3D visualization of mitochondrial solid-phase calcium stores in whole cells
Figures
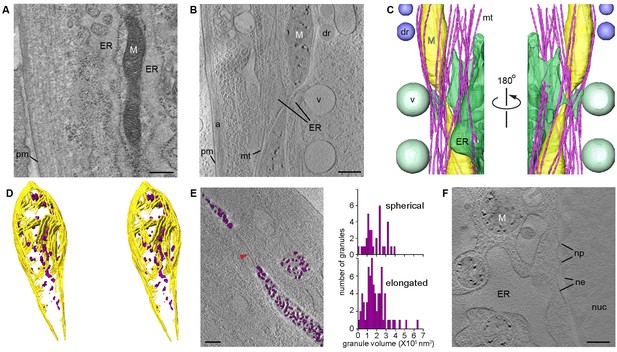
CSTET imaging shows whole mitochondria in situ in mammalian cells.
White M indicates mitochondria; pm, plasma membrane; ER, endoplasmic reticulum; mt, microtubules; dr, lipid droplet; v, vesicle; a, actin; nuc, nucleus; ne, nuclear envelope; np, nuclear pore. Scale bars are 400 nm. (A) Conventional TEM image of a heavy metal stained thin section of a human embryonic lung (WI-38) fibroblast shows staining artifacts, particularly of the mitochondrion. (B) A 30-nm thick section of a CSTET reconstruction ( 750 nm thickness in total) of a WI-38 fibroblast. (C) Segmentation of the CSTET reconstruction. (D) Stereo pair of segmentation of the upper mitochondrion from panels B and C revealing internal ultrastructure. Mitochondrial membranes and cristae are yellow, granules are purple. (E) Elongated and spherical mitochondria show similar granule size distributions. Granule volumes were segmented using an intensity threshold and are displayed in purple above a section from the corresponding tomogram. Red arrowhead indicates a fission tubule. (F) Mitochondria near the nucleus of a human dermal microvasculature endothelial cell (30-nm thick section from a region of 790 nm total thickness).
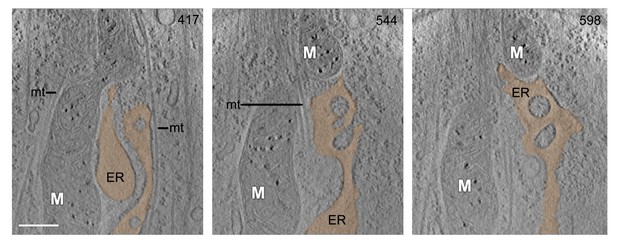
CSTET visualization of mitochondrial interactions.
An additional instance of a mitochondrial/ER junction is displayed through a series of 30-nm thick sections from a CSTET reconstruction of a region in a WI-38 fibroblast. Height (in nm) from the bottom of the cell appears in the upper right corner of each section. Scale bar is 400 nm and applies to all panels. White M indicates mitochondria. ER, endoplasmic reticulum; mt, microtubules. Some microtubules cross the mitochondrial constriction site and others surround the mitochondrion and ER. ER is shaded orange. Total thickness of the cell in the region of the tomogram is 700 nm.
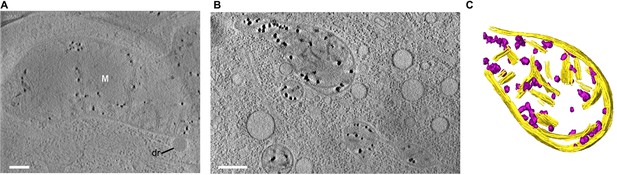
Matrix granules are ubiquitous in mitochondria.
All panels show 30-nm thick sections from CSTET reconstructions. White M indicates mitochondria. dr, lipid droplet. Scale bars 400 nm. (A) U2OS osteosarcoma cell. Thickness of the cell in the region of the tomogram is 440 nm. (B) Primary human dermal microvascular endothelial cell (HDMEC). Thickness of the cell in the region of the tomogram is 415 nm. (C) Segmentation of the largest mitochondrion from the tomogram show in panel B. Mitochondrial membranes and cristae are yellow, granules are purple.
