The role of microglia and their CX3CR1 signaling in adult neurogenesis in the olfactory bulb
Figures
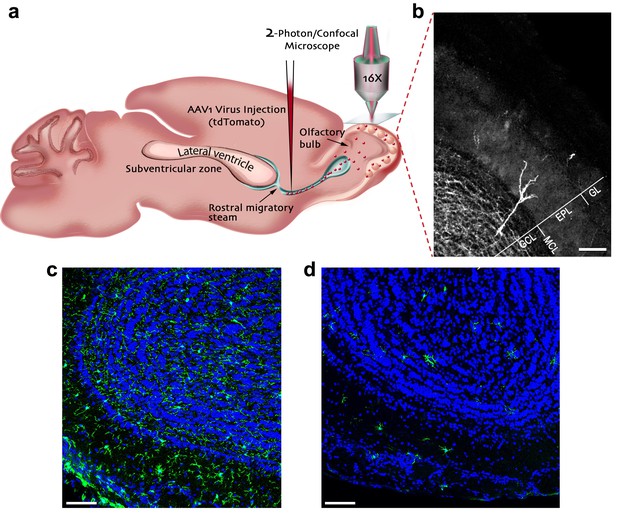
Experimental preparation for investigating the role of microglia in the development, maturation and plasticity of adult-born granule cells in the olfactory bulb.
(a) A scheme of the experimental design: Mice were injected into the rostral migratory stream (RMS) with a virus that induces the expression of TdTomato fluorescent protein in adult-born neurons migrating from the subventricular zone (SVZ) to the OB. (b) A micrograph depicting an adult-born granule cell, expressing TdTomato, with its cell body in the granule cell layer (GCL) and its distal dendrites extending within the external plexiform layer (EPL; situated under the glomerular layer (GL)). Scale bar: 100 µm. (c) Fluorescent micrograph of a coronal section from the OB in a mouse fed with control diet shows the distribution of Iba-1 labeled microglia (green ells) within the OB (blue = DAPI nuclear staining). (d) Fluorescent micrograph of a coronal section from the OB in a mouse fed with PLX5622-containing diet for 28 days, demonstrating a near complete depletion of microglia in the OB. Scale bar: 100 μm.
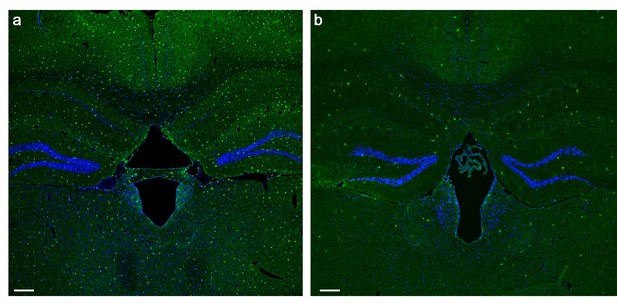
PLX5622 treatment induces brain-wide microglia depletion.
(a) Representative low magnification (X4) projection images of coronal sections from the brain of a mouse fed with a control diet and (b) a mouse fed with a PLX5622-containing diet for 28 days. The marked (>80%) reduction in the normal density of GFP-labeled microglia (green cells) following the PLX5622 treatment is clearly depicted. All cell bodies are stained by DAPI (blue). Scale bar = 200 µm.
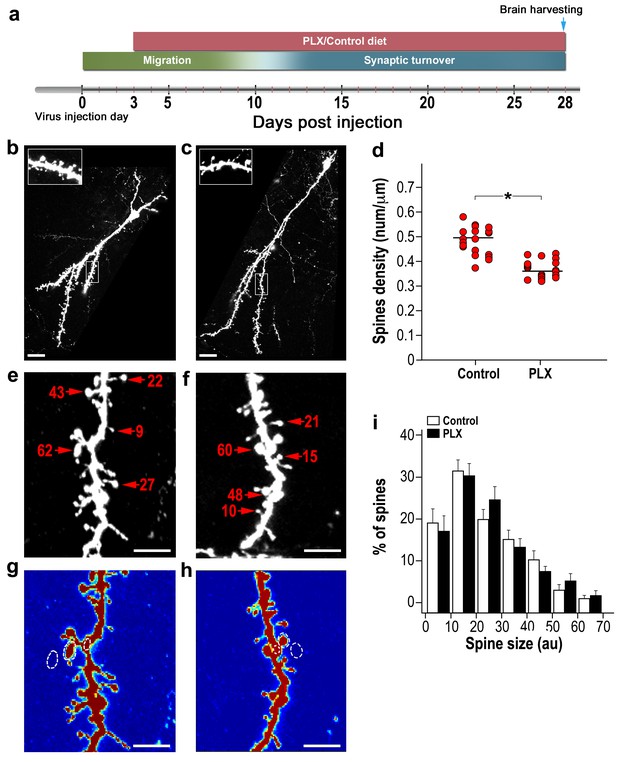
Adult-born granular cells (abGCs) in microglia-depleted mice have lower spine density, but not spine size, compared to abGCs in WT control mice.
(a) Schematic time-line of the experiment. Adult-born cells were transduced by an injection of Td-Tomato-expressing adeno-associated virus 1 (AAV1) into the RMS. PLX5622/control diet feeding commenced 3 days later. Brains were harvested 28 days after the AAV1 injection. (b) High resolution projection images of abGCs and their spiny dendritic branches from a control mouse and (c) a microglia-depleted (PLX5622-treated) mouse. Scale bar: 20 µm. Insets: enlarged spiny dendritic branches. (d) abGCs spine density in groups fed with a control diet was significantly higher than in the PLX5622 diet group (t(38)=6.9, *p=2.98e-08, two-sample t-test). Each dot represents the spine density of an individual GC. The mean of each group is sown by a horizontal line. n = 20 cells from four mice for each of the two groups. Overall, approximately 4000 spines were analyzed. (e) A representative high resolution projection image of a dendritic segment with five representative spine heads, marked by an arrowhead along with their measured sizes (in arbitrary units (AU)) in a control mouse, and (f) a PLX5622-treated mouse. Scale bar: 10 µm. (g) An example of the analyzed dendritic segment and the analysis of spine size in a control mouse and (h) a PLX5622-treated mouse. Color intensity reflects the fluorescence intensity. The regions marked for analysis are: the spine head (middle), the adjacent background (left), and the adjacent dendritic shaft (right) (see Methods section for a detailed explanation of the analysis). (i) Distributions of abGCs spine sizes in control and PLX5622-treated mice. n = 720 spines from 13 cells from four mice from each group. Two sample Kolmogorov–Smirnov test for probability distributions revealed no group differences (Dn,n’=0.071, p=0.978).
