A zebrafish and mouse model for selective pruritus via direct activation of TRPA1
Figures
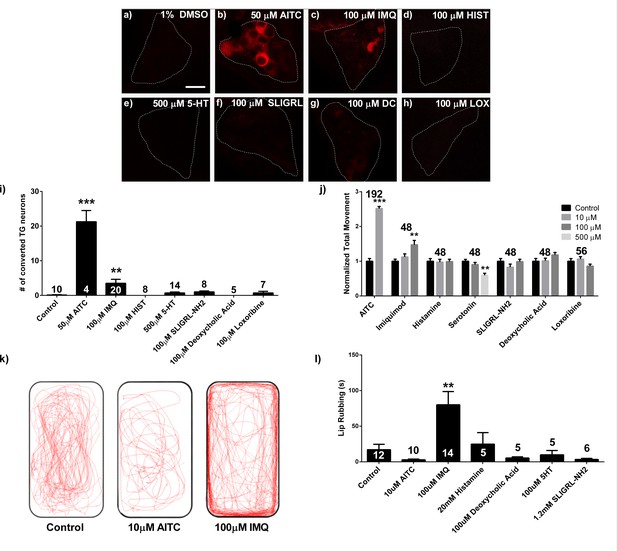
Imiquimod produces behavioral and neuronal responses in the zebrafish.
(A–H) 3dpf live elavl3:CaMPARI TG imaging of larvae exposed to 405 nm light and control (A), 50 μM allyl isothiocyanate (AITC) (B), 100 μM imiquimod (IMQ) (C), 100 μM histamine (HIST) (D), 500 μM serotonin (5-HT) (E), 100 μM SLIGRL-NH2 (F), 100 μM deoxycholic acid (DCA) (G), and 100 μM loxoribine (LOX) solutions (H). (I), Counts of photoconverted cells from experiments (A-H). (J), 5dpf WT larval locomotor behavior screen. (K) Representative traces of adult behavior. (L) Adult lip-rubbing behavioral assay. (I, J, L) ***p<0.001, **p<0.01, one-way ANOVA. Bars represent mean ± s.e.m.
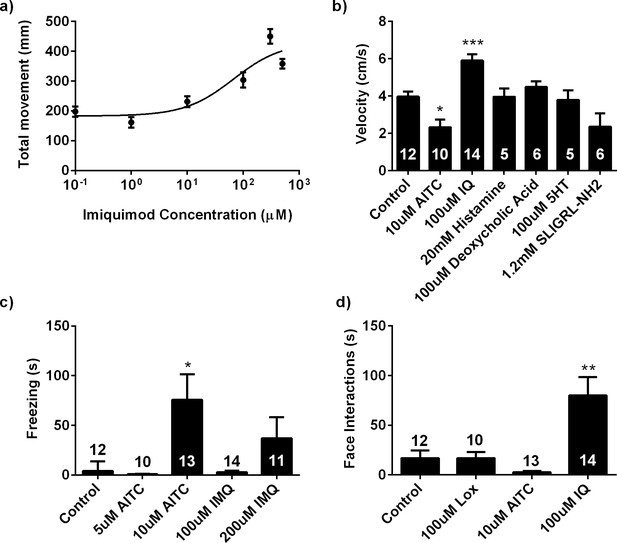
Behavioral effects of noxious pruritic and algogenic stimuli in the zebrafish.
(A) Dose-response curve for IMQ in larval locomotion assay, n = 48 for all conditions. (B) Average velocity of adult zebrafish injected with AITC or known pruritogens. 10 μM AITC induced a significant decrease in swimming velocity, whereas 100 μM IMQ elicited increased velocity. (C) Quantification of freezing behavior in the adult behavioral assay. Of the stimuli tested, only 10 μM AITC elicited significant freezing behaviors in adult zebrafish, although fish injected with 200 μM IMQ displayed a trend towards elevated freezing behaviors. (D) A comparison of lip-rubbing behavior in adult zebrafish injected with 10 μM AITC, 100 μM IMQ, and 100 μM loxoribine. Only IMQ evoked significant lip-rubbing behavior. ***p<0.001, **p<0.01, one-way ANOVA.
