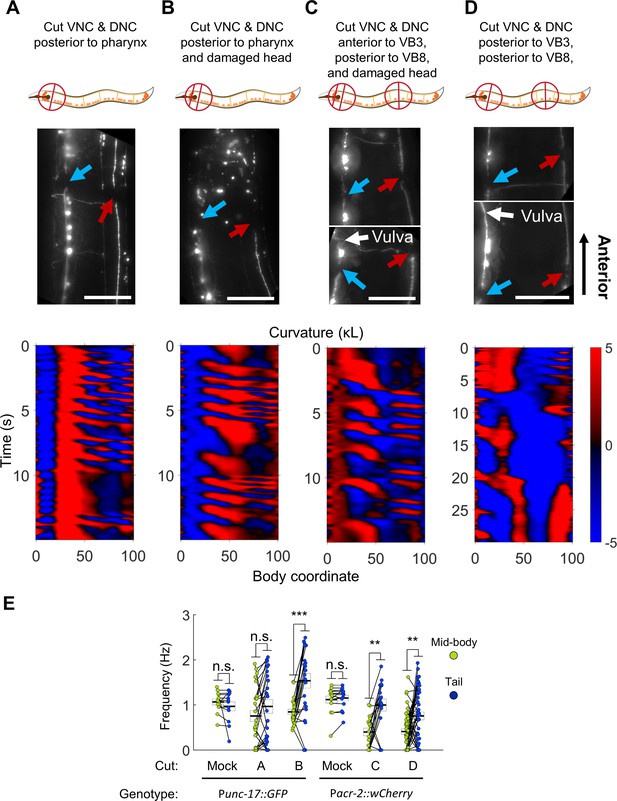
Undulations generated in the tail after severing the dorsal and ventral nerve cords.
(A) The VNC (blue arrow) and DNC (red arrow) were severed in a Punc-17::GFP worm using a pulsed infrared laser. Several other dorso-ventral processes also appear cut. Nonetheless, robust bending waves are generated in the mid-body (lower pane). All scale bars: 50 μm. (B) The VNC and DNC are severed, and additional damage has been applied to the nerve ring to suppress head movements. Robust bending waves are generated in both the neck and tail (lower pane). (C) The VNC and DNC are severed posterior to the head and vulva (Pacr-2::wCherry), and the nerve ring is lesioned to suppress head movements. Robust bending waves are generated in both the neck and tail (lower pane). (D) The VNC and DNC are severed posterior to the head and vulva, but the nerve ring was not targeted. Low-frequency head undulation and high-frequency tail undulation were observed separately in this animal. (E) Frequency of undulation in the mid-body and tail for all ablation conditions and mock controls. Each colored circle pair represents one bout of forward locomotion lasting at least 2 s. For each condition, data are only considered for worms in which the VNC and DNC are clearly severed in the indicated locations. Mid-body and tail frequencies were measured at body coordinates 45 and 85, respectively. Error boxes represent the mean and SEM. N = 3, 7, 3, 4, 5, and seven worms per condition, respectively. (*) p<0.05; (**) p<0.01; (***) p<0.001; paired t-test.
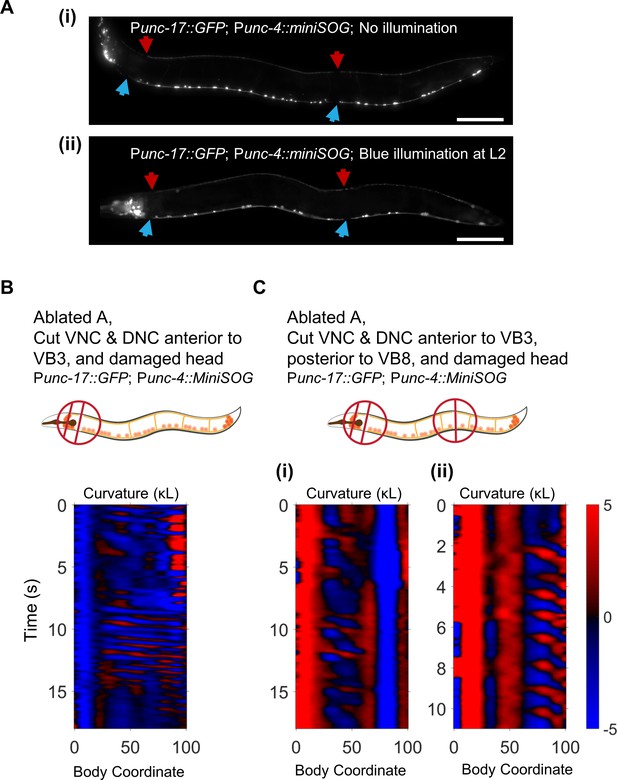
Body undulations after severing the VNC and DNC do not require the A motor neurons.