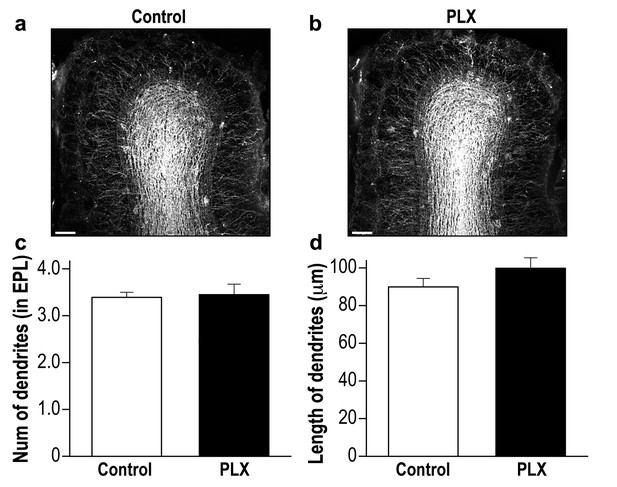
PLX5622-treated and control mice display a similar global neurogenesis process.
(a) Representative low magnification (X10) projection images of the olfactory bulb of an AAV1-injected control mouse and (b) a PLX5622-treated mouse. The images demonstrate the similarity of the distributions of abGCs within the granule cell layer of the control and PLX5622-treated mice, as well as the similar extension of the abGCs' dendrites within the EPL. (c) The average number of dendrites branching from the main dendritic shaft per abGC in control and PLX5622-treated mice. Only dendrites studded with spines were included in this analysis. n = 15 cells from four mice in each group. p=0.78, two-sample t-test. (d) The average length of the dendrites analyzed in (c). p=0.16, two-sample t-test. Scale bar = 100 µm.
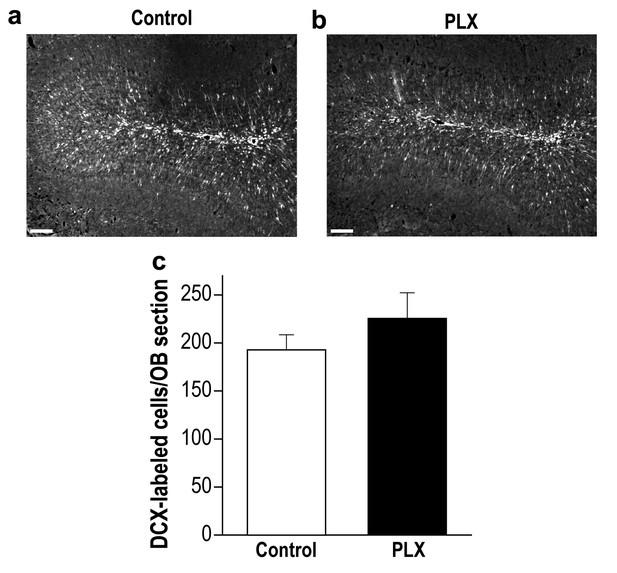
PLX5622-treated and control mice display a similar number of young new-born neurons in the OB.
(a) Representative low magnification (X10) projection images of a DCX-stained olfactory bulb from a control mouse and (b) a PLX5622-treated mouse. (c) There was no difference in the average number of DCX-labeled neurons in control and PLX5622-treated mice. n = 5 mice in each group. p=0.26, two-sample t-test. Scale bar = 100 µm.
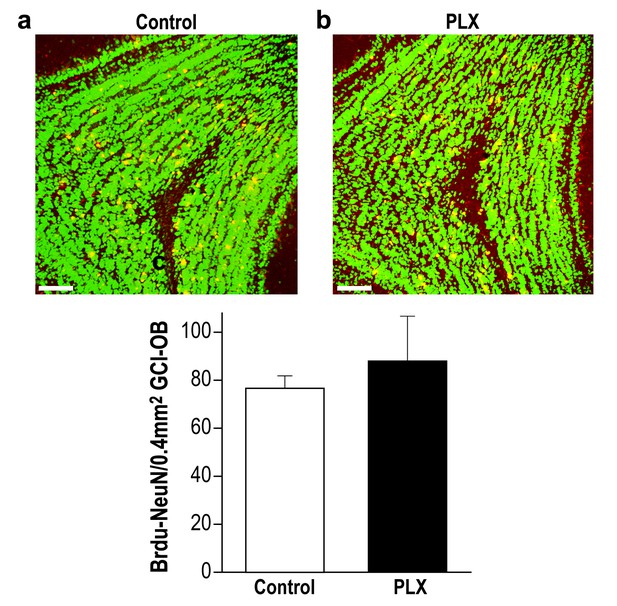
PLX5622-treated and control mice display similar survival rate of 28-day-old new-born neurons in the OB.
(a) Representative high magnification (X20) projection images of a Brdu-NeuN stained granule cell layer (GCL) in the olfactory bulb from a control mouse and (b) a PLX5622-treated mouse. (c) There was no difference in the average number of 28-day-old Brdu-NeuN neurons in control and PLX5622-treated mice. n = 3 mice in each group. p=0.6, two-sample t-test. NeuN = Green; Brdu = Red; Double labeling = Yellow. Scale bar = 65 µm.