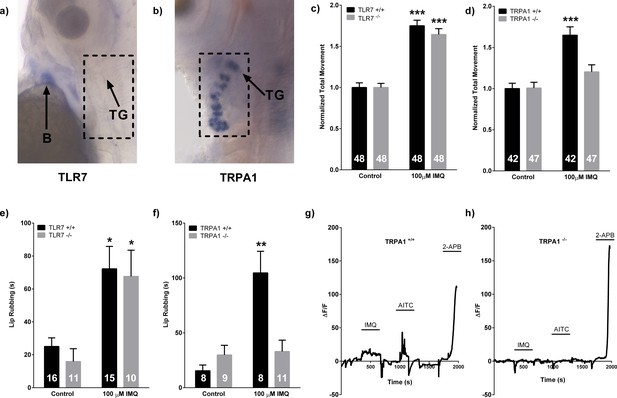
Trpa1b, but not Tlr7, is required for both neuronal and behavioral responses to IMQ.
(A, B) In situ hybridization in 3dpf WT larvae probing for tlr7 and trpa1b mRNA respectively. (C, D) Larval locomotor assay of 5dpf tlr7+/+ and tlr7-/- (C) or trpa1b+/+ and trpa1b-/- (D) larvae. (E, F) Adult lip-rubbing behavioral assay of tlr7+/+/tlr7-/- (E) and trpa1b+/+/trpa1b-/-(F) fish. (C), (D), (E), (F), 100 μM IMQ used. ***p<0.001, **p<0.01, Student’s t-test. Bars represent mean ± s.e.m. (G, H) Representative calcium imaging traces of 3dpf trpa1b+/+ (G) and trpa1b-/- (H) larvae in a transgenic elavl3HuC:GCaMP5 background exposed to 100 μM IMQ, 50 μM AITC, and 1 mM 2-APB. B = blood, TG = trigeminal ganglion.
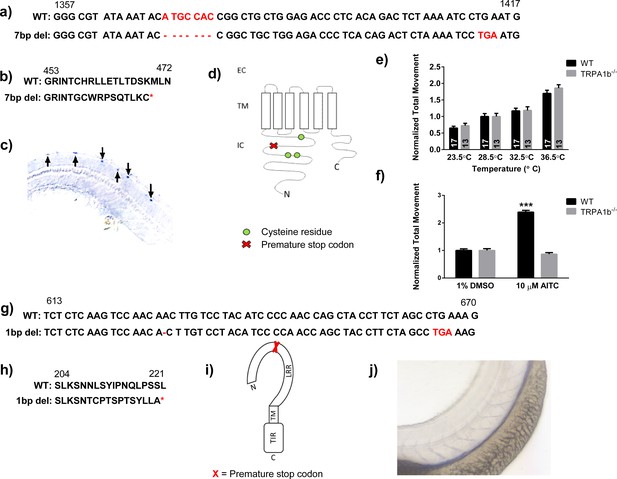
TRPA1b and TLR7 nonsense mutations in the zebrafish.
(A) The 7 bp deletion in the trpa1b coding sequence generated a premature stop codon at 1412 bp. (B) Amino acid sequences of the WT and mutant Trpa1b proteins. (C) In situ hybridization probing for trpa1b mRNA in WT 3dpf larval zebrafish, demonstrating expression in RB neurons. (D) Schematic of the Trpa1b protein structure, demonstrating that a truncated protein (in the unlikely event that it was translated) would lack a critical cysteine residue required for agonist binding (Macpherson et al., 2007). (E) Trpa1b-/- nonsense mutants locomoted more in response to increasing temperatures at levels equivalent to their WT/heterozygous siblings. (F) Normal AITC behavioral responses (increased locomotion) are abolished in trpa1b-/- nonsense mutants. (G) The 1 bp deletion in the tlr7 coding sequence generated a premature stop codon at bp 665. (H) Amino acid sequences of WT and mutant Tlr7 proteins. (I) Schematic of the Tlr7 protein, demonstrating that a truncated protein (in the unlikely event of translation) would lack critical functional domains. (J) In situ hybridization probing for tlr7 mRNA in WT 3dpf larval zebrafish. No tlr7 expression was observed in RB somatosensory neurons. Premature stops are denoted with red highlighting. EC = extracellular, IC = intracellular, TM = transmembrane domain, LRR = leucine rich repeat, TIR = Toll Interleukin-1 Resistance domain. C and N denote c- and n-termini. (E), Student’s t-test.