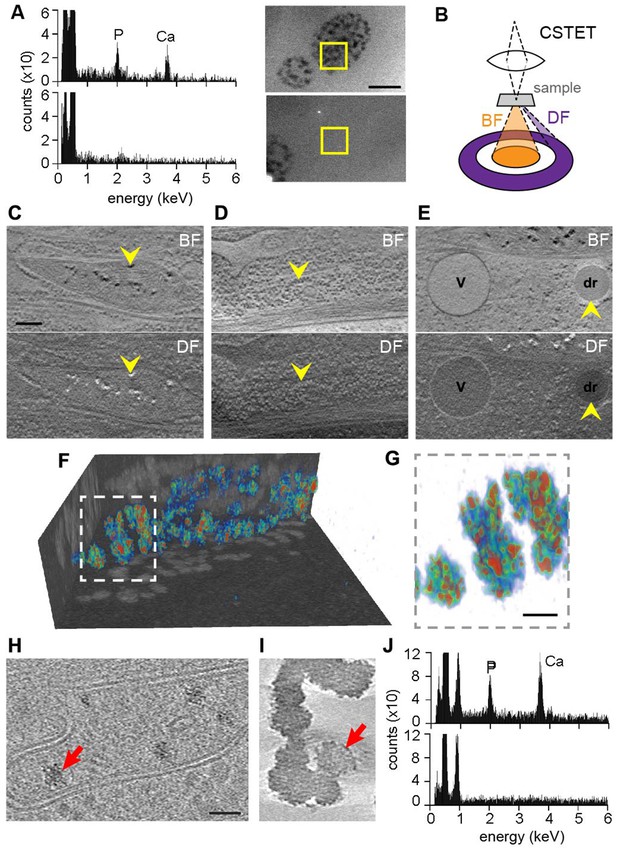
Elemental characterization of mitochondrial granules.
(A) EDX identifies calcium (Ca) and phosphorus (P) enrichment in mitochondria. Areas of a WI-38 fibroblast subjected to EDX (boxed) were imaged prior to spectroscopic analysis. Scale bar is 400 nm. (B) Schematic (not to scale) showing collection of BF and DF CSTET data. (C–E) Scale bar is 200 nm. Sections of BF (top) and DF (bottom) reconstructions display (C) mitochondrial granules (arrowheads), (D) a polyribosome (arrowheads), and (E) a lipid droplet (dr; arrowheads) and a vesicle (v). (F) Color-coded 3D volume rendering (red: high density; blue: low density) showing heterogeneity of density in granules of a WI-38 fibroblast. Granules are presented against backdrops of projected BF volume densities with inverted contrast. (G) Zoom in on the boxed region of F. Scale bar is 50 nm. Sections 10-nm thick from zlTEM tomographic reconstructions are shown for (H) a thin region of a WI-38 fibroblast cell displaying mitochondrial granules and (I) synthetic amorphous calcium phosphate. The synthetic particles shown here were obtained 1.5 min after mixing of calcium and phosphate solutions. Red arrows in panels H and I highlight particles for comparison. Scale bar in H is 50 nm and applies also to I. (J) EDX of synthetic amorphous calcium phosphate (top) compared to an adjacent region of vitrified solution (bottom).
