Clearance of senescent decidual cells by uterine natural killer cells in cycling human endometrium
Figures
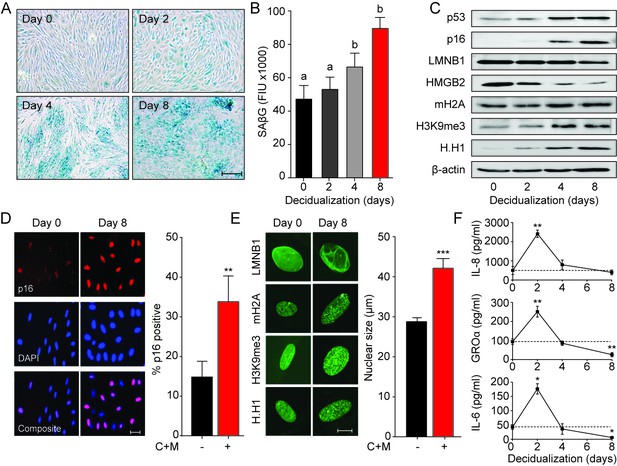
Decidualization induces acute senescence in a subpopulation of EnSCs.
(A) Representative SAβG staining in undifferentiated EnSCs (Day 0) or cells decidualized for the indicated time points with 8-bromo-cAMP and MPA. Scale bar = 100 µm. (B) SAβG activity, expressed in fluorescence intensity units (FIU), in undifferentiated EnSCs (day 0) or cells decidualized for the indicated time points. (C) Representative Western blot analysis of p53, p16, LMNB1, HMGB2, mH2A, H3K9me3 and H.H1 levels in undifferentiated EnSCs and cells decidualized for the indicated time points. β-actin served as a loading control. (D) Left panel: representative immunofluorescence staining for p16 expression in undifferentiated cells and cells decidualized for 8 days. Nuclei were counterstained with DAPI. Scale bar = 50 µm. Right panel: percentage of p16+ cells. (E) Left panel: representative confocal microscopy images of undifferentiated (Day 0) or decidualized (Day 8) EnSCs immune-probed for LMNB1, mH2A, H3K9me3 and H.H1. Scale bar = 10 µm. Right panel: nuclear size of undifferentiated EnSCs (n = 48) and of cells first decidualized for 8 days with 8-br-cAMP and MPA (C + M) (n = 48) was measured in three primary cultures. (F) Secretion of IL-8, GROα, and IL-6 was measured in the supernatant of primary EnSCs collected every 48 hr over an 8 day decidualization time-course. Data are mean ±SEM of 3 biological replicates unless stated otherwise. **p<0.01, ***p<0.001. Different letters above the error bars indicate that those groups are significantly different from each other at p<0.05.
-
Figure 1—source data 1
Decidualization induces acute senescence in a subpopulation of EnSCs.
- https://doi.org/10.7554/eLife.31274.004
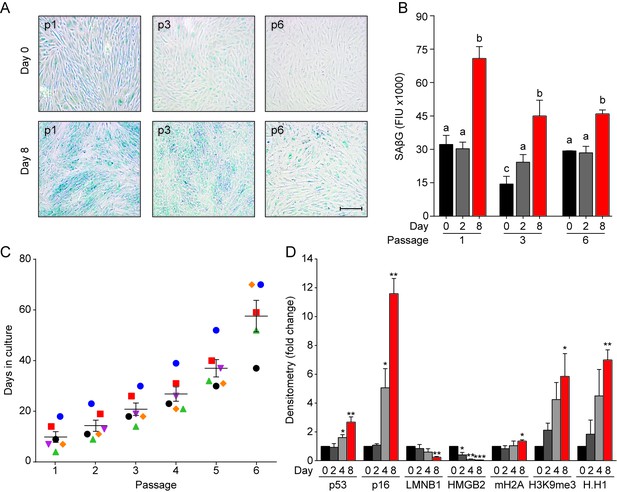
Decidualization-associated acute senescence in primary EnSCs.