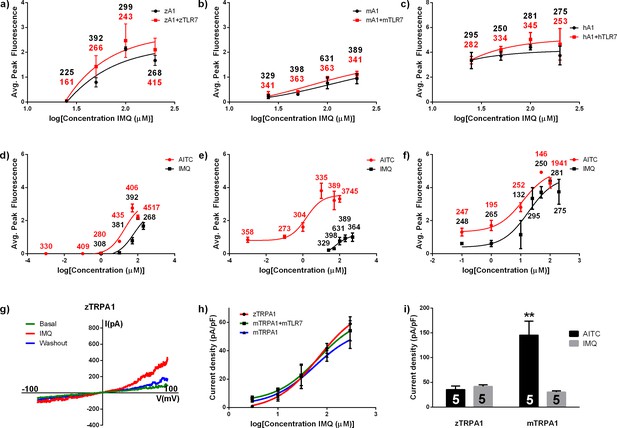
Imiquimod directly activates TRPA1.
(A–C) Calcium imaging of HEK cells transfected with zebrafish (A), mouse (B), and human (C) Trpa1 or Trpa1 + Tlr7. (D, E, F) Calcium imaging dose response curves of HEK cells transfected with zebrafish (D), mouse (E), and human (F) Trpa1, exposed to IMQ or AITC. (A–F) Numbers represent total cell counts per condition. (G) Patch clamp of HEK cell transfected with zebrafish trpa1b exposed to 100 μM IMQ. (H) Patch clamp dose response curve for HEK cells transfected with zebrafish trpa1b, mouse Trpa1, or mouse Trpa1 + mouse Tlr7. (I) Current density values of HEK cells exposed transfected with zebrafish trpa1b or mouse Trpa1 and exposed to 100 μM AITC or 100 μM IMQ. (G–I) n = 5 cells per condition. **p<0.01, Student’s t-test. Bars represent mean ± s.e.m.
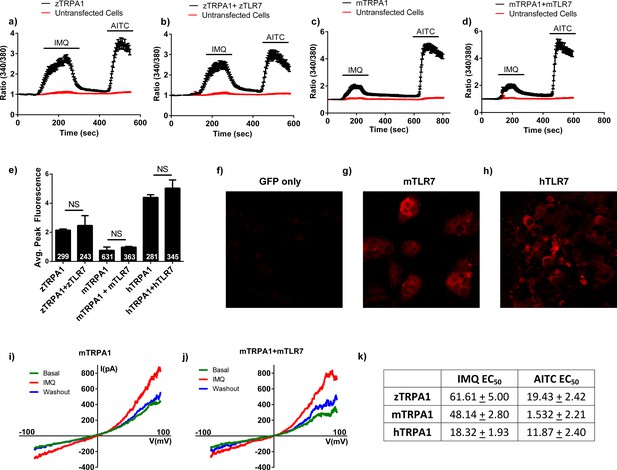
Transfected HEK cell gene expression and functionality.