(A) Representative images of the VNC motor neurons in adult worms without (i) and with (ii) illumination with blue light at the L2 larval stage. After illumination, only B and AS neurons were visible along most of the body. In all worms tested, however (N = 20), at least one A motor neuron was visible at the posterior end of the VNC, corresponding to either VA12, DA8, or DA9. The behavior of the worm in (ii) is shown in C(i). Scale bars: 100 μm. (B) Rhythmic waves visible posterior to the head after removal of the A motor neurons, severing the VNC and DNC in the neck, and damaging the head. (C) Rhythmic waves visible in the mid-body (i) and tail (ii) after removal of the A motor neurons, severing the VNC and DNC in the neck, severing the VNC and DNC posterior to the vulva, and damaging the head.
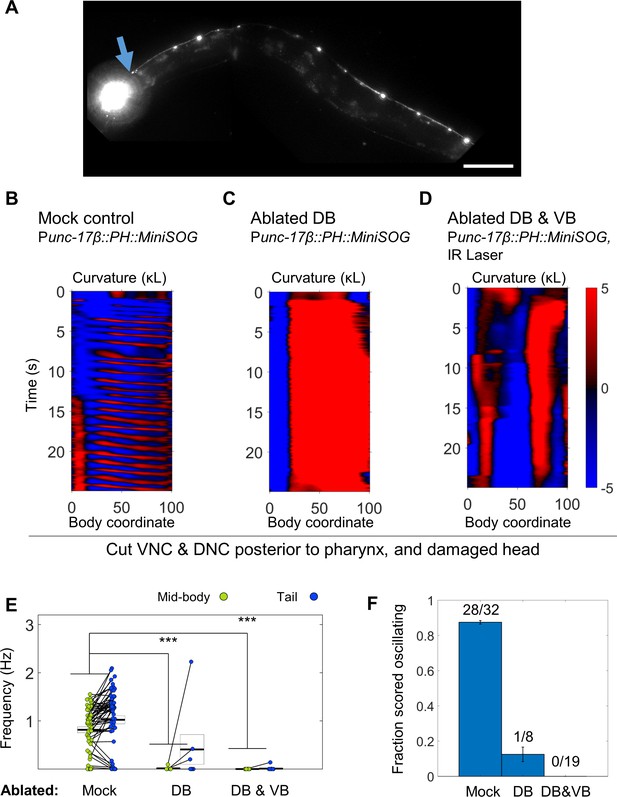
B motor neurons are required for body undulations after severing the VNC and DNC.
(A) Fluorescence image of the worm in (D), for which DB have been removed by MiniSOG and VB have been removed by laser ablation. The remaining VA neurons and processes allow visualization of the VNC, including the cut posterior to the pharynx (blue arrow). DA and DB commissures are not visible. Scale bar: 100 μm. (B–D) behavior during periods of mechanical vibration in worms for which the VNC and DNC were severed posterior to the pharynx. Control worms exhibit robust undulation. Worms lacking DB nearly always coiled in response to the stimulus, but in a minority of cases, very faint movements, potentially oscillations, could be seen in the tip of the tail. Worms lacking DB and VB also reacted to the stimulus by at least partially coiling, but rhythmic body undulations were not observed. (E) Recorded frequency during bouts of well-segmented non-reversal lasting at least two seconds during periods of mechanical stimulation. Control worms routinely exhibited oscillations in the mid-body and tail. DB ablated worms had no mid-body oscillations, but in one case produced very faint high-frequency tail oscillations (see C). DB and VB ablated worms lacked any rhythmic oscillations. N = 58, 7, and 16 bouts from 15, 4, and 9 worms, respectively Mid-body and tail frequency are measured at body coordinates 45 and 85, respectively. (F) Subjective scoring of worm behavior in each condition. Fractions indicate the number of trials blindly scored as oscillating during 20 s periods of mechanical stimulus (anywhere along the body) over the total number of trials. Oscillation was also not observed in DB or DB and VB ablated worms outside periods of stimulus (not shown). Each animal was subjected to 1–2 such stimulus trials. Error bars represent standard error of the sample proportion.