(A) Representative SAβG staining in undifferentiated (Day 0) and decidualized (Day 8) EnSCs at the indicated passage numbers. Scale bar = 100 µm. (B) Quantitative SAβG activity at indicated passage in undifferentiated EnSCs (day 0) or cells decidualized for the specified time points (n = 6). (C) Days in culture in relation to passage number. (D) Densitometric analysis of senescence marker Western blots shown in Figure 1C. Significance for individual markers was determined by t-test in comparison to day 0. *=P < 0.05, **=P < 0.01 and ***=P < 0.001. All data are mean ±SEM of 3 biological replicates, unless indicated otherwise. Different letters above the error bars indicate that those groups are significantly different from each other at p<0.05.
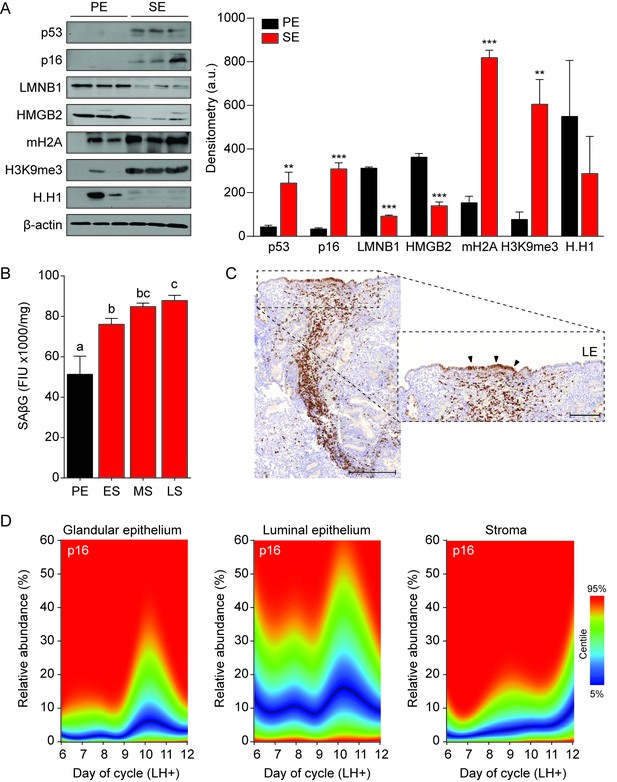
Senescent cells in cycling human endometrium.
(A) Left panel: representative Western blot analysis of p53, p16, LMNB1, HMGB2, mH2A, H3K9me3 and H.H1 levels in whole tissue biopsies from proliferative endometrium (PE) and secretory endometrium (SE). β-actin served as a loading control. Right panel: protein levels quantified relative to β-actin by densitometry and expressed as arbitrary units (a.u.). (B) SAβG activity, expressed in fluorescence intensity units (FIU)/mg protein, was measured in biopsies from proliferative endometrium (PE; n = 7), early-secretory (ES; n = 9), mid-secretory (MS; n = 38) and late-secretory (LS; n = 19) endometrium. (C) Immunohistochemistry demonstrating distribution of p16+ cells in the stromal compartment and luminal epithelium. Scale bars = 200 µm. (D) The abundance of p16+ cells during the luteal phase in glandular epithelium, luminal epithelium and stroma compartment was analyzed by color deconvolution using ImageJ software in 308 LH-timed endometrial biopsies (average 48 samples per time point; range: 22 to 69). The centile graphs depict the distribution of p16+ cells across the peri-implantation window in each cellular compartment. Color key is on the right. Data are mean ±SEM of 3 biological replicates unless stated otherwise. **p<0.01, ***p<0.001. Different letters above the error bars indicate that those groups are significantly different from each other at p<0.05.
-
Figure 2—source data 1
Senescent cells in cycling human endometrium.