(A–D) Average traces from calcium imaging experiments with HEK cells transiently transfected with zebrafish trpa1b (n = 85) (A), zebrafish trpa1b + zebrafish tlr7 (n = 80) (B), mouse Trpa1 (n = 134) (C), or mouse Trpa1+ mouse Tlr7 (n = 89) (D). n = 22, 23, 10, and 19 untransfected control HEK cells per experiment, respectively. No gross differences were observed between the two conditions for each species. (E) Quantification of peak fluorescence intensity achieved during stimulation with 100 μM IMQ across all HEK cell transfection conditions. In all species examined, no significant difference was observed between cells transfected with only Trpa1 and cells transfected with Trpa1 plus the corresponding Tlr7. (F–H), Immunohistochemistry performed on HEK cells transfected with pIRES-eGFP only (F), mouse Tlr7 (G), or human TLR7 (H). As shown, TLR7 labeling (red) was only observed HEK cells transfected with Tlr7 constructs. (I–J), I/V curves from voltage clamp experiments using cells transfected with mTRPA1 (I) and mouse Trpa1 + mouse Tlr7 (J). As shown in (I), 100 μM IMQ elicited greater current influx in transfected cells than under basal conditions, an effect that disappeared during washout. Cells transfected with both mouse Trpa1 and mouse Tlr7 also showed significant current flux during IMQ stimulus, but this was not different from cells transfected only with mTRPA1. n = 5 cells per condition. (K) a table of EC50 values for both IMQ and AITC stimuli in cells transfected with zebrafish trpa1b, mouse Trpa1, and human TRPA1 in Figure 3D–F. As shown, the EC50 values for IMQ are much greater than those of AITC for all species of TRPA1. ΔF/F (fluorescence intensity change) is expressed as a normalized 340 nm/380 nm intensity ratio. (*p<0.05, **p<0.01, ***p<0.001, Student’s t-test. Bars are expressed as means ± s.e.m.

Loxoribine effectively stimulates Tlr7-transfected HEK cells but does not evoke intracellular calcium flux.
(A) A comparison of average peak fluorescence values obtained from Trpa1 or Trpa1 + Tlr7 transfected HEK cells in calcium imaging experiments following stimulation by 100 μM IMQ or 100 μM loxoribine. (B, C) Average traces from calcium experiments in which HEK cells were transfected with zebrafish trpa1b + zebrafish tlr7 (n = 102) (B) or mouse Trpa1 + mouse Tlr7 (n = 45) (C) and treated with 100 μM loxoribine and 100 μM AITC. 15 and 21 untransfected control cells were present in each respective experiment. As shown, loxoribine did not elicit any calcium flux. (D) I/V curve from voltage clamp experiments in which HEK cells were transfected with mouse Trpa1 + mouse Tlr7 and stimulated with both loxoribine (100 μM) and AITC (100 μM). While AITC elicited remarkable current influx, current change associated with application of loxoribine did not change current flow above baseline levels. (E) A dual luciferase assay to verify the functionality of transfected TLR7 and loxoribine. Cells transfected with only the two luciferase constructs and pIRES-eGFP did not demonstrate any NF-kB induction following stimulation with 200 μM loxoribine, as expected. Notable NF-kB induction was observed in cells transfected with the two luciferase constructs and either mouse Tlr7 or human TLR7 following stimulation with loxoribine. Intriguingly, cells transfected with the zebrafish tlr7 did not exhibit any significant NF-kB induction following application of loxoribine. (F) A dual luciferase assay in which both pIRES-eGFP-transfected control HEK cells and zebrafish tlr7-transfected cells were treated with TLR7 agonists loxoribine (500 μM and 1 mM) and IMQ (100 μM and 500 μM). There was no difference in NF-kB induction between pIRES-eGFP-only and zebrafish tlr7-transfected cells, potentially indicating that zebrafish Tlr7 is unresponsive to typical mammalian TLR7 agonists. ΔF/F (fluorescence intensity change) is expressed as a normalized 340 nm/380 nm intensity ratio. Luminosity values are expressed as the firefly/renilla ratio of relative light units in counts per second (CPS) following background subtraction. *p<0.05, **p<0.01, ***p<0.001, Student’s t-test (A, E) or one-way ANOVA (F). Bars are expressed as means ± s.e.m.