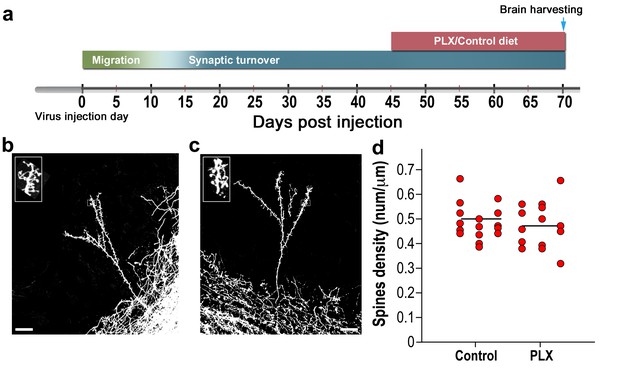
Spine density in mature abGCs is not affected by microglia depletion.
(a) Schematic time-line of the experiment. Adult-born cells were transduced by an injection of Td-Tomato-expressing AAV1 into the RMS. PLX5622/control diet feeding commenced 45 days later, i.e., when the cells were fully mature, and was continued for 25 days. Brains were harvested 70 days after the AAV1 injection. (b) High resolution projection images of mature abGCs and their spiny dendritic branches from a control mouse and (c) a microglia-depleted (PLX5622-treated) mouse. Scale bar: 20 µm. Insets: enlarged spiny dendritic branches. (d) The spine density in mature GCs in mice fed with control diet was similar to the spine density in mice treated with PLX5622 diet (t(34)=0.82, p=0.41, two-sample t-test). Each dot represents the spine density of an individual abGC. The mean of each group is depicted by a horizontal line. n = 18 mature abGCs from four mice for each of the two groups. Overall, approximately 3600 spines were analyzed.
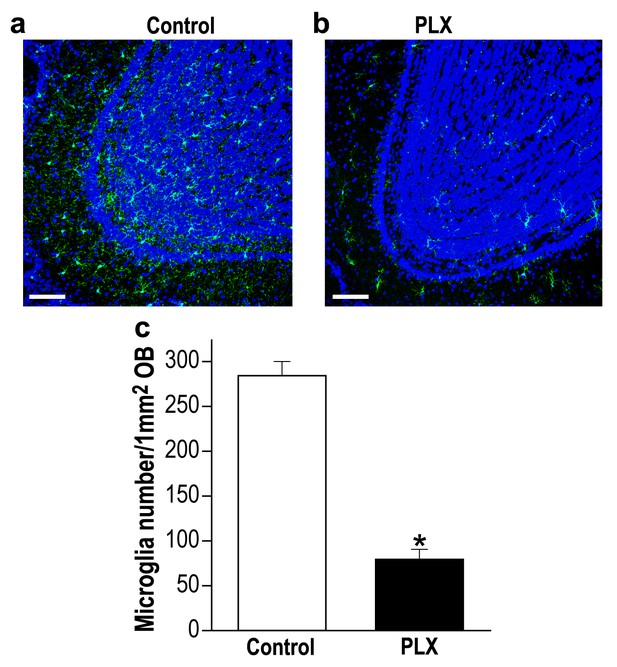
Delayed PLX5622 treatment (from 45 to 70 d.p.i) induces microglia depletion in the OB.
(a) Representative low magnification (X20) projection images of the olfactory bulb of an Iba-1 stained control mouse and (b) a mouse treated with PLX5622, from 45 to 70 d.p.i. (c) The average number of microglia in the OB was significantly reduced in the PLX5622-treated mice as compared to control diet-treated mice (t(4)=10.47, *p=4.7e-04, two-sampled t-test). n = 3 mice in each group. (DAPI = Blue; Iba1 = Green). Scale bar = 100 µm.
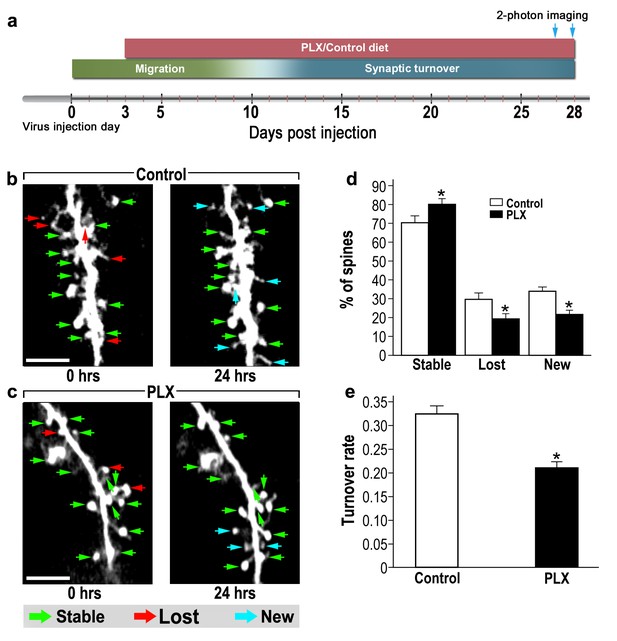
abGCs in microglia-depleted mice have lower spine formation and elimination compared with control mice.