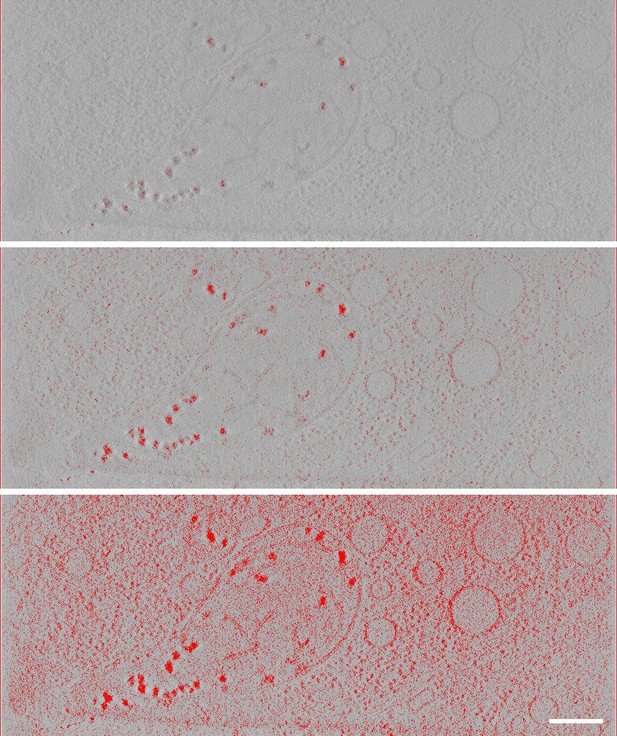
Intensity thresholding for quantitative estimation of mitochondrial granule scattering.
The BF tomogram obtained from the HDMEC cell imaged in Figure 1—figure supplement 2B was used for analysis. A movie of the tilt series and reconstruction of this cell region are shown in Videos 7 and 8. Mean intensity in the selected slice is 9.7, with standard deviation 2.4. The aqueous medium filled in at a threshold of 16 (not shown). With a threshold of 3.0, dense parts of mitochondrial granules were selected (top). The densest peaks reached an intensity value of 0.3 (not shown). With a threshold of 5.3, most of the granule volumes and the densest regions of ribosomes were selected (middle). A threshold of 7.5 captures most of the ribosomal volumes (bottom). Scale bar is 400 nm. These data are used in Table 3 to estimate the material densities of granules.
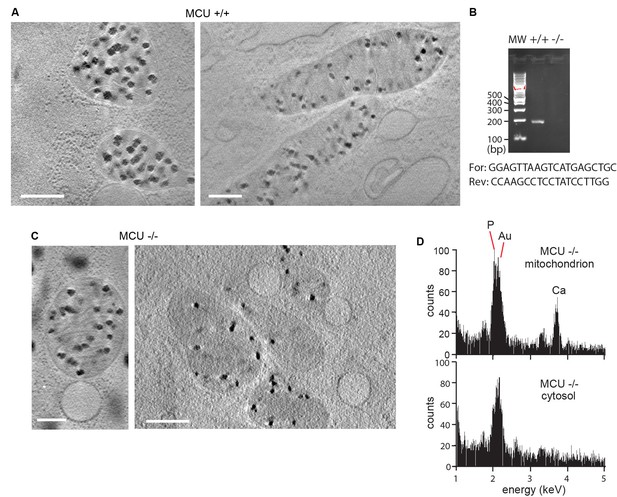
Large granules are observed in mitochondria of murine embryonic fibroblasts (MEFs) and are independent of the presence of the mitochondrial calcium uniporter MCU.
(A) Mitochondria in wild-type MEFs. Scale bars are 400 nm. (B) PCR was used to confirm the MCU -/- and +/+genotypes (Pan et al., 2013). (C) Mitochondria from MCU -/- MEFs contain granules. Scale bars are 200 nm in the left panel and 400 nm in the right. (D) EDX confirms the presence of calcium and phosphorus in granules of MCU -/- MEFs. The gold (Au) peak partially overlapping the phosphorus peak is present in this spectrum but not that shown in main Figure 2A due to a change in the microscope aperture hardware that occurred between the two experiments.