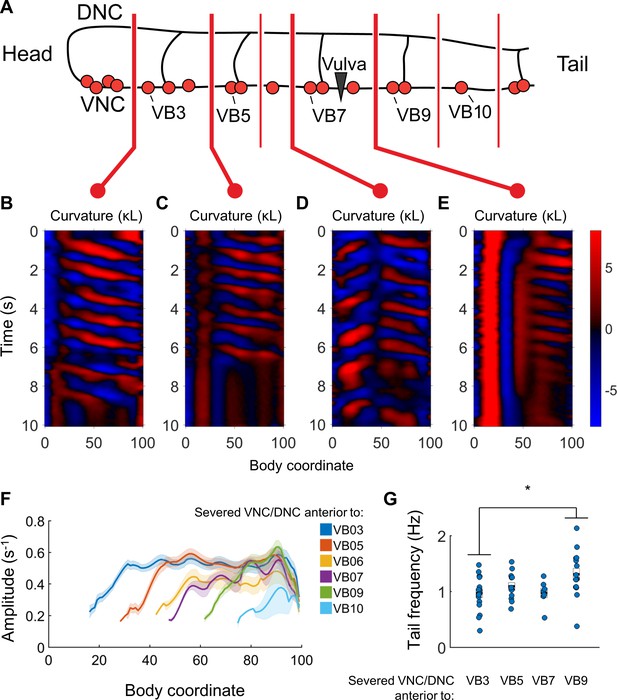
Undulations are generated after VNC/DNC lesioning in arbitrary locations.
(A) Schematic indicating all regions at which we severed the VNC and DNC in relation to the B motor neurons. Each animal’s nerve cords were severed at only one of these locations. The nerve ring of each worm was also damaged to restrict head movements as in Figure 5B. (B–E) Representative curvature maps for worms subject to four of the tested conditions. Note that anterior-to-posterior waves begin progressively more posterior for each cut location. In some cases, the head and tail exhibited waves propagating in opposite directions (D). (F) Amplitude of bending as a function of body coordinate after severing the VNC and DNC anterior to the indicated motor neuron. Only the portion of the curve posterior to the amplitude minima (the cut location) is shown. No bouts of locomotion with anterior-to-posterior waves were discernible posterior to the cut at VB11 either subjectively or by our analysis software. For cut locations at VB3, VB5, VB6, VB7, VB9, VB10, and VB11 we studied N = 15, 9, 6, 10, 14, 9, and 6 worms and observed 25, 16, 7, 12, 30, 19, and 14 bouts of forward locomotion (lasting at least 3 s), respectively. Shaded outline represents ±SEM. (G) Frequency of undulation at body coordinate 75 for four cut conditions. Boxes represent mean and SEM. Each colored circle indicates the frequency during one bout of forward locomotion. *p<0.05, one-way ANOVA with Bonferroni post-hoc comparisons.
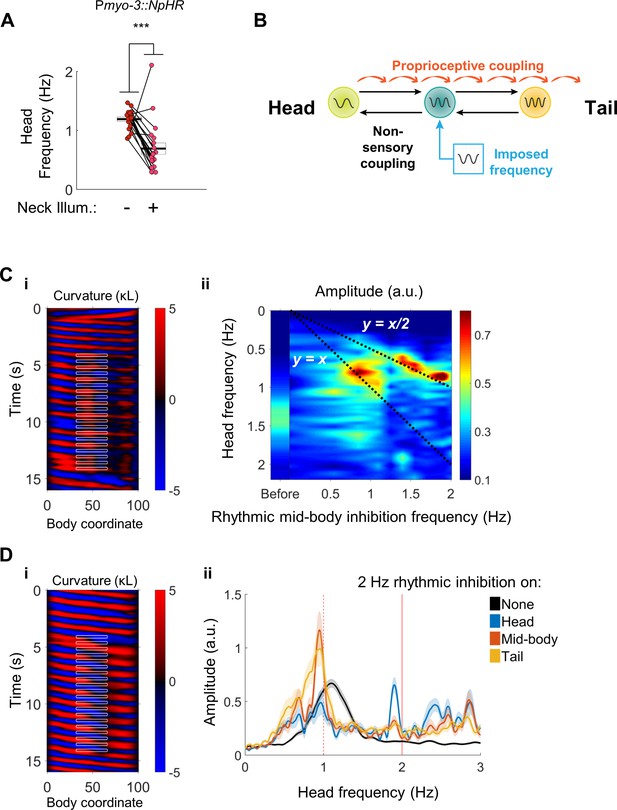
Head undulation frequency can be entrained by mid-body optogenetic manipulation.