(a) Schematic time-line of the experiment. Adult-born cells were transduced by administration of Td-Tomato-expressing AAV1 into the RMS. PLX5622/control diet feeding commenced 3 days later. Time-lapse two-photon imaging was performed on days 27 and 28 after the injection. (b) Two projection images of the same abGC dendritic segment, imaged in vivo at a 24 hr interval in a control mouse, and (c) a microglia-depleted (PLX5622-treated) mouse. The green, red and blue arrowheads mark stable, lost and new spines, respectively. Scale bars: 10 µm. (d) Analysis of the in vivo spine dynamics over the 24 hr time lapse (n = 17 dendritic segments from 14 cells in four control mice (for a total of 560 spines), n = 17 dendritic segments from 15 cells in 4 PLX5622-treated mice (for a total of 440 spines)). ANOVA with the group as a between subjects factor and the spine category (Stable, Lost, New) as a within-subjects repeated measure factor revealed an overall group by category interaction (F(2,64)=18.1, *p=7.73e-07). Specific comparisons using two sample t-tests with Bonferroni's correction revealed significant differences between the control group and the PLX5622-treated group within each category (Stable: t(32)=3.3, *p=0.0018; Lost: t(32)=3.38,*p=0.0023; New: t(32)=5.76, *p=2.1-e06). (e) Mean turnover rate (TOR) of abGCs spines over the 24 hr interval. A significant difference was found between the two groups (t(32) =6.78), *p=1.15e-07), two sample t-test. Data are presented as the mean ±SEM.
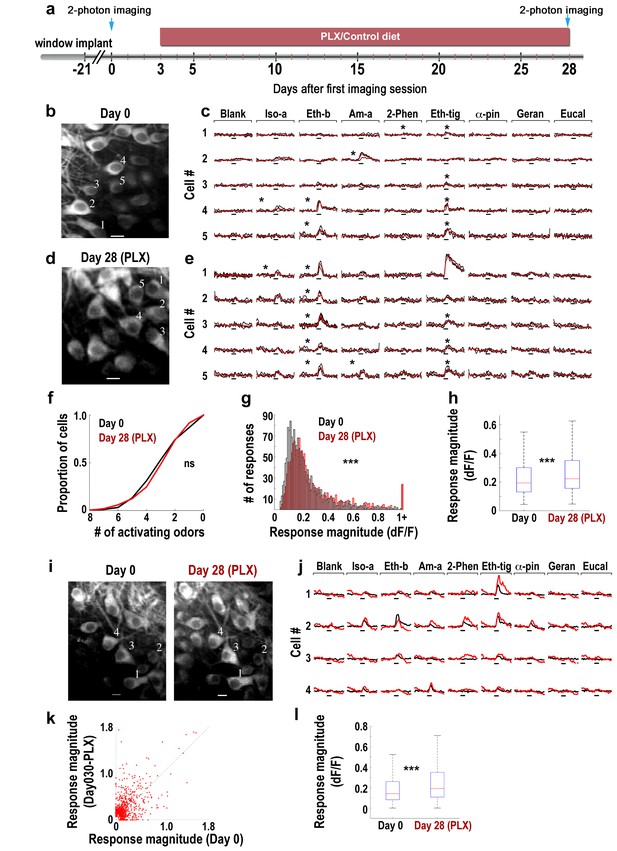
Response magnitude of MCs increases following microglia depletion.
(a) Schematic time-line of the experiment. Three Thy1-GCamp3 mice with implanted cranial windows were imaged for calcium responses of MCs to eight monomolecular odors, both at baseline (day 0) and 30 days later, following microglia depletion induced by PLX5622 treatment. (b) In vivo two-photon micrograph of a representative field of MCs at the first imaging session (day 0). Scale bar = 10 μm. (c) Examples of calcium transients from the five neurons marked on the left image in response to eight odors during baseline. Odor stimulation was applied for 2 s, denoted as a black line under each trace. Black traces represent the three single trials and the red trace is the average. Asterisks denote a statistically significant response to that odor. Scale - 100% ∆F/F. Iso-a = Isoamyl acetate, Eth-b = Ethyl butyrate, Am-a = Amyl Acetate, 2-Phen = 2 phenylethanol, Eth-tig = Ethyl tiglate, α-pin = Alpha Pinen, Geran = Geraniol, Eucal = Eucalypyol. (d) Same as in panel b but for data collected from a different mouse, following 30 days of PLX5622 administration. (e) Examples of calcium transients from five neurons marked on the left image in response to eight odors. (f) Cumulative distribution of the proportion of MCs responding to 0–8 odors, before (Day 0; n = 622 MCs) and after (Day 30; n = 575 MCs) microglia depletion. The distributions in the two groups were not significantly different (Dn,n’=0.0649, p=0.155, two-sample Kolmogorov-Smirnov test). (g) Histograms showing the response magnitudes of all the significant cell odor pair responses. Data is based on imaging 622 neurons for a total of n = 1641 odor pairs on Day 0 (black bars), and 575 neurons for a total of n = 1492 odor pairs on Day 30 (red bars). Distributions are significantly different (Dn,n’=0.1114, ***p=6.30e-09, two-sample Kolmogorov–Smirnov test). (h) Box plots for the distributions shown in (g) (t(3131) = −4.9771, ***p=6.8e-07, two sample t-test). (i) In vivo two-photon micrographs of the same field of MCs before and after microglia depletion. Scale bar: 10 µm. (j) Representative examples of the mean calcium transients evoked by eight odors before (black) and after (red) microglia depletion. Data is from the four neurons marked on the image in F. Scale - 100% ∆F/F. Odors are the same as in A. (k) Scatter plot of the individual cell-odor pair responses from the neurons at day 0 (x-axis) and at day 30 (y-axis). n = 180 neurons with a total of n = 654 cell-odor pairs. (l) Box plots of the repeated neuronal responses before and after microglia depletion (t(653)=6.3525, ***p=3.97e-10, paired t-test).
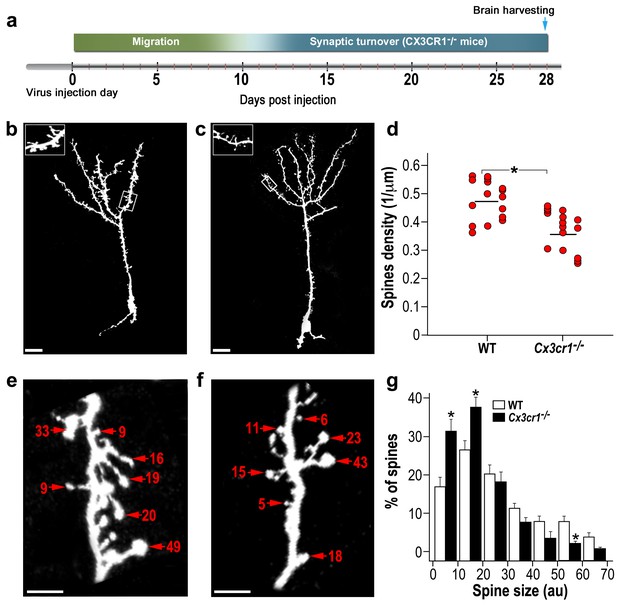
abGCs in Cx3cr1−/− mice have lower spine density and smaller spine sizes compared to WT mice.