- https://doi.org/10.7554/eLife.31274.006
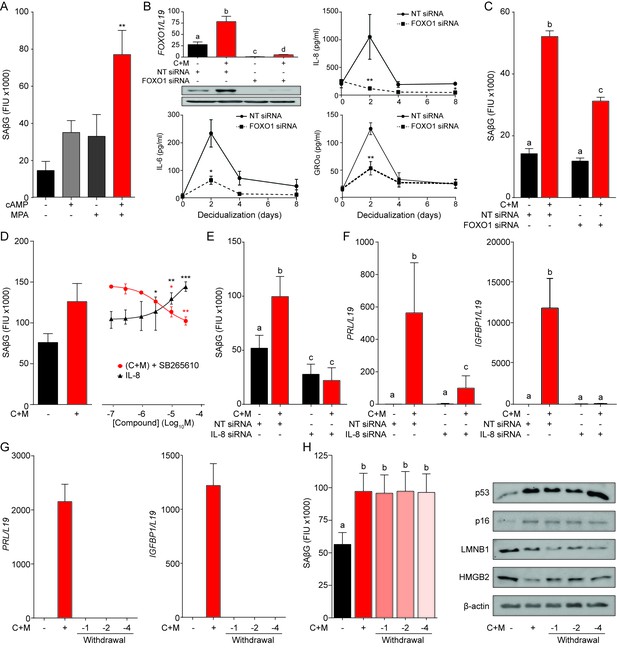
A FOXO1/IL-8 axis drives EnSC differentiation and senescence.
(A) SAβG activity in EnSCs either undifferentiated, or decidualized for 8 days with 8-bromo-cAMP, MPA, or a combination. (B) Top left panel: FOXO1 mRNA levels in undifferentiated EnSCs and cells treated with 8-br-cAMP and MPA (C + M) following transfection with non-targeting (NT) or FOXO1 siRNA. Other panels: Secretion of IL-8, IL-6 and GROα was measured following FOXO1 knockdown in the supernatant of primary EnSCs every 48 hr over an 8 day decidualization time-course. (C) SAβG activity in EnSCs following transfection with NT or FOXO1 siRNA. The cultures either remain untreated or decidualized for 8 days. (D) SAβG activity in undifferentiated EnSCs treated for 8 days with increasing concentrations of recombinant IL-8 and in cells decidualized for 8 days in the presence of increasing concentrations of the CXCR2 antagonist, SB265610. (E) SAβG activity in EnSCs following transfection with IL-8 siRNA. The cultures either remain untreated or decidualized for 8 days. (F) PRL and IGFBP1 transcript levels in EnSCs following transfection with IL-8 siRNA. The cultures either remain untreated or decidualized for 8 days. (G) PRL and IGFBP1 expression in undifferentiated EnSCs, cells decidualized for 8 days, and upon withdrawal of 8-br-cAMP and MPA (C + M) for the indicated days. (H) Left panel: SAβG activity in undifferentiated EnSCs, cells decidualized for 8 days, and following withdrawal of C + M for the indicated days. Right panel: representative Western blot analysis of p53, p16, LMNB1 and HMGB2 levels in undifferentiated EnSCs, cells decidualized for 8 days, and following withdrawal of C + M for the indicated days. β-actin served as a loading control. Data are mean ±SEM of 3 biological replicates unless stated otherwise. *p<0.05, **p<0.01 and ***p<0.005. Different letters above the error bars indicate that those groups are significantly different from each other at p<0.05.
-
Figure 3—source data 1
A FOXO1/IL-8 axis drives EnSC differentiation and senescence.
- https://doi.org/10.7554/eLife.31274.009
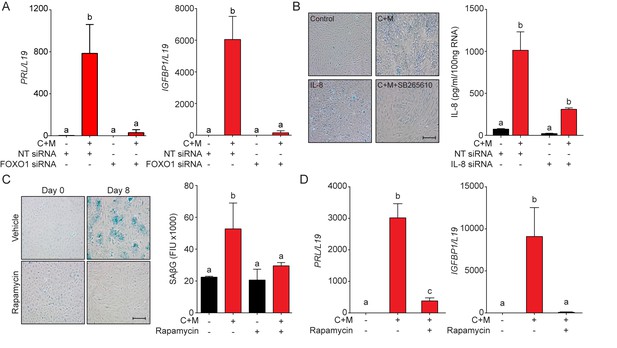
EnSC differentiation and senescence is driven by FOXO1, IL-8 and mTOR.
