BMP and FGF signaling interact to pattern mesoderm by controlling basic helix-loop-helix transcription factor activity
Figures
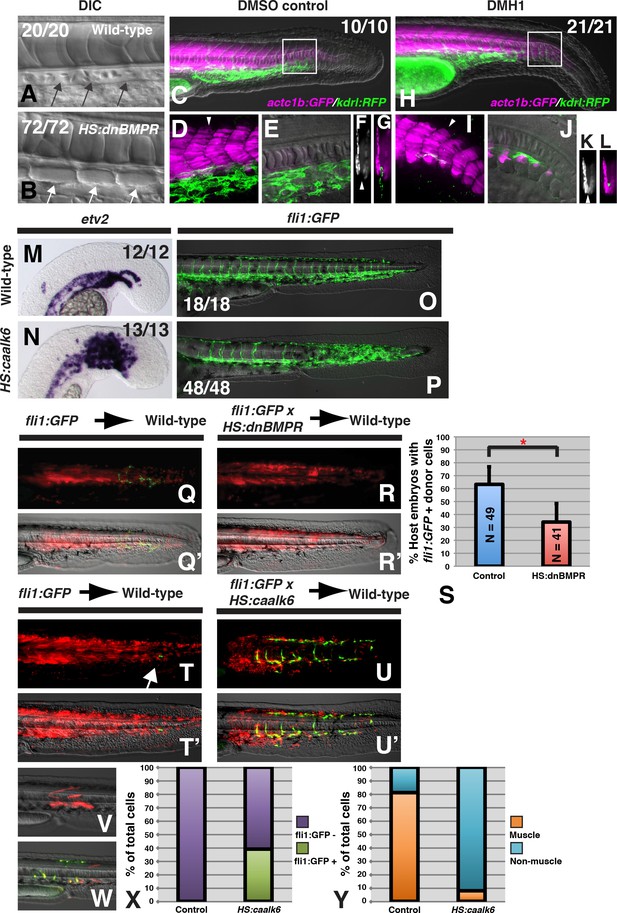
BMP signaling is necessary and sufficient for endothelial fate specification in tailbud-derived mesoderm.
(A) Wild-type sibling embryos heat-shocked at the 12-somite stage exhibit normal formation of the dorsal aorta (black arrows, 20/20 normal). (B) HS:dnbmpr embryos heat-shocked at the 12-somite stage have ectopic segmented somite tissue where the dorsal aorta normally forms (white arrows, 72/72 with ectopic somite tissue). (C–L) Loss of BMP signaling using the small molecule DMH1 phenocopies HS:dnbmpr embryos. Embryos transgenic for both the actc1b:GFP (muscle, magenta) and kdrl:rfp (endothelium, green) transgenes were treated with DMSO (C–G) or DMH1 (H–L). A confocal Z-projection of the boxed region in C shows the presence of both muscle and endothelium in control DMSO treatment. A single z-slice at the midline shows the presence of endothelium and absence of muscle, which can also be observed in a digital cross section at the level of the white arrowhead in panel D. A confocal z-projection of the boxed region in H shows the presence of muscle and large reduction in endothelium (I). A single z-section at the midline shows the reduction of endothelium is accompanied by ectopic midline muscle formation, also observed in the digital cross-section at the level of the white arrowhead in panel I. (M, N) Transgenic HS:caalk6 embryos heat-shocked at the 12-somite stage exhibit expansion of the endothelial marker etv2 into the pre-somitic mesoderm 5 hr after the heat-shock (control N = 12, HS:caalk6 N = 13). (O, P) At 36 hpf, HS:caalk6 embryos heat-shocked at 12-somite stage have a dramatic expansion of fli1:GFP expression in posterior regions that would normally form somites, whereas there is no effect on anterior somites that formed before the heat-shock (Control N = 18, HS:caalk6 N = 48). (Q–R’) Rhodamine dextran (red) labeled fli1:GFP donor cells were transplanted into unlabeled wild-type host embryos to monitor for contribution of transplanted cells to endothelium. (Q, Q’, S) Control cells contribute to endothelium in 63% of host embryos (N = 49). (R, R’, S) Heat-shock induction of dnbmpr at the 12-somite stage significantly (p=0.0107) reduces the percentage of host embryos (34%) that have donor-derived endothelium (N = 41). (T–U’) Induction of endothelium by BMP signaling is cell-autonomous, as exhibited in HS:caalk6 x fli1:GFP cells transplanted wild-type host embryos. Host embryos were heat-shocked at the 12-somite stage and assayed for fli1:GFP expression at 36 hpf. (U, U’) HS:caalk6 transgenic cells do not contribute to somites and instead give rise to endothelium. One-cell transplants were done to quantify fate changes after BMP activation (W) compared to controls (V). 12-somite stage BMP activation resulted in 39% fli:GFP positive cells (four embryos, 49 cells), compared to 0% in control transplants (three embryos, 36 cells, p<0.0001) (X). The fate of control transplanted cells was 81% muscle, whereas only 8% of HS:caalk6 cells adopted a muscle fate (p<0.0001) (Y). All embryos are pictured from a lateral view with the head to the left, except for F, G, K, and L which are digital transverse sections.