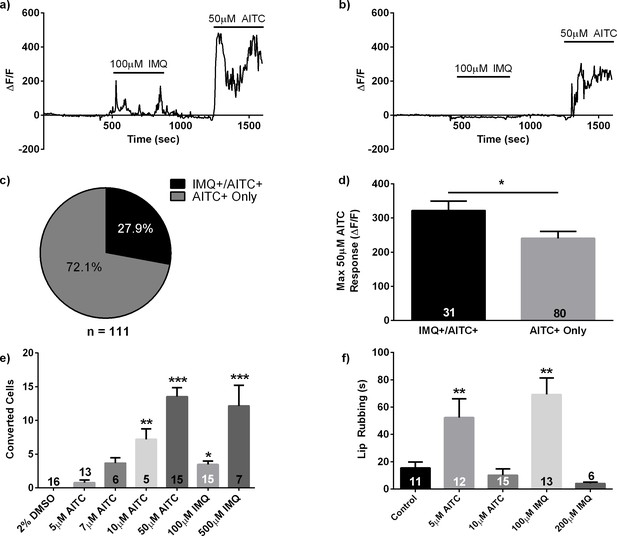
Imiquimod responsive cells are a primed subpopulation of TRPA1 positive cells and stimulus intensity affects behavioral and neuronal responses.
(A, B) Representative traces from calcium imaging experiments in 3dpf elavl3:H2BGCaMP6 zebrafish exposed to 100 μM IMQ and 50 μM AITC. An IMQ+/AITC+ TG neuron is shown in (A), while an AITC+ only neuron is shown in (B). (C) Quantification of neuronal subtypes within AITC+ neurons. n = 31 IMQ+/AITC+ neurons, n = 80 AITC+ only neurons. (D) Comparison of the maximum ΔF/F during the 50 μM AITC stimulus between neuronal subtypes. (E) Number of photoconverted neurons in 3dpf elavl3:CaMPARI zebrafish TG following stimulation with TRPA1 agonists. (F) Quantification of lip-rubbing behavior in adult zebrafish following exposure to TRPA1 agonists. Bars represent means ± s.e.m. *p<0.05, **p<0.01, ***p<0.001, Student’s t-test (D), one-way ANOVA (E, F).
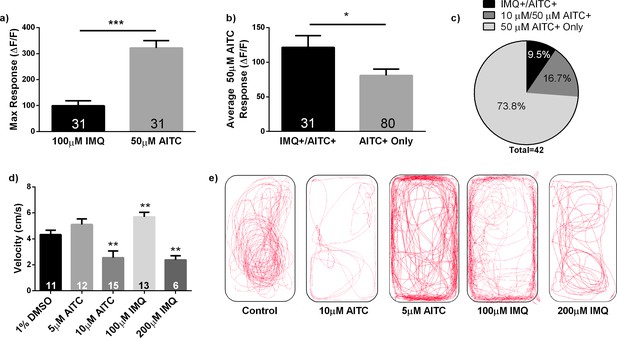
Further effects of stimulus intensity on zebrafish neuronal activity and behavior.
(A) IMQ is a lower intensity stimulus than AITC. Application of 100 μM IMQ elicited a lower maximal ΔF/F response than application of 50 μM AITC in IMQ+/AITC+ TG neurons in 3dpf larval zebrafish. (B) In an effort to ensure that our analyses in Figure 4D were accurate reflections of neuronal activity, we also examined the average ΔF/F across the stimulus period. We found that IMQ+/AITC+ cells exhibited a significantly higher average fluorescence change across the entire 50 μM AITC stimulus period than AITC + only responding neurons. (C) Sequential application of 100 μM IMQ, 10 μM AITC, and 50 μM AITC revealed that IMQ+ neurons (n = 4) were only found in a subset of neurons that responded to 10 μM AITC (n = 11), which themselves were included in a larger population of 50 μM AITC+ neurons (n = 42). 50 μM AITC results were consistent with previous experiments. (D) Swimming velocity in adult zebrafish also varied as a function of stimulus intensity. Fish that were injected with 100 μM IMQ (n = 13) exhibited a significant increase in swimming velocity as compared to fish injected with 1% DMSO vehicle (n = 11). Fish injected with 10 μM AITC (n = 15) and 200 μM IMQ (n = 6) exhibited significant reductions in swimming velocity as compared to vehicle-injected controls, suggesting that these stimuli at these particular concentrations are effectually algogenic. 5 μM AITC evoked no significant change in swimming velocity (n = 12). ΔF/F (fluorescence intensity change) is calculated as the percent change over baseline fluorescence. Bars are expressed as means ± s.e.m. *p<0.05, **p<0.01, ***p<0.001, Student’s t-test (A, B), one-way ANOVA (D). (E) Representative traces of adult behavior.