(A) PRL and IGFBP1 transcript levels in EnSCs following transfection with FOXO1 siRNA. The cultures either remained untreated or were decidualized for 8 days. (B) Left panel: SAβG staining in undifferentiated EnSCs that remained untreated (control) or were incubated with recombinant IL-8 (30 μM) for 8 days. SAβG staining was also performed in parallel cultures decidualized with 8-bromo-cAMP and MPA (C + M) in the absence or presence of the CXCR2 antagonist SB265610 (10 μM). Right panel: IL-8 concentration in conditioned media from decidualized EnSCs following siRNA-mediated CXCL8 (IL-8) knockdown. (C) SAβG staining (left panel) and activity (right panel) in undifferentiated (day 0) and decidualized (day 8) EnSCs in the presence of the mTOR inhibitor rapamycin. FIU: fluorescence intensity units. (D) PRL and IGFPB1 transcripts in undifferentiated EnSCs and cells decidualized for 8 days in the presence or absence of rapamycin (100 nM). All data are mean ±SEM of 3 biological replicates. Different letters above the error bars indicate that those groups are significantly different from each other at p<0.05. Scale bars = 100 μm.
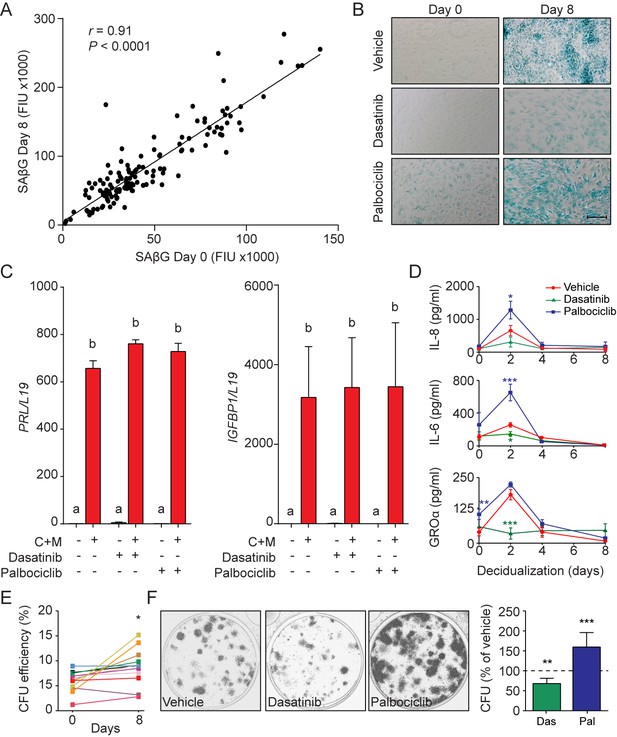
Functions of senescent decidual cells.
(A) Pearson’s correlation analysis of SAβG activity in 75 matched undifferentiated primary cultures and cultures decidualized for 8 days. (B) Representative SAβG staining in undifferentiated (Day 0) and decidualizing EnSCs (Day 8) following 4 days of pretreatment with vehicle, dasatinib (250 nM) or palbociclib (1 μM). Scale bar = 100 µm. (C) PRL and IGFBP1 mRNA expression in response to pretreatment with vehicle, dasatinib or palbociclib. The cultures then remained undifferentiated or were decidualized for 8 days. (D) IL-8, IL-6 and GROα secretion was measured every 48 hr in the supernatant of primary EnSCs decidualized for the indicated time-points following pretreatment with vehicle, dasatinib or palbociclib. (E) Colony forming unit (CFU) activity in paired EnSC cultures that either remain undifferentiated (Day 0) or were decidualized for 8 days (n = 10). (F) Left panel: representative clonogenic assays established from EnSC cultures first pretreated with vehicle, dasatinib or palbociclib and then decidualized for 8 days. Right panel: CFU activity in EnSC cultures first pretreated with vehicle, dasatinib or palbociclib and then decidualized for 8 days. Data are mean ±SEM of 3 biological replicates unless stated otherwise. *p<0.05, **p<0.01 and ***p<0.001. Different letters above the error bars indicate that those groups are significantly different from each other at p<0.05.