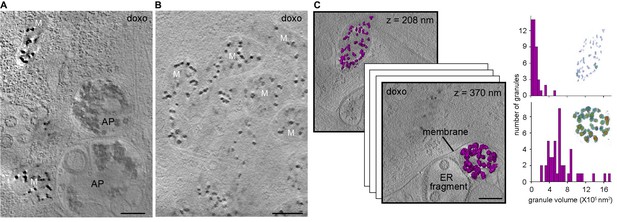
Effect of cell stress on mitochondrial granules.
Sections 30-nm thick from BF CSTET reconstructions of doxorubicin-treated WI-38 cells are displayed. Scale bars are 400 nm. (A) Mitochondria in the vicinity of autophagosomes (AP) (cell is 890-nm thick in this region). (B) Aggregated mitochondria (cell is 1-μm thick). (C) Different granule sizes in two nearby mitochondria separated by a membrane. Granule volumes were segmented using an intensity threshold and are displayed in purple. Height (in nm) from the bottom of the cell (which is 800-nm thick in this region) is in the upper right corner of each section. Histograms of granule sizes are displayed for the two mitochondria. Insets show granules colored as in Figure 1F. Tilt series and reconstruction movies of a doxorubicin-treated cell are in Videos 10 and 11.

Region of a doxorubicin-treated fibroblast cell.
A region of a WI-38 fibroblast shows multivesicular bodies and irregular membrane structures. Organelles consistent with the size of round mitochondria contain dense material with a fibrous or crystalline needle form (yellow arrowheads). Z-sections 30-nm thick from a CSTET reconstruction are shown. Height (in nm) from the bottom of the cell appears in the upper right corner of each section. Scale bar is 400 nm.
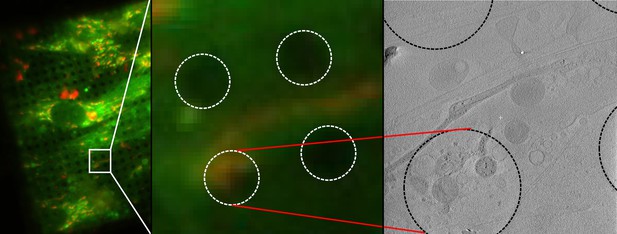
Correlative imaging of mitochondria using fluorescence from a membrane-potential reporter.
Mitochondria in WI-38 fibroblasts were stained with JC-1, cryo-preserved, and imaged successively by fluorescence microscopy and CSTET. A field of JC-1 stained cells is shown on the left. The section of the field corresponding to the tomogram is enlarged in the middle panel. The right panel shows a slice of the tomogram. Dashed circles indicate the holes (3.5 μm diameter) in the carbon support on which the cells were grown. Mitochondria show irregular morphology and partial dissolution of granules.
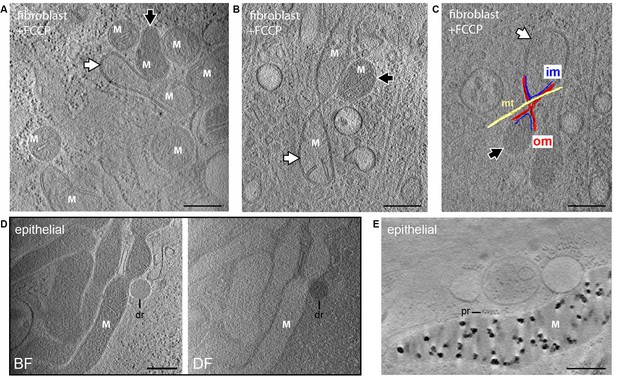
Dissipation of matrix granules.
All panels show 30-nm thick sections from CSTET reconstructions. White M indicates mitochondria. Scale bars are 400 nm. (A,B) Fibroblasts treated with FCCP show no granules. White and black arrows indicate mitochondria with weakly and strongly scattering matrices, respectively. (C) Distinct matrix densities in mitochondria sharing contiguous outer membranes in FCCP-treated cells. om, outer membrane; im, inner membrane; mt, microtubule. (D) Group of granule-free mitochondria in MCF10A cells. dr, lipid droplet. Cell is 800-nm thick in this region. (E) MCF10A mitochondria containing granules. pr, polyribosome.