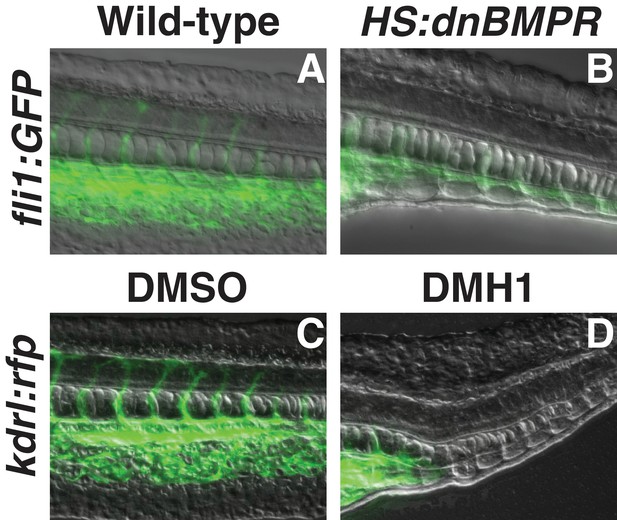
BMP signaling inhibition at bud stage medializes tail mesoderm.
Transgenic fli1:gfp embryos were heat-shocked at the bud stage. Embryos that also had the HS:dnbmpr transgene exhibited a loss of endothelium and gain of somite tissue compared to control embryos (B compared to A). Similarly, kdrl:GFP embryos treated with the BMP inhibitor DMH1 showed a loss of endothelium and expansion of ectopic somite tissue compared to DMSO-treated control embryos (D compared to C).
Blood flow in a 48 hpf control embryo that was heat-shocked at the 12-somite stage.
https://doi.org/10.7554/eLife.31018.004A movie illustrating the complete lack of posterior blood flow in a 48 hpf HS:dnBMPR transgenic embryo that was heat-shocked at the 12-somite stage.
The ectopic somite tissue is visible just ventral to the notochord.
Blood flow in a 48 hpf wild-type embryos heat shocked at the 12-somite stage.
https://doi.org/10.7554/eLife.31018.006Blood flow in a 48 hpf HS:caalk6 transgenic embryo heat-shocked at the 12-somite stage.
Posterior regions where somites should normally reside show extensive blood flowing through ectopic vasculature.
High-magnification view of blood flow in the tail of a 48 hpf wild-type embryo that was heat-shocked at the 12-somite stage.
https://doi.org/10.7554/eLife.31018.008High-magnification view of blood flow in the tail of a 48 hpf HS:caalk6 transgenic embryo heat-shocked at the 12-somite stage, focusing on a position where the skeletal muscle of somites would normally exist in a wild-type embryo.
https://doi.org/10.7554/eLife.31018.009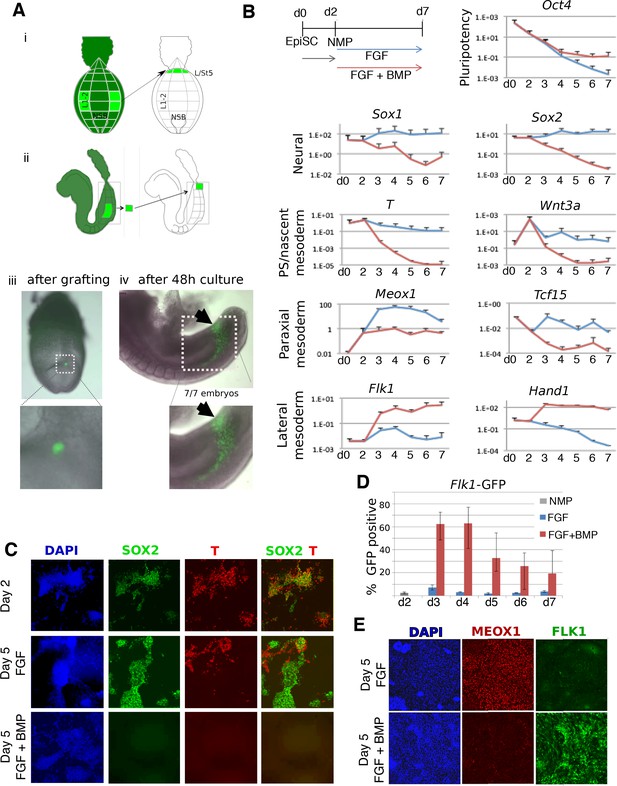
BMP redirects fate of mouse NMPs from paraxial to lateral mesoderm.
(A) Heterotopic grafting from ubiquitous GFP embryos (Gilchrist et al., 2003) of NMP region fated for paraxial mesoderm at 2–5 somite stage (E8.0) into the posterior primitive streak region fated for lateral and ventral mesoderm, followed by 48 hr culture. (i) Posterior view (ii) Lateral view (iii) Representative embryo immediately after grafting showing position of GFP +grafted cells (iv) Representative embryo after 48 culture showing that descendants of grafted cells have adopted a lateral fate (arrowheads). (B) qPCR at indicated time points during the differentiation of EpiSCs into NMPs then treated with FGF2 or FGF2 +BMP4. Data shown relative to the housekeeping gene TBP. (C) Immunofluorescence detection of indicated markers in in vitro derived NMPs and their differentiating derivatives. (D) Flow cytometry of Flk1-GFP in the differentiating derivatives of in-vitro derived NMPs. (E) Immunofluorescence detection of indicated markers in the differentiating derivatives of in-vitro derived NMPs.
