Non-invasive detection of urothelial cancer through the analysis of driver gene mutations and aneuploidy
Figures
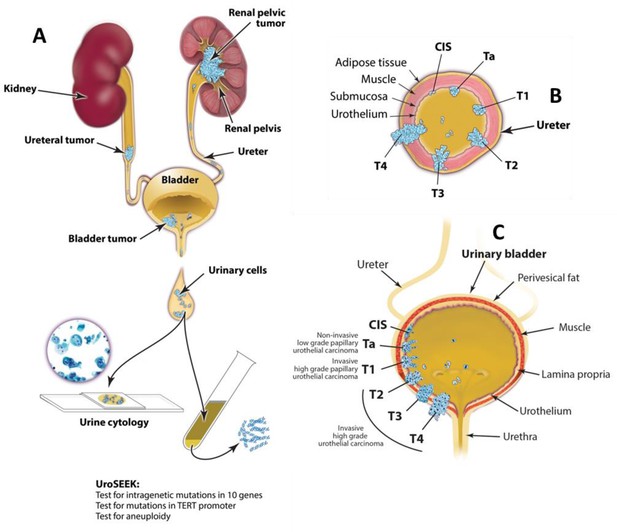
Schematic of the approach used to evaluate urinary cells in this study.
UroSEEK assay is designed to detect urothelial neoplasms that are in direct contact with urine (A) of variable pathologic stages originating in upper urinary tract (B) or bladder (C).
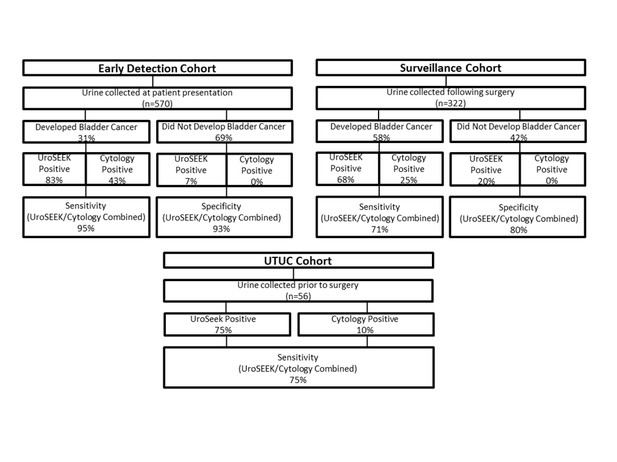
Flow diagram indicating the number of patients in the three cohorts evaluated in this study and summarizing the salient findings.
Cytology was performed on only a subset of the patients (see main text).
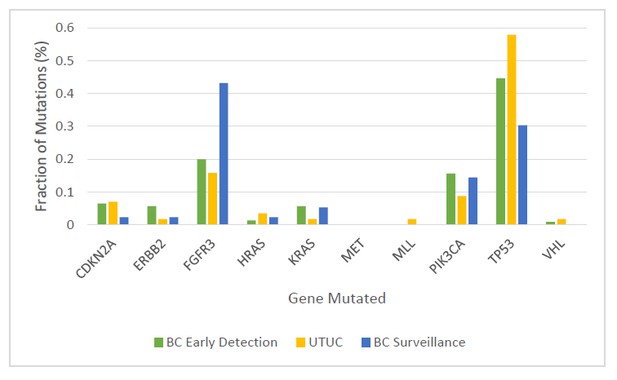
Fraction of mutations found in the ten-gene panel in 231 urinary cell samples assessed in the BC early detection cohort, 56 urinary cell samples assessed in the UTUC cohort, and 132 urinary cell samples assessed in the BC surveillance cohort.
https://doi.org/10.7554/eLife.32143.005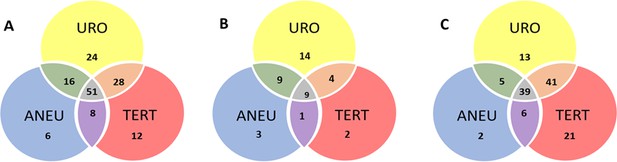
Venn diagram showing the distribution of positive results for each of the three UroSEEK assays for the (A) BC early detection (B) UTUC and (C) BC surveillance cohorts.
URO = Ten gene panel, TERT = TERT promoter region, ANEU = Aneuploidy test.