-
Figure 4—source data 1
Functions of senescent decidual cells.
- https://doi.org/10.7554/eLife.31274.012
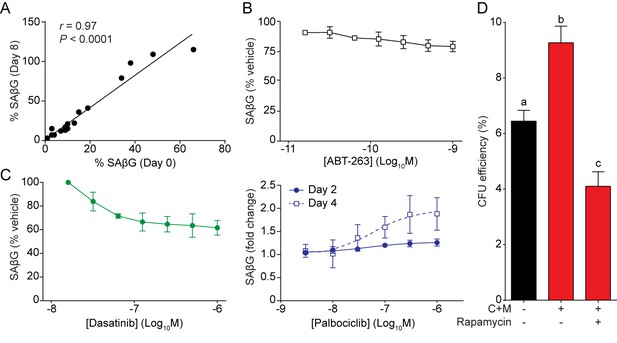
Modulation of senescence in EnSC cultures.
(A) Pearson’s correlation analysis of SAβG staining in 18 matched undifferentiated and decidualized EnSC cultures. (B–C) Quantitative analysis of SAβG in EnSCs following exposure to senolytics ABT-263 (B) and Dasatinib (C, left panel) for 3 days, or the CDK4/CDK6-inhibitor Palbociclib for times indicated (C, right panel). (D) Colony forming unit (CFU) efficiency of EnSC following decidualization for 8 days in the absence or presence of rapamycin (100 nM). Different letters above the error bars indicates that those groups are significantly different from each other at p<0.05. Data are mean ±SEM, n = 3.
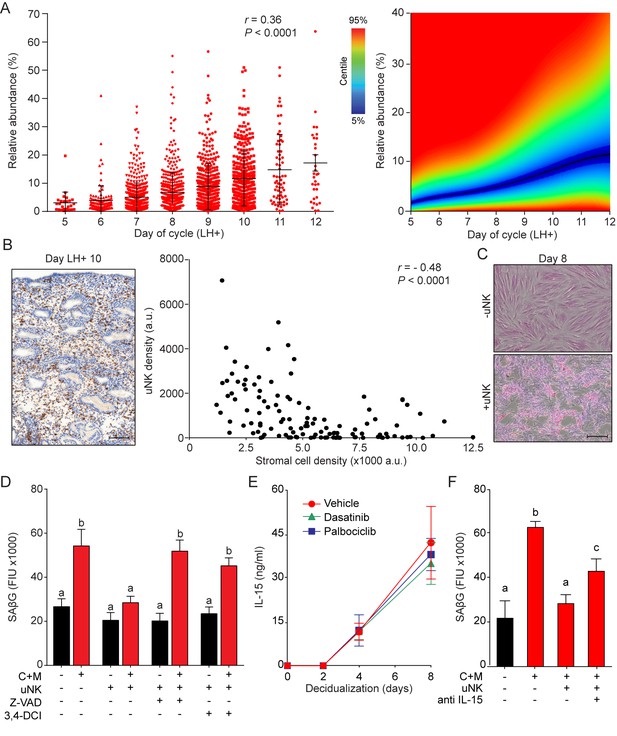
uNK cell mediated immune surveillance and clearance of senescent cells.