-
Figure 2—source data 1
Raw data for the qPCR experiments in Figure 2.
- https://doi.org/10.7554/eLife.31018.011
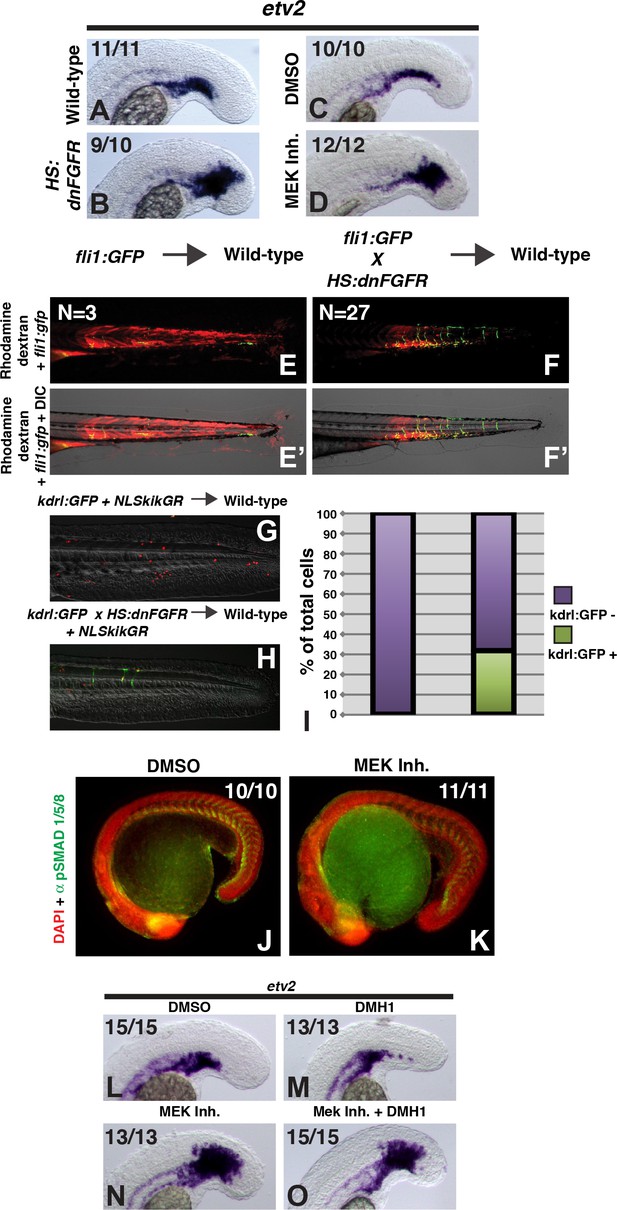
FGF signaling is necessary to maintain paraxial mesoderm fate and inhibit a default endothelial fate.
Heat-shock induction of dnfgfr (B) or treatment with a MEK inhibitor (D) at the 12-somite stage causes an expansion of the endothelial marker etv2 into the pre-somitic mesoderm 5 hr later compared to controls (A, C). (F, F’) Transplanted HS:dnfgfr x fli1:GFP show a cell-autonomous shift from somite to endothelial fate when heat-shocked at the 12-somite stage, whereas fli:GFP transplants mostly contribute to muscle with minor endothelial contribution (E, E’). The same effect is seen with HS:dnfgfr x kdrl:GFP transplanted cells when heat-shocked at the 12-somite stage (G, H). NLS-kikume was injected into donor embryos to quantify cell fate changes. 12-somite stage FGF inhibition resulted in 31% kdrl:GFP-positive cells (13 embryos, 308 cells), compared to 0% in control transplants (seven embryos, 587 cells, p<0.0001) (I). Expansion of endothelium 5 hr after MEK inhibitor treatment is not due to an expansion of BMP signaling, as revealed by pSMAD 1/5/8 staining (K compared to J, green staining, red color is DAPI staining). (L–O) Similarly, treatment with the BMP inhibitor DMH1 does not prevent MEK-inhibitor-induced expansion of endothelium. Embryos were treated at the 12-somite stage and fixed 6 hr later. The expansion of the endothelial marker etv2 into the PSM after MEK inhibitor treatment (N) is not inhibited by the addition of DMH1 (O).
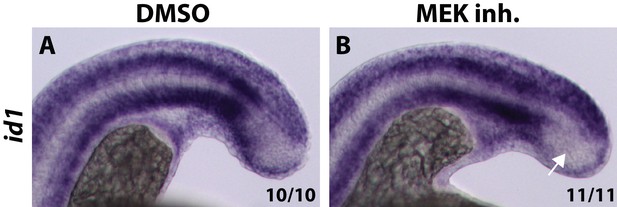
MEK inhibitor does not cause an expansion of id1 expression into the PSM.
Embryos were treated with a MEK inhibitor at the 12-somite stage and fixed and analyzed for id1 expression six hours later. The expression of id1, which is a direct BMP target gene, does not expand into the PSM after MEK inhibition (B, arrow, compared to A).