(a) Schematic time-line of the experiment. Adult-born cells were transduced with a TdTomato-expressing AAV1 injection into the RMS. (b) High resolution projection images of abGCs and their spiny dendritic branches from a WT control mouse, and (c) a Cx3cr1−/− mouse. Scale bar: 20 µm. Insets: enlarged spiny dendritic branches. (d) abGCs spine density in the WT group was significantly higher than in the Cx3cr1−/− group (t(38)=5.1; *p=9.71e-06, two-sample t-test). Each dot represents the spine density of an individual GC. The mean of each group is shown by a horizontal line. n = 20 cells from five mice for each of the two groups. Overall, approximately 4000 spines were analyzed. (e) Representative high resolution projection images of dendritic segments from a WT and (f) a Cx3cr1−/− mouse, with seven representative spine heads, marked with arrowheads along with their measured sizes (in arbitrary units (AU). Scale bar: 10 µm. (g) The overall distributions of abGCs spine sizes (based on n = 760 spines from 13 and 14 cells from four mice from each group) was significantly different between WT and Cx3cr1−/− mice (Dn,n’=0.237; p=0.0078 in the Kolmogorov–Smirnov test for probability distributions), and specifically for spine sizes 0–10 au (t(25)=3.91, *p=6.2e-04), 10–20 au (t(25)=3.14, *p=0.004), and 50–60 au (t(25)=3.69, *p=0.001), two-sample t-tests (with Bonferroni's correction).
Cx3cr1−/− and WT mice display a similar global neurogenesis process.
(a) Representative low magnification (X10) projection images of the olfactory bulb of an AAV1-injected control mouse and (b) a Cx3cr1−/− mouse. The images demonstrate the similarity of the distributions of abGCs within the granule cell layer of the control and Cx3cr1−/− mice, as well as the similar extension of the abGCs' dendrites within the EPL. (c) The average number of dendrites branching from the main dendritic shaft per abGC in control and Cx3cr1−/− mice. Only dendrites studded with spines were included in this analysis. n = 15 cells from five mice in each group. p=0.825, two-sampled t-test. (d) The average length of the dendrites analyzed in (c). p=0.4, two-sampled t-test. Scale bar = 100 µm.
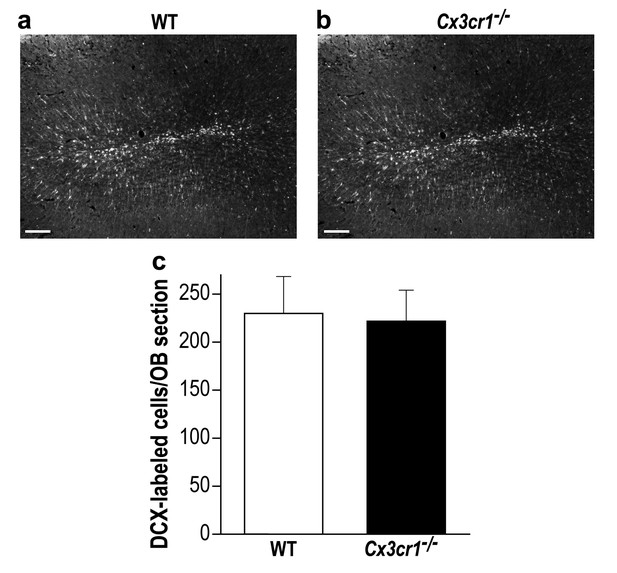
Cx3cr1−/− and control mice display similar young new-born neurons number in the OB.
(a) Representative low magnification (X10) projection images of a DCX-stained olfactory bulb from a WT mouse and (b) a Cx3cr1−/− mouse. (c) There was no difference in the average number of OB DCX-labeled neurons in control and Cx3cr1−/− mice (in contrast with previous findings from our group and others, showing reduced number of adult-born neurons in the hippocampus of the Cx3cr1−/− mice (Maggi et al., 2011; Gemma and Bachstetter, 2013 ; Reshef et al., 2014). n = 4 mice in each group. p=0.85, two-sample t-test. Scale bar = 100 µm.
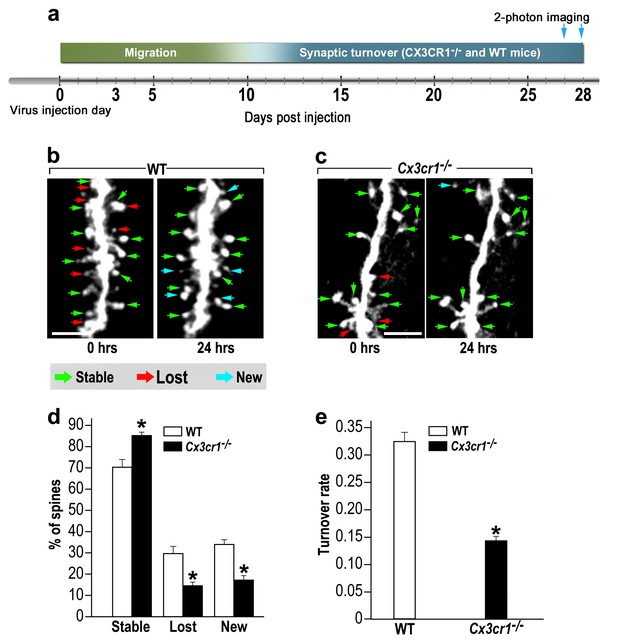
abGCs in Cx3cr1−/− mice have lower spine formation and elimination compared with WT mice.