(A) Left panel: uNK cell density in the subluminal stroma was quantified using a standardized immunohistochemistry protocol in LH timed endometrial biopsies (n = 1,997). Right panel: corresponding centile graph. Color code on the left. (B) Left panel: example of the tissue distribution of CD56+ uNK cells (brown staining) at LH + 10. Scale bar = 250 μm. Right panel: Pearson’s correlation analysis of stromal cell and uNK cell densities. A total of 80 randomly selected images from 20 biopsies were analyzed. (C) Representative images of an eosin stained primary culture decidualized for 8 days incubated for 18 hr with or without uNK cells isolated from luteal phase endometrium. Scale bar = 100 μm. (D) SAβG activity in undifferentiated or day eight decidualized EnSCs co-cultured with or without uNK cells in the presence or absence of the apoptosis inhibitor Z-VAD-FMK (Z-VAD, 10 μM) or the granzyme activity inhibitor 3,4-DCI (25 μM). (E) Secretion of IL-15 secretion was measured every 48 hr in the supernatant of primary EnSCs decidualized for the indicated time-points following pretreatment with vehicle, dasatinib (250 nM) or palbociclib (1 μM). (F) SAβG activity in undifferentiated or day eight decidualized EnSCs co-cultured with or without uNK cells in the presence or absence of an IL-15 blocking antibody (1 μg/ml). Data are mean ±SEM of 3 biological replicates unless stated otherwise. Different letters above the error bars indicate that those groups are significantly different from each other at p<0.05.
-
Figure 5—source data 1
uNK cell mediated immune surveillance and clearance of senescent cells.
- https://doi.org/10.7554/eLife.31274.015
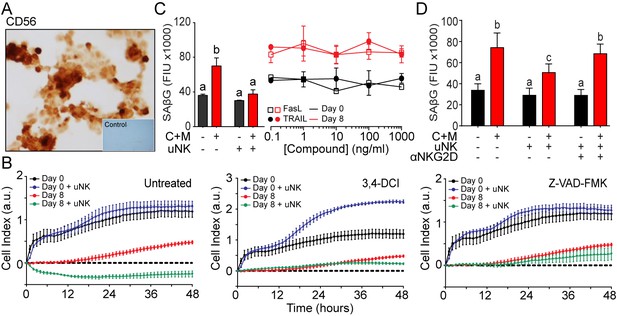
uNK cell mediated immune surveillance and clearance of senescent cells.
(A) CD56 staining on cytospin preparations of isolated uNK cells. Inset: primary antibody was omitted. (B) Real-time analysis of EnSC viability using xCELLigence in the presence or absence of uNKs following treatment with 25 μM 3,4-DCI or 10 μM Z-VAD_FMK as indicated. Data are mean ±SEM from technical replicates and representative of two further biological repeat experiments. (C) SAβG activity following exposure of EnSCs to increasing concentrations of FasL or TRAIL as indicated. Parallel co-cultures of EnSCs with uNK cells are shown for comparison. (D) SAβG activity in undifferentiated or day eight decidualized EnSCs co-cultured with or without uNK cells in the presence or absence an inhibitory NKG2D receptor antibody. Different letters above the error bars indicates that those groups are significantly different from each other at p<0.05. Data are mean ±SEM, n = 3.
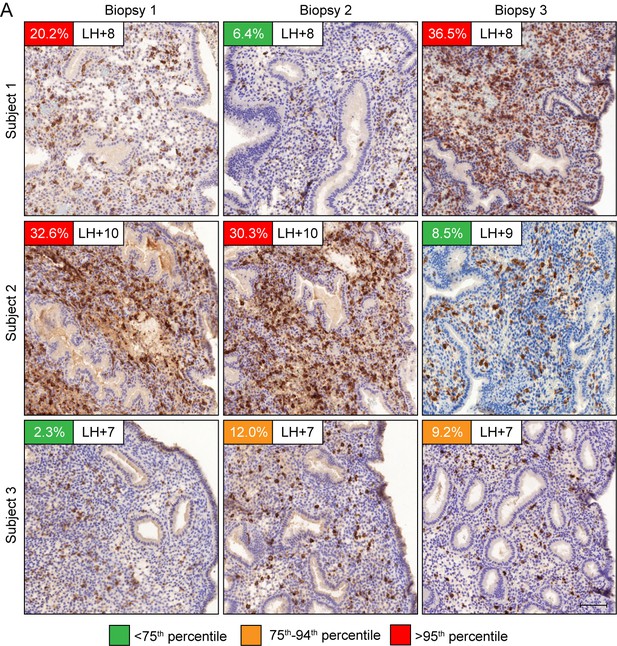
Dynamic inter-cycle fluctuations in uNK cell levels.