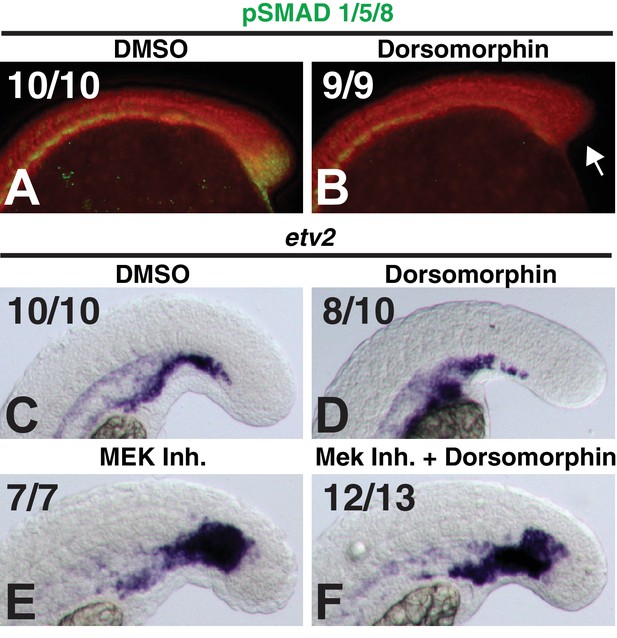
Dorsomorphin does not rescue MEK-inhibitor-induced endothelial expansion.
Embryos were treated at bud stage with dorsomorphin and a subset of them were assayed at the 12-somite stage for the loss of pSMAD 1/5/8 staining in the tailbud (A, B, arrow indicates loss of pSMAD staining, red color is DAPI staining). (C–F) A subset of the remaining embryos were treated at the 12-somite stage with the MEK inhibitor, fixed 6 hr later and stained for mRNA expression of the endothelial marker etv2. Dorsomorphin did not rescue MEK-inhibitor-induced expansion of endothelium (F compared to E).
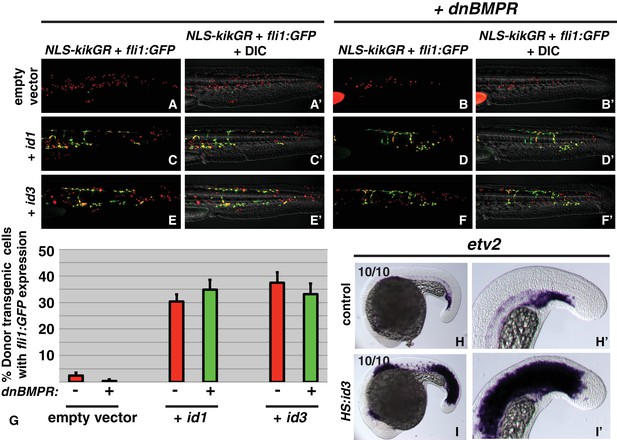
id genes are the essential BMP targets mediating endothelial induction.
An assay was developed to quantify the percent of transplanted cells that adopt an endothelial fate (see text for details). Control cells transplanted to the ventral margin of host embryos and heat-shocked at the 12-somite stage exhibit a small percentage contribution to endothelium (green cells, A, A’, G, empty vector Nembryos = 19, Ncells = 500), which is significantly reduced when BMP signaling is inhibited in transplanted cells (B, B’, G, empty vector +dnbmpr Nembryos=19, Ncells = 1022, p=0.006). Activation of id1 or id3 causes a significantly larger percentage of transplanted cells to adopt an endothelial fate (C, C’, E, E’, G, id1 Nembryos = 16, Ncells = 1159, p<0.0001, id3 Nembryos = 18, Ncells = 574, p<0.0001), and this effect is unchanged in cells that also lack BMP signaling (D, D’, F, F’, G, id1 +dnbmpr Nembryos=16, Ncells = 614, p<0.0001, id3 +dnbmpr Nembryos=12, Ncells = 531, p<0.0001). Cell fate quantification from these experiments is represented in panel G. A stable HS:id3 transgenic line heat-shocked at the 12-somite stage and fixed 5 hr later exhibits a large expansion of the endothelial marker etv2 (I, I’) compared to heat-shocked wild-type embryos (H, H’).
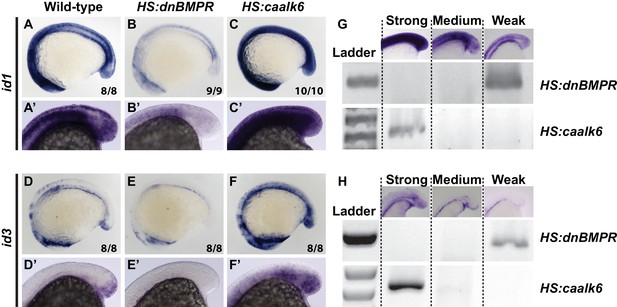
BMP signaling is necessary and sufficient for id1 and id3 expression.