(A) Neck muscle hyperpolarization (Figure 3A) causes a significant decrease in head bending frequency. This decrease is not predicted by either model discussed in Figure 1. (B) A multi-oscillator model of forward locomotion allowing for motor coupling in both the anterior and posterior directions. To test this model, we sought to impose a new frequency on the mid-body of a freely moving worm, and test whether head bending also adopts the new frequency. (C) Head bending frequency can be entrained by rhythmically inhibiting the mid-body BWMs. (i) A curvature map showing a representative trial. Green light was pulsed on coordinates 33–66 at a frequency of 2 Hz onto a Pmyo-3::NpHR worm. Note that the head frequency slows to half of the imposed frequency, although some instances of a 1:1 correlation between a laser pulse and a head bend are also evident (e.g. around t = 13 s). (ii) Mean head frequency power spectra of Pmyo-3::NpHR worms before manipulation (left bar, worms from all conditions are pooled) and while subject to rhythmic mid-body paralysis. Frequencies tested were 0 (with laser on), 0.5, 0.85, 1.1, 1.25, 1.4, 1.55, 1.7, 1.9, and 2.0 Hz. Frequency data are interpolated between these points. N ≥ 11 trials per condition, with each worm supplying at most two trials. For high-frequency inhibition (f > 1.1 Hz), the head is entrained to half of the inhibition frequency (bright peaks lie along y = x/2). For lower frequencies of inhibition (f ~ 0.85 Hz), the head is entrained to the inhibition frequency (bright peaks lie along y = x). (D) Head bending frequency can be entrained by rhythmically inhibiting the head, mid-body, or tail cholinergic neurons. (i) A curvature map showing a representative trial. Green light was pulsed on coordinates 33–66 at a frequency of 2 Hz onto a Punc-17::NpHR worm. (ii) Mean head frequency spectra before manipulation (black, all conditions pooled), and after rhythmically inhibiting the head (blue, body coordinates 0–33), mid-body (orange, 33–66), or tail (yellow, 66–99) neurons at 2 Hz. Rhythmic inhibition of the mid-body or tail increases the frequency power at 1 Hz and decreases the power at the original undulation frequency, mirroring (C). N ≥ 16 trials per condition, with each worm supplying at most two trials. Shaded outlines are the SEM. Vertical red lines indicate the imposed frequency (solid) or one-half of the imposed frequency (dashed).
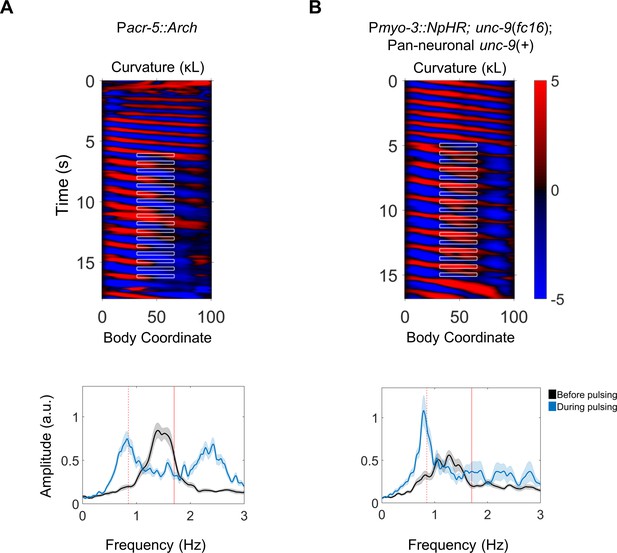
Rhythmic activity in the mid-body B motor neurons is sufficient for posterior-to-anterior entrainment, and UNC-9 muscle-to-muscle gap junctions are not required.