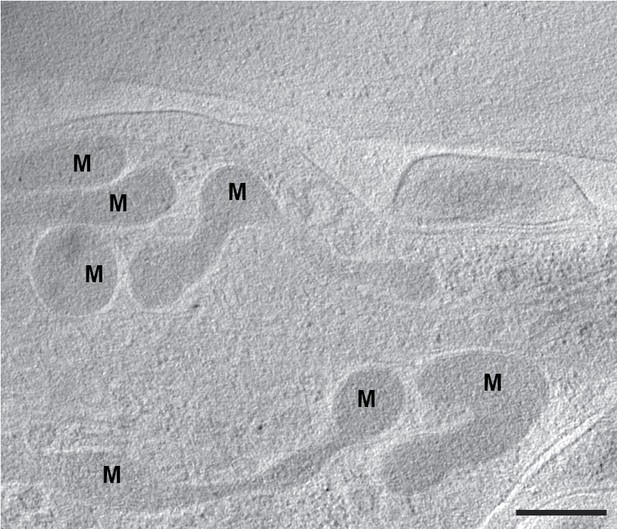
Granule-free mitochondria in fibroblasts nearing confluence.
A section of a tomogram of WI-38 fibroblasts that had grown to near confluence on the grid shows mitochondria lacking granules. Black M indicates mitochondria. Scale bar is 400 nm.
Videos
Aligned tilt series of CSTET BF images from a region 750-nm thick within a WI-38 fibroblast.
The tilt series corresponds to Figure 1B. Scale bar is 400 nm.
Aligned tilt series of CSTET DF images from a region 750-nm thick within a WI-38 fibroblast.
The data for this tilt series were collected simultaneously with those shown in Video 1. Scale bar is 400 nm.
BF tomographic reconstruction of a region within a WI-38 fibroblast.
The reconstruction corresponds to Figure 1B and was done based on the tilt series shown in Video 1. Scale bar is 400 nm.
DF tomographic reconstruction of a region within a WI-38 fibroblast.
The reconstruction was done based on the tilt series shown in Video 2.
Animation of segmentation shown in Figure 1C, overlaid on the reconstruction shown in Figure 1B and Video 3.
The reconstruction contrast has been inverted.
Animation of segmentation shown in Figure 1D.
https://doi.org/10.7554/eLife.29929.010Aligned tilt series of CSTET BF images from a region within a human dermal microvascular endothelial cell, shown in Figure 1—figure supplement 2B.
Scale bar is 400 nm.
BF tomographic reconstruction of a region within a human dermal microvascular endothelial cell, shown in Figure 1—figure supplement 2B.
The reconstruction was done based on the tilt series shown in Video 7. Scale bar is 400 nm.
Animation of segmentation shown in Figure 1—figure supplement 2C, overlaid on the reconstruction shown in Figure 1—figure supplement 2B and Video 8.
The BF reconstruction contrast has been inverted.
Aligned tilt series of CSTET BF images from a region within a WI-38 fibroblast treated with doxorubicin.
Scale bar is 400 nm.
BF tomographic reconstruction of the tilt series in Video 10.
Scale bar is 400 nm.