HS:dnbmpr and HS:caalk6 transgenic lines were used to inhibit or activate BMP signaling, respectively, at the 12-somite stage and embryos were fixed 3 hr later. id1 and id3 are normally expressed in the tailbud and areas of vasculogenesis, as well as other regions of the body (A, A’, D, D’). Loss of BMP signaling results in a near total loss of expression of both id1 (B, B’) and id3 (E, E’) throughout the body. Activation of BMP signaling has the opposite result, with a broad expansion of id1 (C, C’) and id3 (F, F’) throughout the body. The analysis was repeated to perform an unbiased blind assessment of expression changes in the different genetic backgrounds. Embryos from HS:dnbmpr and HS:caalk6 outcrosses were heat-shocked at the 12-somite stage and fixed three hours later. Embryos were mixed together and in situ hybridization was performed for id1 or id3. Embryos were sorted based on expression patterns (strong, medium, or weak) and PCR genotyped using primers specific for the HS:caalk6 or HS:dnbmpr transgenes. Strong expression correlated with presence of the HS:caalk6 transgene, weak expression with the presence of the HS:dnbmpr transgene, and medium expression with the absence of both transgenes. The correlation held for 14/15 genotyped id1 stained embryos (5/5 strong, 5/5 medium, 4/5 weak) and 13/16 id3 stained embryos (4/5 strong, 4/5 medium, 5/6 weak). Primers for the HS:dnbmpr transgene amplified the Xenopus laevis BMP receptor within the transgene (forward primer: 5’ ATTCATGCCCAAGGACAGGA 3’, reverse primer: 5’ CTCCATCTGCGATCTTTGGC 3’, amplicon size is 382 bp), while primers for the HS:caalk6 transgene amplified the kikume sequence within the transgene (forward primer: 5’ GTAAACGGGCACAAGTTCGT 3’, reverse primer: 5’ CAGCCCGGAATGAGCTTTAG 3’, amplicon size is 615 bp).
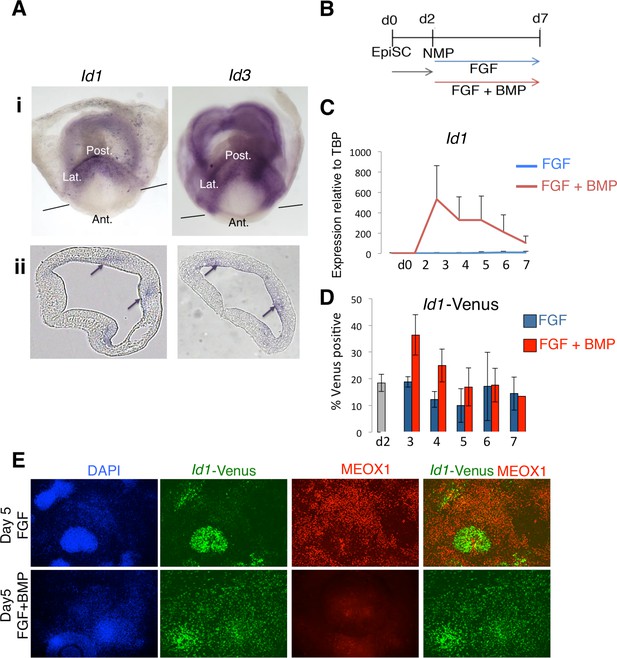
Id1 and Id3 are expressed in prospective lateral/ventral mesoderm at early somite stages in vivo, and are induced by BMP in NMPs in vitro.
(A) In situ hybridisation for Id1 and Id3 in wholemount (i) and sections (ii) showing Id1 and Id3 expression restricted to the posterior (labelled 'Post.') and lateral (labeled 'Lat.') regions of the primitive streak. Id1/3 are not detected in the anterior primitive streak (labeled 'Ant'). Lines in (i) indicate the plane of section. Arrows in (ii) indicate regions of expression in the posterior lateral regions of the primitive streak. (B) In vitro differentiation protocol. (C) Id1 mRNA is expressed in response to BMP4 but not FGF2 during differentiation of NMP in culture (D) an Id1-Venus reporter is activated in response to BMP4 but not FGF2 during differentiation of NMP in culture (E) Immunofluorescence for indicated markers during differentiation of NMP in culture: expression of MEOX1 is mutually exclusive from expression of ID1-Venus, and is suppressed by addition of BMP4.
-
Figure 5—source data 1
Raw data for the qPCR experiments in Figure 5.
- https://doi.org/10.7554/eLife.31018.018
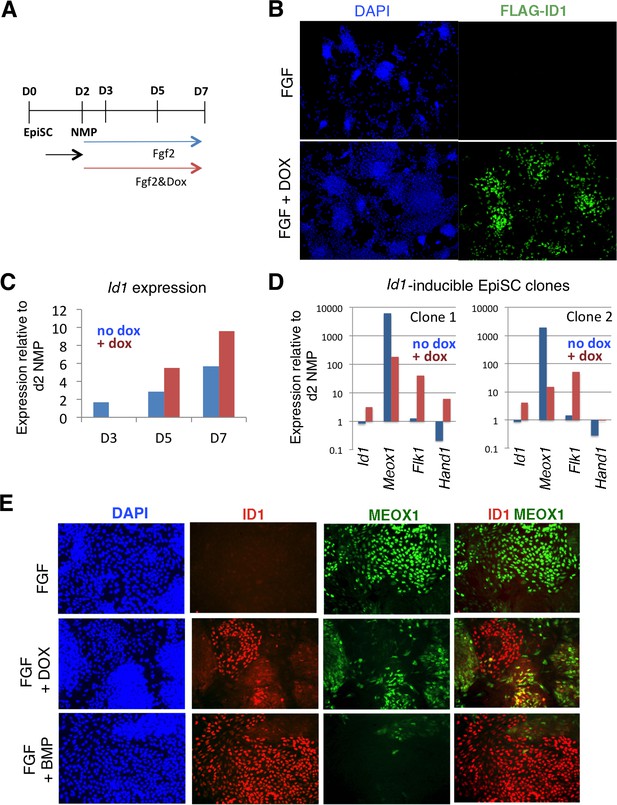
Id1 GOF drives differentiation of lateral mesoderm at the expense of paraxial mesoderm.