(a) Schematic time-line of the experiment. Adult-born cells were transduced with a TdTomato-expressing AAV1 injection into the RMS. (b) Two projection images of the same abGC dendritic segment are depicted, imaged in vivo at a 24 hr interval in a WT mouse, and (c) a Cx3cr1−/− mouse. The green, red and blue arrowheads mark stable, lost and new spines, respectively. Scale bars: 10 µm. (d) Analysis of the in vivo spine dynamics over the 24 hr time lapse (n = 16 segments from 14 cells in 4 WT mice, n = 538 spines; n = 18 segments from 16 cells in 4 Cx3cr1−/− mice n = 450 spines). ANOVA with the group as a between subjects factor and the spine category (Stable, Lost, New) as a within-subjects repeated-measures factor revealed an overall group by category interaction (F(2,64)= 48.1, p=1.77e-13). Specific comparisons using two sample t-tests with Bonferroni's correction revealed significant differences between the WT group and the Cx3cr1−/− group within each category (Stable: t(32)=6.0, *p=1.08e-06; Lost: t(32)=6.1, *p=8.1e-071; New: t(32)=8.7,*p=6.9e-10), two sample t-test. (e) Mean turnover rate (TOR) of abGCs spines over the 24 hr interval. A significant difference was found between the two groups (t(32)=9.6; *p=6.03e-11). Data presented as the mean ±S.E.M.
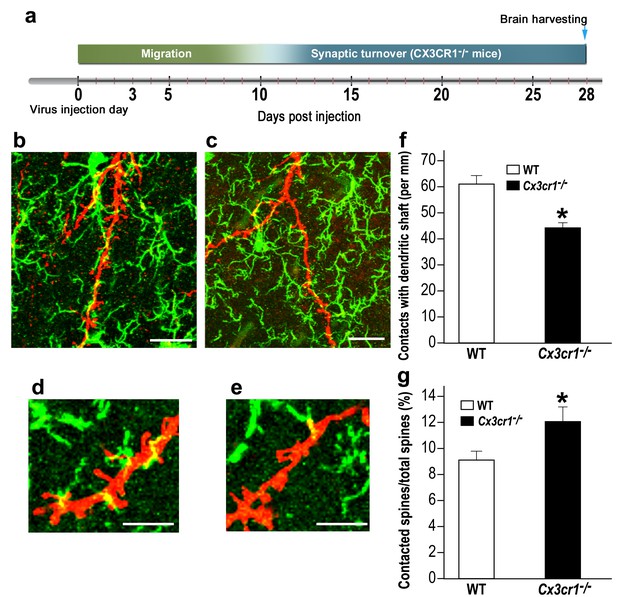
Cx3cr1 deficient microglia show aberrant interactions with abGCs.
(a) Schematic time-line of the experiment. Adult-born cells were transduced with a TdTomato-expressing AAV1 injection into the RMS, and the pattern of microglia-dendrites contacts were analyzed 28 days later. (b) High resolution projection images showing the interaction between microglia and an abGC dendrite from a WT mouse, and (c) a Cx3cr1−/− mouse (abGC dendrite = red; microglia = green). Scale bar: 20 µm. (d) High magnification of the interaction between microglial processes and a single dendritic segment from a WT mouse, and (e) a Cx3cr1−/− mouse. Scale bar: 10 µm. (f) Density of the contacts between microglia and dendritic shaft (expressed as the contact number per mm length of the shaft) in the WT and Cx3cr1−/− groups. n = 13 cells from four animals in each group. Overall, 70 dendrites were analyzed (t(24)=4.1, *p=4.1e-04, two-sample t-test). (g) The percentage of spines contacted by microglia out of the total spine population in the WT and Cx3cr1−/− groups. n = 13 cells from four animals in each group. Overall, approximately 2400 spines were analyzed (t(24)=2.2, *p=0.03, two-sample t-test).
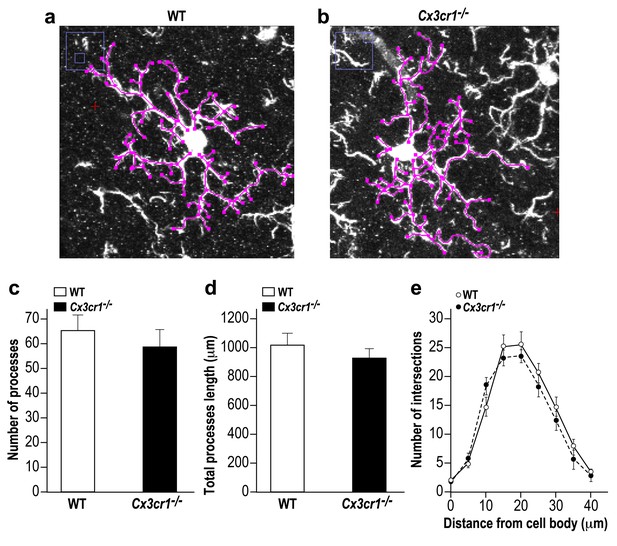
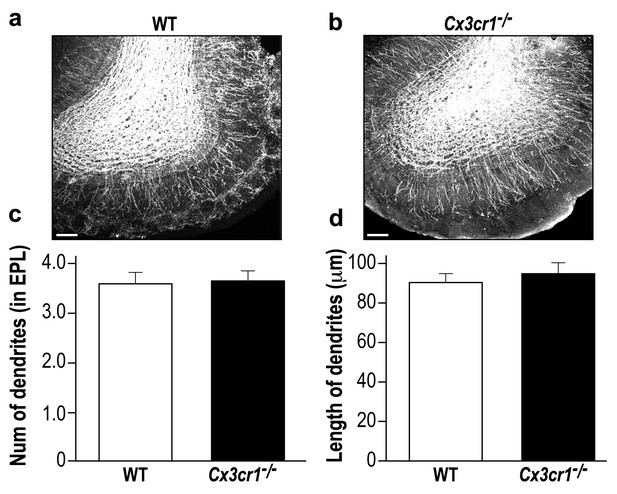
Microglia in Cx3cr1 deficient and WT mice have similar morphology.