CD56 immunohistochemistry of LH-timed endometrial biopsies obtained in three different cycles in three subjects. The day of the biopsy and the percentage of CD56+ uNK cells versus stromal cells are indicated. The color of the box indicates the percentile range of uNK when adjusted for the day of biopsy. Scale = 200 μm.
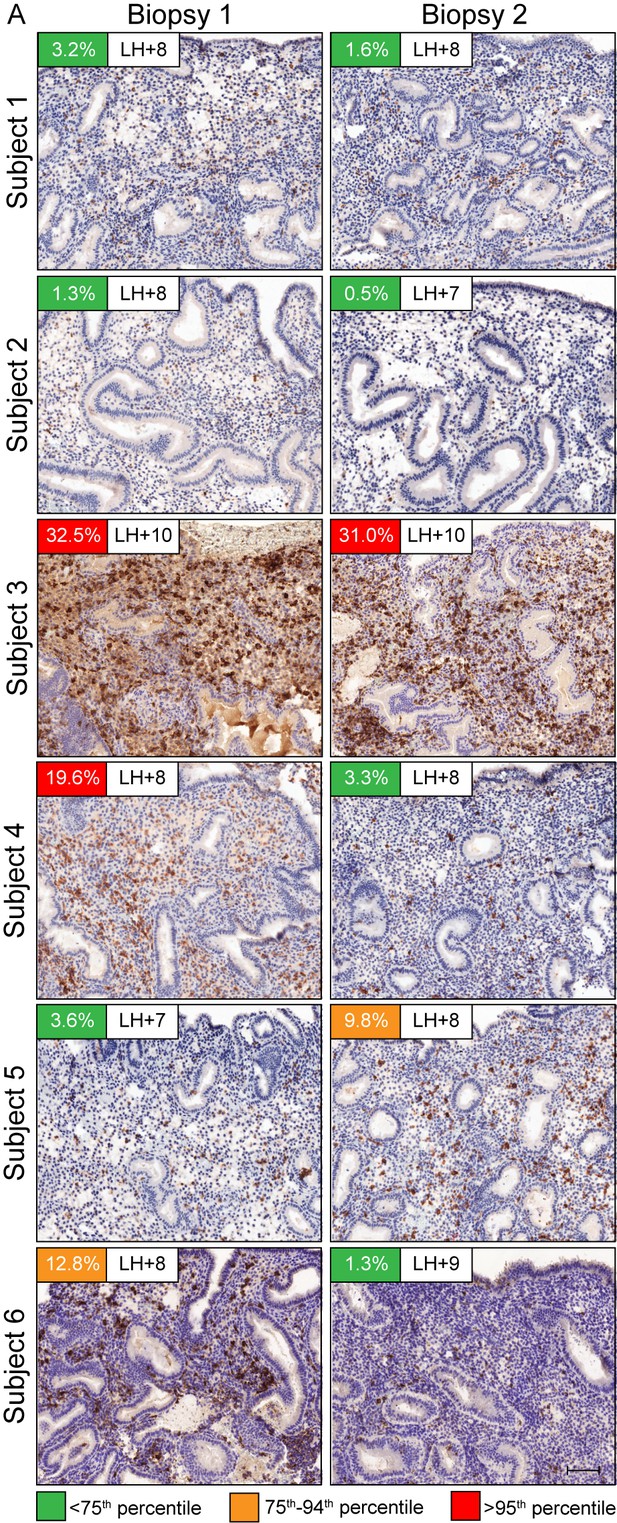
uNK cells in repeat biopsies.