A: Differentiation protocol. B: Immunofluorescence detection for the Flag epitope in Flag-Id1 inducible EpiSC indicates that addition of dox induces Flag-ID1 in a subset of cells. C: qPCR to detect Id1 mRNA in the absence and presence of dox in Flag-Id1 inducible EpiSC. D: qPCR to detect the indicated mesoderm markers in Flag-Id1 inducible EpiSC the absence and presence of dox: data from two independent clonal lines is shown. D: Immunofluorescence detection of indicated markers: induction of Id1 suppresses expression of MEOX1, recapitulating the effect of adding BMP4.
-
Figure 6—source data 1
Raw data for the qPCR experiments in Figure 6.
- https://doi.org/10.7554/eLife.31018.020
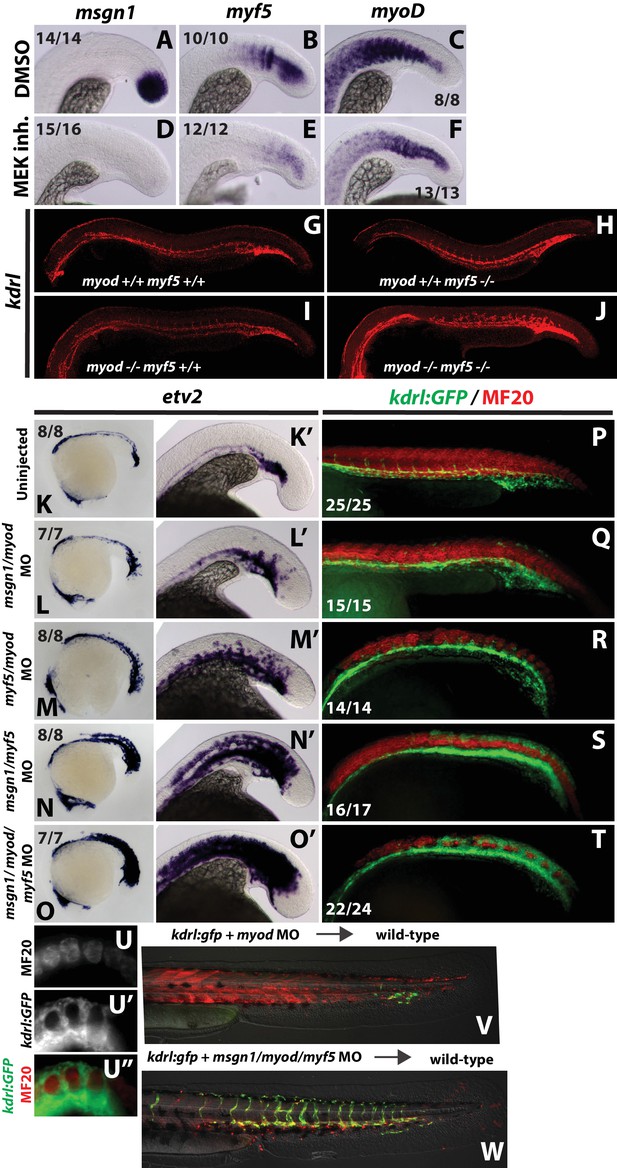
FGF signaling maintains paraxial mesoderm fate and inhibits endothelial fate through positive regulation of bHLH transcription factors.
Wild-type embryos were treated with the MEK inhibitor or DMSO at the 12-somite stage and fixed five hours later. Expression of msgn1 (D) and myf5 (E) were significantly downregulated compared to controls (A, B), whereas myod (F) exhibited only a minor reduction in expression compared to controls (C). Expression of kdrl (red) is expanded into somitic territories in myod;myf5 double mutants compared to controls (G). n = 44 controls (pooled +/+;+/+, +/+;+/-; +/-;+/+, and +/-;±genotyped embryos). 0/44 controls have expanded kdrl. n = 4 mutants (-/-;-/- genotyped embryos). 4/4 show expanded kdrl (representative embryos shown). MO-mediated loss of function of msgn1/myod (L, L’) or myf5/myod (M, M’) results in a moderate expansion of etv2 expression at the 22 somite stage, whereas loss of msgn1/myf5 causes a broad expansion of etv2 (N, N’). Loss of function of all three genes further enhances etv2 expansion (O, O’). MF20 (muscle, red) antibody staining in 30 hpf kdrl:GFP embryos demonstrates the gain of differentiated vasculature at the expense of differentiated muscle (P–T). U-U’’ are high-magnification views of MF20 staining and kdrl:GFP expression in a msgn1/myf5 loss of function embryo. Transplanted kdrl:GFP cells lacking myod/myf5/msgn1 fail to join host somites and instead contribute predominantly to endothelium (W), whereas cells lacking myod behave normally, with most transplanted cells joining the somites and forming muscle (V).