(a) A Z-projection image of a microglia in the olfactory bulb of a WT mouse, and (b) a Cx3cr1−/− mouse. The colored tracings exemplify the analysis of the processes morphology. (c) The number of processes per microglia cell was similar in Cx3cr1−/− and WT mice. n = 10 cells from four mice in each group. p=0.44, two-sample t-test. (d) There was no difference in the total processes length between the groups. p=0.43, two-sample t-test. (e) Sholl analysis revealed that there were also no differences in the spatial dispersion of the microglial processes. p=0.305, ANOVA with group as a between subjects factor and the distance from the soma as a repeated measures, within subjects factor.
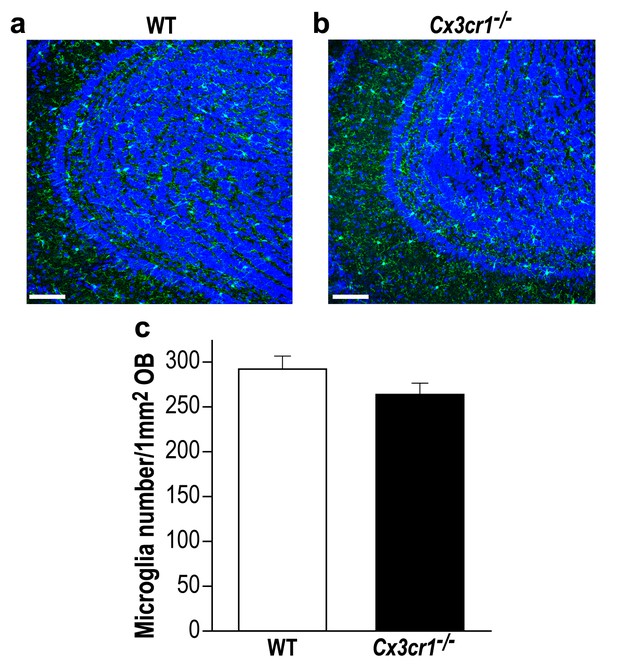
Cx3cr1 deficient and WT mice display similar microglial density in the OB.
(a) Representative low magnification (X20) projection images of the olfactory bulb of an Iba-1 stained control mouse and (b) a Cx3cr1−/− mouse. (c) There was no difference in the average number of Iba-1 labeled microglia in control and Cx3cr1−/− mice. n = 3 mice in each group. p=0.19, two-sample t-test. (DAPI = Blue; Iba1 = Green). Scale bar = 65 µm.
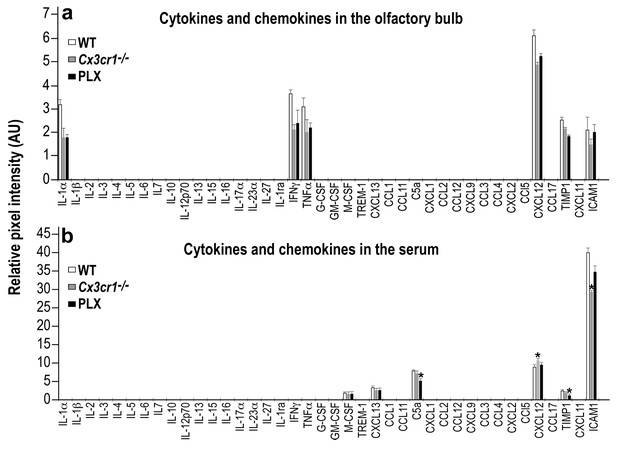
Cytokine and Chemokine expression in the OB and serum of WT, Cx3cr1−/− and PLX5622-treated mice.
(a) Cytokine and Chemokine microarray assay performed on total tissue lysates of OBs from WT, Cx3cr1 −/−, and PLX5622-terated mice. Expression levels are presented as arbitrary units, measured by densitometry. Using the Bonferroni procedure to correct for multiple comparisons, no significant changes were found between the expressed cytokines. n = 5, pooled in each group. Two-sample t-test. (b) Cytokine and Chemokine microarray assay performed on serum from WT, Cx3cr1−/−, and PLX5622-terated mice. Cytokine and Chemokine expression levels were measured by densitometry, and are presented as arbitrary units. Using the Bonferroni procedure to correct for multiple comparisons, significant changes were found in the following molecules: (C5a: WT vs PLX, t(2)=12.1, *p=0.0067; CXCL12: WT vs KO, t(2)=11.7, *p=0.007; TIMP1: WT vs PLX, t(2)=8.1, *p=0.014; ICAM1: WT vs KO, t(2)=10.4, *p=0.009). n = 5 pooled in each group. Two-sample t-test.
-
Figure 9—source data 1
Data of Cytokine and Chemokine expression in the OB and serum of WT, Cx3cr1−/− and PLX5622-treated mice.
- https://doi.org/10.7554/eLife.30809.022
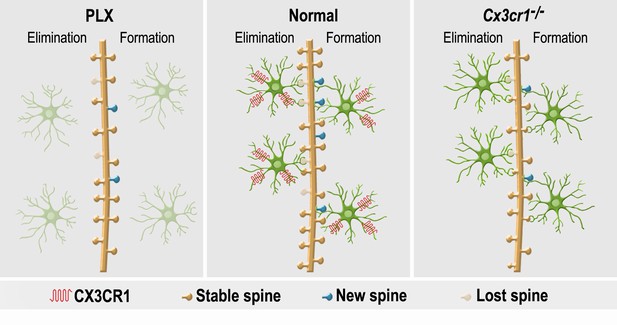
A summary of the effects of microglial depletion and Cx3cr1 deficiency on the formation, elimination, head size and density of spines on abGCs.