CD56 immunochemistry of LH-timed endometrial biopsies in two consecutive cycles. Day of biopsy relative to LH surge and the percentage of CD56+ uNK cells versus stromal cells are indicated. The color of the box indicates the percentile range of uNK when adjusted for the day of biopsy. Scale = 200 μm.
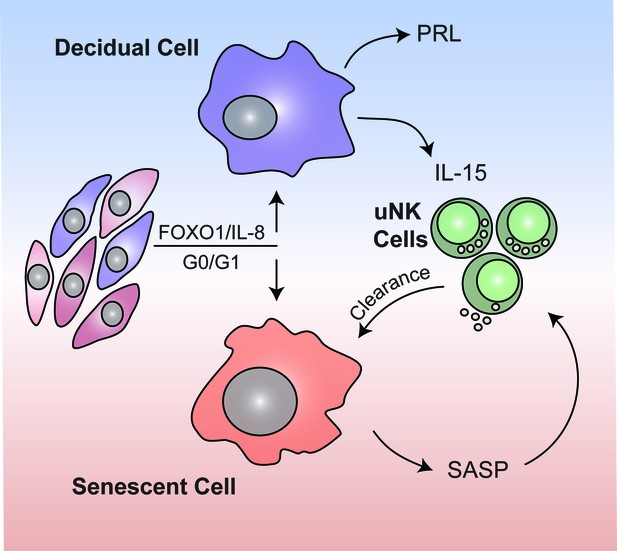
Schematic summary.
We propose that rapid endometrial growth during the proliferative phase is important for implantation as it imparts replication stress in a subpopulation of EnSCs. Upon cell cycle exit at G0/G1, this subpopulation of stressed EnSCs do not differentiate into specialist decidual cells but undergo acute cellular senescence and secrete a host of inflammatory mediators (senescence associated secretory phenotype; SASP) involved in endometrial receptivity. In parallel, Il-15 secreted by differentiated decidual cells activates uNK cells, which then target and eliminate senescent cells through granule exocytosis. Systematic clearance of acutely senescent decidual cells by uNK cells not only remodels but also rejuvenates the endometrium at the time of embryo implantation.
Videos
EnSCs and uNK co-cultures.
Time-lapse microscopy of 8 day decidualized EnSCs and uNK co-cultures. Images captured at a rate of 1 frame per 10 min. Time (hours) and scale as indicated.
Tables
| Reagent type | Designation | Source | Identifiers |
|---|---|---|---|
| antibody | p53 | Dako | RRID:AB_2206626 |
| antibody | P16INK4a; p16 | Abcam | RRID:AB_10858268 |
| antibody | Lamin B1; LMN1 | Abcam | RRID:AB_443298 |
| antibody | HMBG2 | Abcam | RRID:AB_1140885 |
| antibody | macro.H2A; mH2A | Abcam | RRID:AB_2716576 |
| antibody | Histone H1; H.H1 | Abcam | RRID:AB_11000797 |
| antibody | H3K9me3 | Abcam | RRID:AB_306848 |
| antibody | β-actin | Sigma-Aldrich | RRID:AB_476744 |
| antibody | IL-15 | R and D Systems | RRID:AB_2124578 |
| antibody | NKG2D | R and D Systems | RRID:AB_2133263 |
| antibody | PE-conjugated anti-CD56 | Biolegend | RRID:AB_2563924 |
Additional files
-
Supplementary file 1
Patient demographics.
Data are related to primary cultures or individual figures as indicated. Primary cultures refer to all biopsies from which EnSCs were isolated and propagated in culture. *Values are presented as mean ±SD. ^Values are presented as median (range). N/A: not applicable.
- https://doi.org/10.7554/eLife.31274.020
-
Transparent reporting form
- https://doi.org/10.7554/eLife.31274.021