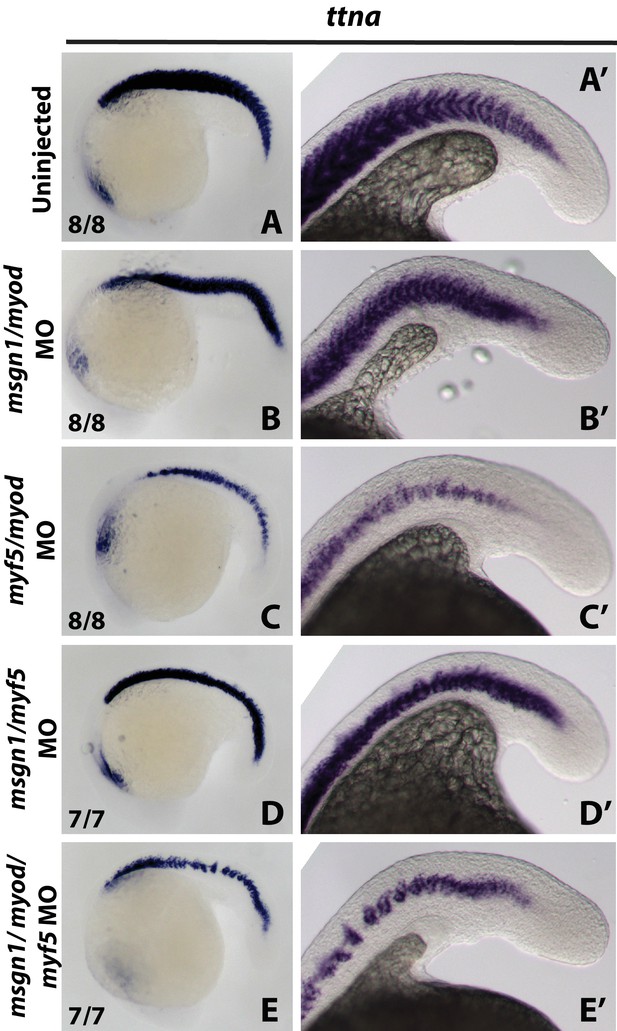
bHLH transcription factor knockdown inhibits skeletal muscle specification.
A probe for titin-a (ttna) was used to label differentiating cardiac and skeletal muscle at the 22-somite stage. Loss of msgn1 and myod function produced only a minor loss of skeletal muscle. Loss of myf5 and myod, msgn1 and myf5, or msgn1, myod, and myf5 caused a substantial loss of skeletal muscle, with the triple knockdown having the strongest effect.
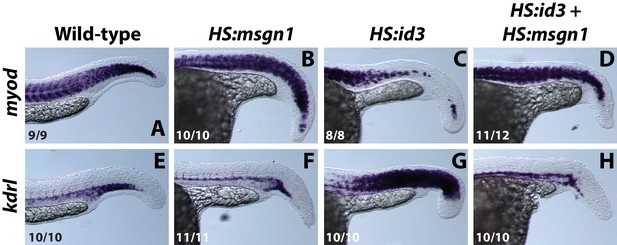
Over-expression of msgn1 rescues id3 over-expression.
HS:id3 and HS:msgn1 lines were crossed to each other and heat-shocked at the 12-somite stage and fixed at 24 hpf for analysis of myod and kdrl expression. The activation of msgn1 alone results in relatively normal myod expression and a posterior loss of kdrl. Activation of id3 causes a strong loss of posterior myod expression and gain of kdrl expression throughout regions where somites normally form. Activation of id3 and msgn1 largely restores normal patterning of muscle and vasculature.
Blood flow in a 30 hpf host embryos that received donor kdrl:GFP cells that were injected with msgn1, myod, and myf5 MOs.
Donor cells that contributed to the host endothelium appear as green GFP fluorescing cells. The donor-derived endothelium appears to function completely normally.
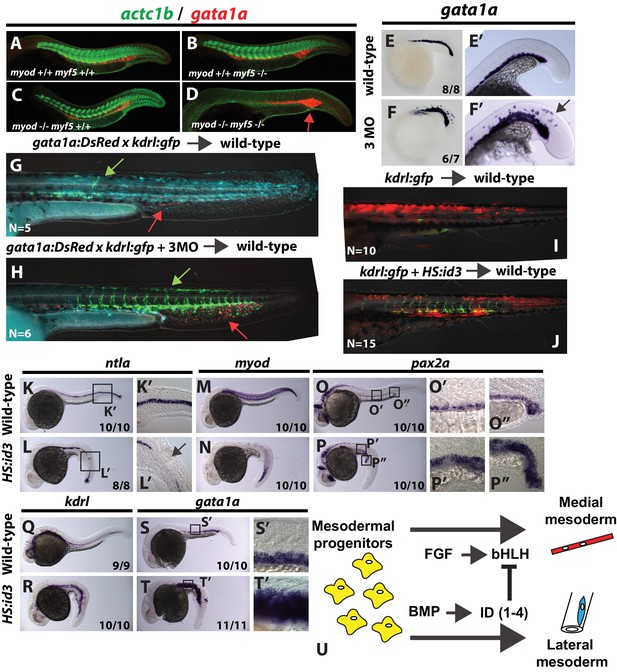
bHLH transcription factor activity provides mediolateral pattern to the entire mesodermal germ layer.