Tables
Computation of atom number densities for ribosomes and calcium phosphate.
https://doi.org/10.7554/eLife.29929.017| Element | # of atoms | Mole fraction | # atoms/nm3 | |
|---|---|---|---|---|
| ribosomal RNA* | C | 68671 | 0.295 | 31.2 |
| H | 78158 | 0.336 | 35.5 | |
| N | 27884 | 0.120 | 12.7 | |
| O | 50462 | 0.217 | 22.9 | |
| P | 7216 | 0.031 | 3.3 | |
| Mg | 239 | 0.001 | 0.24 | |
| Ribosomes† | C | 135061 | 0.186 | 19.3 |
| H‡ | 372416 | 0.514 | 53.2 | |
| N | 48041 | 0.0663 | 6.86 | |
| O‡ | 161037 | 0.222 | 23.0 | |
| S | 501 | 0.000692 | 0.072 | |
| P | 7216 | 0.00996 | 1.03 | |
| Mg | 239 | 0.000330 | 0.034 | |
| Ca3(PO4)2 | Ca | 3 | 0.231 | 18.4 |
| as crystalline TCP§ | P | 2 | 0.154 | 12.2 |
| O | 8 | 0.615 | 49.1 |
-
* The partial specific volume of RNA was taken to be 0.569 cm3/g (Voss and Gerstein, 2005).
† A volume of 7000 nm3 was estimated to enclose the ribosome (Protein Data Bank ID 4UG0) based on a ~ 2 nm-resolution isosurface calculated using Chimera (Pettersen et al., 2004). Solvent within this isosurface (42%) was treated as bulk vitreous ice for atom number density summations.
-
‡ Includes solvent atoms.
§ A density of 3.14 g/cm3 was taken for crystalline TCP.
Prediction of BF signals for conditions used in tomographic data collection.
From these data, we obtain the predicted intensity ratios between ribosomes and water, ribosomal RNA and water, and TCP and water.
| Scattering cross-sections per atom (>5 mrad) | |
|---|---|
| H* | - |
| C | 0.0042 |
| N | 0.0047 |
| O | 0.0051 |
| P | 0.0165 |
| Mg | 0.0097 |
| S | 0.0182 |
| Ca | 0.0278 |
| Estimated scattering signal per nm3 material | |
| Water | 0.159 |
| Ribosome | 0.249 |
| ribosomal RNA | 0.364 |
| TCP | 0.963 |
| Predicted scattering intensity ratios | |
| Ribosome/water | 1.57 |
| RNA/water | 2.29 |
| TCP/water | 6.06 |
-
* Hydrogen does not scatter electrons above the cutoff angle for the BF detector.
Granule density evaluation.
https://doi.org/10.7554/eLife.29929.019| Component | Threshold level in tomogram | Ratio to water | Ratio to TCP |
|---|---|---|---|
| Granule (peak) | 0.3 | 2.89 (2.05)† | 0.48 (0.34)† |
| Granule (inclusive) | 3.0 | 2.57 (1.87) | 0.42 (0.31) |
| Ribosome (peak) | 5.3 | 2.29 (1.72) | 0.38 (0.28) |
| Ribosome (inclusive) | 7.5 | 2.02 (1.57) | 0.33 (0.26) |
| Water (cytosol) | 16 | 1.00 | 0.16 |
| Extrapolated background | 24.3* (30.9)† | ||
| TCPc | −26.0‡ (−59.4) |
-
‡ The threshold for TCP is predicted based on the scattering intensity ratio given in Table 2.
| Reagent type (species) or resource | Designation | Source or reference | Identifiers |
|---|---|---|---|
| Cell line | WI-38 | Coriell Institute | NA06814 |
| Cell line | Human Dermal Microvascular Endothelial Cells (HDMEC) juvenile foreskin | Promocell | C-22020 |
| Cell line | U2OS | ATCC | HTB-96 |
| Cell line | MCF10A | ATCC | CRL-10317 |
| Cell line | mouse embryonic fibroblasts | Weizmann Institute Stem Cell Core Unit | MEFs |
| Cell line | mouse embryonic fibroblasts (MCU -/-) | Pan et al., 2013 | MEFs MCU -/- |
| Cell line | mouse embryonic fibroblasts (MCU +/+) | Pan et al., 2013 | MEFs MCU +/+ |
Additional files
-
Transparent reporting form
- https://doi.org/10.7554/eLife.29929.027