(A) Representative kymogram from a Pacr-5::Arch worm that was subject to rhythmic (1.7 Hz) hyperpolarization of the mid-body B motor neurons (top), and average frequency spectra of the head before and during rhythmic inhibition (bottom). Note an increase in amplitude at 0.85 Hz, one-half of the imposed frequency. N = 22 trials were analyzed from 11 worms. Vertical red lines indicate the imposed mid-body frequency (solid) or one-half of the imposed frequency (dashed). (B) Equivalent analysis for worms in which the mid-body muscles were rhythmically inhibited and muscle-to-muscle gap junctions are disrupted. Expression of UNC-9 in neurons, but not muscles, was transgenically restored in an unc-9 worm (Wen et al., 2012). Note an increase in amplitude at 0.85 Hz, one half of the imposed frequency. N = 22 trials were analyzed from 11 worms.
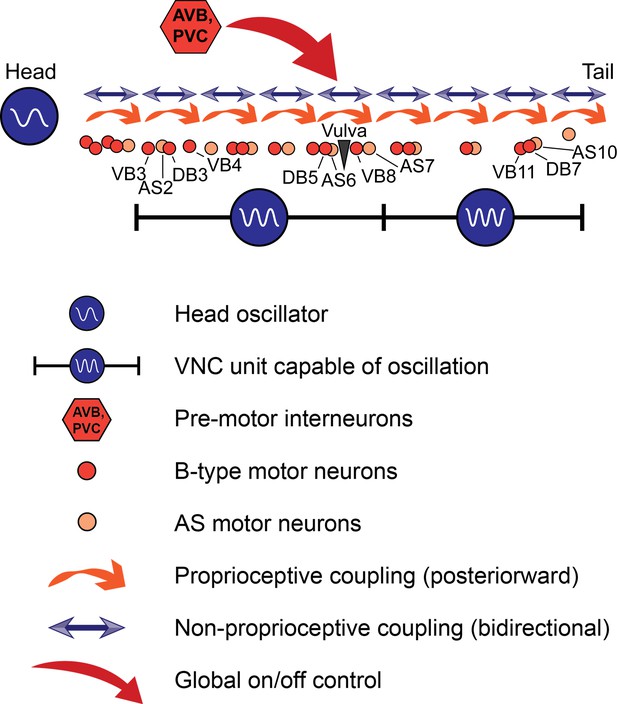
A model for C. elegans forward locomotion.
Two units of the VNC motor neurons (and potentially more subsections) are capable of independent rhythm generation. However, all oscillating units are coupled by proprioceptive coupling (Wen et al., 2012) and another unknown, likely non-proprioceptive coupling mechanism that allows signaling in the anteriorward direction, and potentially also in the posteriorward direction. Pre-motor interneurons activate or suppress this circuit. AVB may have an additional, unexplained role in rhythm generation.
Videos
Posterior undulations after optogenetic inhibition of anterior body wall muscles or cholinergic neurons.
https://doi.org/10.7554/eLife.29913.005Two-frequency undulation during optogenetic inhibition of neck BWM, cholinergic neurons, or B motor neurons.
https://doi.org/10.7554/eLife.29913.008Additional manipulations that evoke two-frequency undulation (2FU): Stimulation of B and AS during tail paralysis; inhomogenous mechanical environment.
https://doi.org/10.7554/eLife.29913.009Removal of B motor neurons by miniSOG and laser ablation eliminates 2FU.
https://doi.org/10.7554/eLife.29913.018Posterior undulations occur after severing the ventral and dorsal nerve cords with an infrared laser (VNC-isolated animals).
https://doi.org/10.7554/eLife.29913.022Removal of B motor neurons by miniSOG and laser ablation eliminates undulations in VNC-isolated animals.
https://doi.org/10.7554/eLife.29913.023Head undulation frequency can be entrained by rhythmic mid-body optogenetic manipulation of the muscles or cholinergic neurons.