Under Normal conditions (middle panel), microglia (green cells) play an important role in both facilitation of spine formation and in elimination of nonfunctional spines, leading to normal maturation and plasticity of adult-born granule cells (abGCs) in the OB. These effects are at least partly dependent on the microglial expression of CX3CR1, which mediates neuronal-microglial communication. Microglial depletion in PLX5622-treated mice (left panel) reduced the formation and elimination of spines on abGCs. Because in control mice spine formation exceeds spine elimination during the initial developmental period of abGCs, the reduced synaptic turnover in microglia-depleted mice resulted in lower spine density. Abrogation of normal microglia-neuronal interactions in Cx3cr1−/− mice (right panel) induced a reduction in spine formation, possibly due to the lower number of contacts between microglial processes and dendritic shafts, as well as lower spine head size, possibly due to the increased proportion of spines that were contacted by microglia. Spine elimination was also reduced, suggesting that CX3CL1-CX3CR1-mediated communication between specific spines on abGCs and adjacent microglial processes plays an important role in spune pruning. Similarly to the effects of microglia depletion, spine density was also reduced in the Cx3cr1−/− mice.
Videos
Putative contacts between microglial processes and abGCs in a WT mouse.
A movie demonstrating putative contacts between WT microglia and the dendrites of an abGC. The movie shows the individual focal planes of a Z stack obtained using confocal microscopy. The analysis of the density of microglia-dendritic contacts reported in Figure 8 was conducted based on examination of such individual focal planes.
Putative contacts of Cx3cr1 deficient microglia processes and abGCs.
A movie demonstrating putative contacts between Cx3cr1-deficient microglia and the dendrites of an abGC. The movie shows the individual focal planes of a Z stack obtained using confocal microscopy. The analysis of the density of microglia-dendritic contacts reported in Figure 8 was conducted based on examination of such individual focal planes.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information | |
|---|---|---|---|---|---|
| strain, strain background (Mice) | Cx3cr1−/− | The Jackson Laboratories | B6.129P-Cx3cr1tm1Litt/J. (RRID:IMSR_JAX:005582) | On C57BL/6 background (serving as wild-type control) | |
| strain, strain background (Mice) | Thy1-GCaMP3 | Kindly provided to Adi Mizrahi from Guoping Feng. Chen et al. (2012) | Available in Jackson as B6;CBA-Tg(Thy1-GCaMP3)6Gfng/J. (RRID:IMSR_JAX:017893) | On C57BL/6 background (serving as wild-type control) | |
| antibody | Anti-Iba-1, Rabbit | Wako, Osaka, Japan | Wako Cat. No. 019–19741. (RRID:AB_2665520) | 1:1000, in blocker solution. Shaker, Room temp | |
| antibody | Anti-DCX, Guinea pig | Millipore (Mercury), CA, U.S.A. | Millipore Cat. No. AB2253. (RRID:AB_2230227) | 1:1000, in blocker solution. Shaker, Room temp | |
| antibody | Anti-BrdU, Rat | Harlan Sera-Lab, Loughborough, U.K. | Harlan-Sera Cat. No. OBT0030 (RRID:AB_2314037) | 1:200, in blocker solution. Shaker, Room temp | |
| antibody | Anti-NeuN, Rabbit | Cell signaling, MA, U.S.A. | Cell Signaling Cat. No. 24307 (RRID:AB_2651140) | 1:200, in blocker solution. Shaker, Room temp | |
| antibody | Anti-RFP, Rabbit | Rockland, PA, U.S.A. | Rockland Cat. No. 600-401-379 (RRID:AB_2209751) | 1:1000, in blocker solution. Shaker, Room temp | |
| recombinant DNA reagent | Details for AAV1 | Upenn, PA, U.S.A. | Upenn viral core Cat. No. AV-1-PV3365 | AAV1 under the CAG promoter, expressing TdTomato | |
| commercial assay or kit | Cytokine and chemokine microarray assay | R and D Systems, MN, U.S.A. | Proteome Profiler Mouse Cytokine Array Kit (ARY006) | ||
| cchemical compound, drug | PLX5622 | PLEXXIKON Inc., CA, U.S.A. | AIN-76A Rodent Diet with PLX5622 | 1,200 mg PLX5622 (Free Base)/kg | |
| chemical compound, drug | Control diet | PLEXXIKON Inc., CA, U.S.A. | AIN-76A Rodent Diet | ||
| chemical compound, drug | BrdU | Sigma-Aldrich, MO, U.S.A. | Sigma-Aldrich Cat. NO. B5002 | 10 mg/ml IP injection | |
| software, algorithm | ImageJ/Figi | University of Wisconsin, Madison, WI, U.S.A. | (http://rsb.info.nih.gov/ij/), (http://fiji.sc/Fiji) (RRID:SCR_003070) | ||
| software, algorithm | Matlab | Mathworks Inc., U.S.A. | Matlab, R2014a (RRID:SCR_001622) | ||
| software, algorithm | SPSS | I.B.M., U.S.A. | SPSS, Version 19 (RRID:SCR_002865) | ||
Additional files
-
Supplementary file 1
Gene transcripts significantly differentially regulated in the olfactory bulb of PLX5622-treated mice, compared with control diet-treated mice.
- https://doi.org/10.7554/eLife.30809.024
-
Supplementary file 2
Comparisons between gene transcript expression of 40 cytokines and chemokines in the olfactory bulbs of Cx3cr1−/− (KO) and PLX5622-treated (PLX) mice, as well as their respective wild type (WT) and control diet-treated (CON) mice.
- https://doi.org/10.7554/eLife.30809.025
-
Source code 1
Calculation of spine size from flourscent Z stacks.
- https://doi.org/10.7554/eLife.30809.026
-
Transparent reporting form
- https://doi.org/10.7554/eLife.30809.027