Homozygous myod;myf5 mutant embryos exhibit slightly expanded gata1a expression (D, red staining, arrow) and a complete loss of skeletal muscle marker actc1b (green staining). n = 49 controls (pooled +/+;+/+, +/+;+/-; +/-;+/+, and +/-;±genotyped embryos). 49/49 controls show normal actc1b, 0/49 controls have expanded gata1a. n = 9 mutants (-/-;-/- genotyped embryos). 9/9 show loss of actc1b, 7/9 show expanded gata1a (representative embryos shown). Loss of msgn1/myod/myf5 function results in an expansion of gata1a expression into somitic domains at the 22 s stage (E–F’). Cells from transgenic gata1a:dsRed x kdrl:GFP embryos injected with cascade blue dextran and msgn1/myod/myf5 MOs transplanted into unlabeled host embryos are excluded from somites and contribute extensively to endothelium (H, green arrow) and red blood cell lineages (H, red arrow). Control cascade blue injected gata1a:dsRed x kdrl:GFP transplanted cells contribute primarily to somitic muscle with minor contributions to endothelium (G, green arrow) and red blood cells (G, red arrow). Heat-shock induction of id3 at shield stage in mesodermally targeted transplanted cells that also contain the kdrl:GFP transgene causes a shift from predominantly somitic muscle fate to significant endothelial contribution in the trunk (J compared to I). Whole embryo induction of id3 expression at shield stage and analyzed at 24 hpf indicates a loss of medial mesoderm (notochord and muscle, (K–N), and an expansion of lateral mesoderm (pronephros, vasculature, and blood, (O–T’). Expression of gata1a in the trunk shows broad expansion into somite territories (S’ compared to T’). (U) A model for how FGF and BMP signaling control mediolateral patterning of the mesoderm through modulation of bHLH transcription factor activity.
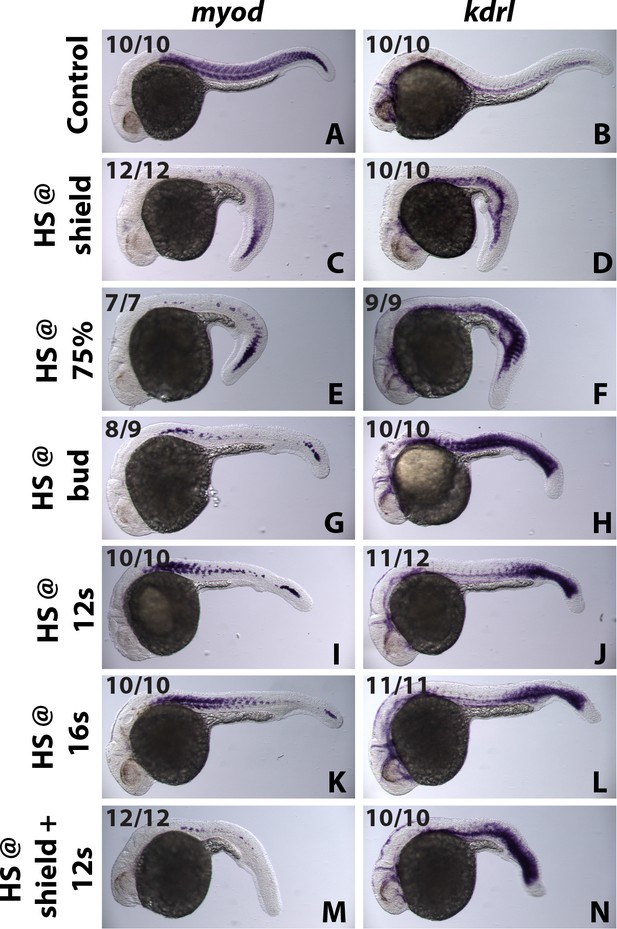
id3 mediated patterning of the mediolateral mesodermal axis is coordinated with AP axis formation.
A stage series of heat-shock inductions using the HS:id3 transgenic line indicates that Id3 patterns mesoderm in coordination with anterior posterior axis formation. Early stage heat-shock inductions inhibit myod and expand kdrl expression mostly in anterior regions but posterior tissues are normal (C–F). An intermediate stage induction at the end of gastrulation inhibits myoD and expands kdrl everywhere except the extreme anterior and posterior regions (G, H). At later stages during somitogenesis, id3 induction inhibits myod and expands kdrl expression in posterior but not anterior regions (I–L). Two heat-shock inductions at early and late stages indicates that recovery of posterior patterning in single early heat-shock inductions is due to turnover of the induced Id3 protein (M, N).
Additional files
-
Transparent reporting form
- https://doi.org/10.7554/eLife.31018.028