https://doi.org/10.7554/eLife.29913.027Tables
Transgenic arrays acquired or generated for this study
https://doi.org/10.7554/eLife.29913.029| Transgene | Plasmid or reference | Description | Purpose | Strain |
|---|---|---|---|---|
| vsIs48 | (Chase et al., 2004) | Punc-17::GFP | Identification of VNC and DNC | LX929 |
| akIs11 | (Zheng et al., 1999) | Pnmr-1::ICE; lin-15(+) | Ablation of INs | VM4770 |
| kyIs36 | (Zheng et al., 1999) | Pglr-1::ICE; lin-15(+) | Ablation of INs | VM4771 |
| wenIs001 | pJH2918 | Pacr-5::ArchT::RFP; lin-15(+) | Inhibition of B motor neurons | WEN001 |
| qhIs1 | (Leifer et al., 2011; Husson et al., 2012) | Pmyo-3::NpHR::ECFP; lin-15(+) | Inhibition of muscles | YX9 |
| qhIs2 | YX10 | |||
| qhIs4 | pJH1841 | Pacr-2::wCherry; dpy-20(+) | Identification of B | YX146 |
| qhIs5 | (Xu and Chisholm, 2016) | Pmyo-3::PH::miniSOG(Q103L)+Pmyo-3::mCherry + Pttx-3::RFP | Ablation of BWM | YX203 |
| qhIs9 | (Xu and Chisholm, 2016) | Punc-17β::PH::miniSOG (Q103L)+Pacr-2::mCherry+ Pttx-3::RFP | Ablation of B | YX234 |
| hpEx803 | (Wen et al., 2012) | unc-9(fc16); hpIs3; Prgef-1-unc-9cDN)+odr-1 | Neuronal unc-9 rescue | ZM2509 |
| zxIs6 | (Liewald et al., 2008) | Punc-17::ChR2(H134R)::YFP;lin-15(+) | Stimulation of cholinergic neurons | ZX460/ ZM3265 |
| hpIs166 | (Gao et al., 2015) | Pglr-1::chop-2(H134R)::YFP; lin-15(+) | Identification of INs | ZM4624 |
| hpIs178 | (Leifer et al., 2011) | Punc-17::NpHR::ECFP; lin-15(+) | Inhibition of cholinergic neurons | ZM5016 |
| hpIs179 | (Kawano et al., 2011) | Psra-11::D3cpv | Identification of AVB | ZM5132 |
| hpIs366 | pJH2843 | Punc-4::tomm-20::miniSOG:: urSL::wCherry; lin-15(+) | Ablation of A and VC | ZM7690 |
| hpIs371 | ZM7691 |
Additional strains generated by combining transgenes in Table 1.
https://doi.org/10.7554/eLife.29913.030| Strain | Transgenes | Description | Purpose |
|---|---|---|---|
| YX119 | qhIs1; unc-49(e407) | Muscle::NpHR, unc-49 | 2FU with D function impaired |
| YX126 | qhIs1; hpIs371 | Muscle::NpHR, A/VC::miniSOG | 2FU with A and VC removed |
| YX127 | hpIs178; hpIs371; zxIs6 | Cholinergic Neurons::NpHR&ChR2, A/VC::miniSOG | Inhibition or excitation of Cholinergic neurons with A removed |
| YX135 | qhIs1; vab-7(e1562) III. | Muscle::NpHR, DB disrupted | 2FU (fails) with DB disrupted |
| YX137 | qhIs1; unc-7(e5) | Muscle::NpHR, unc-7 | 2FU with AVB::B gap junctions disrupted |
| YX139 | qhIs1; unc-9(fc16); hpEx803 | Muscle::NpHR, UNC-9 disruption in muscles only | Entrainment with BWM::BWM gap junctions disrupted |
| YX140 | qhIs1; unc-9(fc16) | Muscle::NpHR, unc-9 | 2FU with AVB::B gap junctions disrupted |
| YX148 | qhIs1; qhIs4 | Muscle::NpHR, AB::RFP | 2FU with some B removed OR undulation with nerve cords severed |
| YX152 | hpIs166; akIs11 | IN::ICE&YFP | Assessment of ICE ablations |
| YX153 | hpIs166; kyIs36 | IN::ICE&YFP | Assessment of ICE ablations |
| YX159 | qhIs2; akIs11 | Muscle::NpHR, IN::ICE | 2FU with PVC removed |
| YX160 | qhIs2; kyIs36 | Muscle::NpHR, IN::ICE | 2FU with PVC removed |
| YX177 | hpIs366; vsIs48 | A/VC::miniSOG, Cholinergic Neurons::GFP | Undulation with nerve cords severed and A removed. |
| YX223 | qhIs1; qhIs9 | Muscle::NpHR, DB ablated | 2FU with B removed OR undulation with nerve cords severed and B removed. |
| YX230 | qhIs1; hpIs179 | Muscle::NpHR, AVB labeled for ablation | 2FU with AVB removed |
Additional files
-
Transparent reporting form
- https://doi.org/10.7554/eLife.29913.031