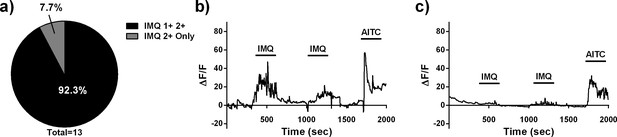
Imiquimod activates a specific subset of neurons in a non-stochastic manner in the zebrafish.
(A) Proportions of neurons responding to two sequential pulses of 100 µM IMQ, only the first pulse, or only the second pulse in 3dpf larval zebrafish. (B) Representative trace of a TG neuron that responded to both pulses of 100 µM IMQ. (C) Representative trace of a TG neuron that responded to only the second pulse of 100 µM IMQ.
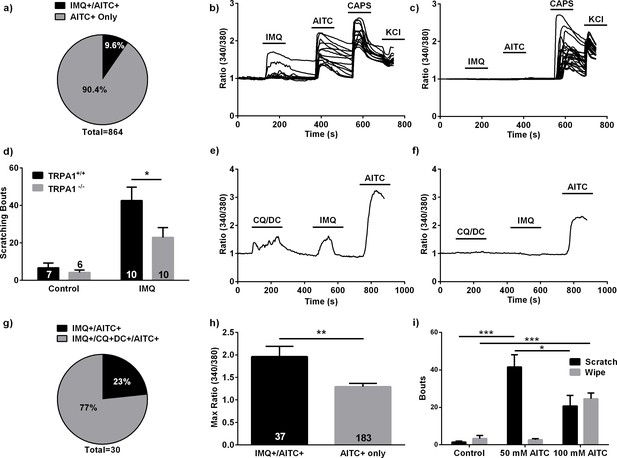
Imiquimod’s effects in the mouse.
(A) Proportions of IMQ+/AITC+ and AITC+ only DRG neurons observed in calcium imaging experiments (n = 83 IMQ+/AITC+ neurons, n = 781 AITC+ only DRG neurons). (B, C) Representative traces from calcium imaging experiments on dissociated mouse DRG neurons from WT (B) (n = 15 neurons) and Trpa1-/- (C) (n = 23 neurons) mice. (A–C), 100 μM IMQ and 100 μM AITC used. (D) Quantification of scratching bouts in WT and Trpa1-/- mice following IMQ (10 μg) injections. (E, F) Representative traces from calcium imaging experiments in which 100 μM IMQ, 100 μM CQ, 100 μM DC, and 100 μM AITC were applied. IMQ+/CQ+DCA+/AITC+ (e) and AITC+ only (f) neurons are shown. (G) Quantification of the IMQ+ neuronal populations from the experiments shown in (E, F) (n = 27 IMQ+/CQ+DCA+/AITC+ neurons, 7 IMQ+/AITC+ neurons). (H) Comparison of maximum ΔF/F between IMQ+/AITC+ and AITC+ only. 100 μM IMQ, 10 μM AITC used. All calcium imaging experiments performed on dissociated mouse DRG neurons. (I) Quantification of scratching bouts in WT mice following injections of varying AITC concentrations. *p<0.05, **p<0.01, ***p<0.001, Student’s t-test. Bars are expressed as mean + s.e.m.
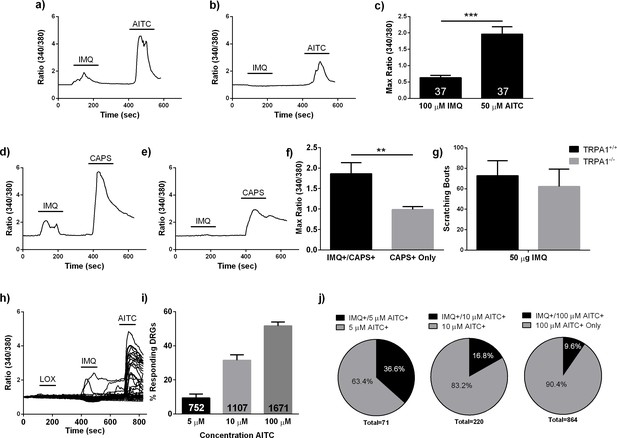
Calcium imaging of discrete neuronal subpopulations in the mouse.
(A, B) Representative traces from calcium imaging experiments featuring dissociated mouse DRG neurons sequentially bathed in 100 μM IMQ and 10 μM AITC. IMQ+/AITC+ (A) and AITC+ only (B) neurons were both identified. As shown in (B), many of the AITC+ only neurons peaked at lower ΔF/F values than the IMQ+/AITC+ neurons. (C) Within the IMQ+/AITC+ population (n = 37 neurons), 100 μM IMQ was demonstrated to be a weaker stimulus than 10 μM AITC. (D, E) Representative traces of calcium imaging experiments where DRG neurons were stimulated with 100 μM IMQ and 100 nM CAPS. Two main functional subpopulations were identified--one that responded to both IMQ and CAPS (D), and another that responded only to 100 nM CAPS (E). Interestingly, many of the CAPS+ only cells peaked at lower ΔF/F values during the CAPS stimulus than the neurons that responded to both IMQ and CAPS. (F) Comparison of maximum ΔF/F between IMQ+/CAPS+ and CAPS+ only neurons. (G) Quantification of scratching bouts in WT and Trpa1-/- mice following IMQ injections. (H) Representative traces from from one calcium imaging experiment in which 100 μM loxoribine was applied to dissociated DRG neurons. As shown, loxoribine did not elicit any fluorescence changes in DRG neurons (n = 51), whereas 100 μM IMQ and 100 μM AITC elicited robust intracellular calcium flux. (I) Increasing the concentration of AITC activates greater proportions of DRG neurons (71/752, 379/1107, and 864/1671 neurons for the 5 μM, 10 μM, and μM AITC experiments). (J) IMQ+ neuron populations are enriched within populations that respond to lower concentrations of AITC (26/71 5 μM AITC+ neurons, 37/220 10 μM AITC+ neurons, 83/864 100 μM AITC+ neurons). Fluorescence intensity is expressed as ΔF/F using the 340/380 ratio values. Bars represented as means + s.e.m., Student’s t-test. *p<0.05, **p<0.01, ***p<0.001.
Videos
Normal adult zebrafish swimming behavior.
This video is an example of typical swimming behavior following a cutaneous injection of 1% DMSO into the upper lip of an adult zebrafish. Note that the zebrafish exhibits minimal interaction with the walls of the tank, and its swimming capacity is not compromised.
Adult zebrafish injected with AITC demonstrate nocifensive behaviors.
This video is an example of typical swimming behavior following a cutaneous injection of 10 µM AITC into the upper lip of an adult zebrafish. The zebrafish exhibits previously described nocifensive behaviors, such as dramatically reduced locomotion, bouts of freezing, and heightened respiration.
Adult zebrafish injected with IMQ demonstrate pruritic behaviors.
This video provides a typical example of swimming behavior observed in adult zebrafish following a cutaneous upper lip injection of 100 µM IMQ. The fish not only exhibits increased swimming velocity in response to this stimulus, but also frequently rubs its lips against the walls of its tank, engaging in a behavior that is potentially analogous to mammalian scratching.
Additional files
-
Transparent reporting form
- https://doi.org/10.7554/eLife.32036.018