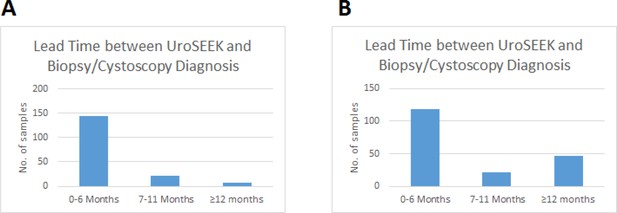
Bar graphs of the lead time between a positive UroSEEK test and the detection of disease at the clinical level in the (A) BC early detection and (B) BC surveillance cohorts.
https://doi.org/10.7554/eLife.32143.007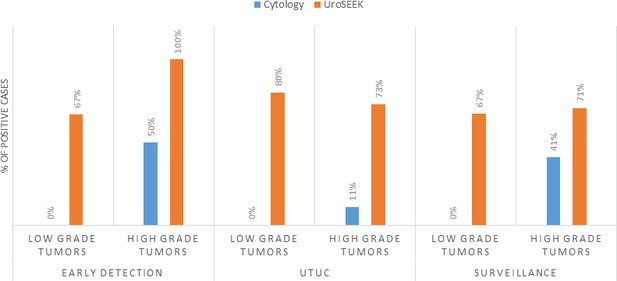
Bar graphs representing the performance of Cytology vs. UroSEEK in diagnosis of low- and high-grade urothelial neoplasms in the early detection and surveillance BC cohorts and the UTUC cohort.
https://doi.org/10.7554/eLife.32143.009Tables
Demographic, clinical and genetic features of the early detection cohort.
https://doi.org/10.7554/eLife.32143.004| Gender | n | % | Ten-gene multiplex positive | TERT positive | Aneuploidy positive | UroSEEK positive | Cytology positive* | Uroseek or cytology positive* |
|---|---|---|---|---|---|---|---|---|
| Table 1a. Demographic, clinical and genetic features of the early detection cohort | ||||||||
| Males without recurrence | 172 | 59% | 3 (2%) | 10 (6%) | 2 (1%) | 13 (8%) | 0 (0%) | 13 (8%) |
| Males with recurrence | 32 | 11% | 26 (81%) | 21 (66%) | 19 (59%) | 29 (91%) | 16 (50%) | 30 (94%) |
| Females without recurrence | 81 | 28% | 2 (2%) | 2 (2%) | 1 (1%) | 5 (6%) | 0 (0%) | 5 (6%) |
| Females with recurrence | 9 | 3% | 4 (44%) | 4 (44%) | 3 (33%) | 6 (67%) | 1 (11%) | 6 (67%) |
| Indication | ||||||||
| Hematuria without recurrence | 346 | 61% | 6 (2%) | 15 (4%) | 5 (1%) | 22 (6%) | 0 (0%) | 17 (5%) |
| Hematuria with recurrence | 163 | 29% | 108 (66%) | 90 (55%) | 76 (47%) | 134 (82%) | 18 (11%) | 32 (2%) |
| LUTS without recurrence | 11 | 2% | 0 (0%) | 2 (18%) | 0 (0%) | 2 (18%) | 0 (0%) | 2 (18%) |
| LUTS with recurrence | 3 | 1% | 2 (67%) | 1 (33%) | 0 (0%) | 2 (67%) | 1 (33%) | 2 (67%) |
| Other without recurrence | 38 | 7% | 1 (3%) | 0 (0%) | 1 (3%) | 2 (5%) | 0 (0%) | 2 (5%) |
| Other with recurrence | 9 | 2% | 9 (100%) | 8 (89%) | 5 (56%) | 9 (100%) | 2 (22%) | 9 (100%) |
| Detected Tumor Diagnosis | ||||||||
| PUNLMP | 2 | 1% | 0 (0%) | 1 (50%) | 0 (0%) | 1 (50%) | 0 (0%) | 0 (0%) |
| CIS | 7 | 5% | 4 (57%) | 4 (57%) | 1 (14%) | 6 (86%) | 3 (43%) | 6 (86%) |
| LGTCC | 31 | 21% | 15 (48%) | 18 (58%) | 9 (29%) | 22 (71%) | 0 (0%) | 4 (13%) |
| HGTCC | 49 | 33% | 34 (69%) | 28 (57%) | 26 (53%) | 40 (82%) | 4 (8%) | 11 (22%) |
| INTCC | 61 | 41% | 48 (79%) | 36 (59%) | 35 (57%) | 57 (93%) | 9 (15%) | 16 (26%) |
| Cytology diagnosis* | ||||||||
| Positive | 21 | 6% | 16 (76%) | 12 (57%) | 16 (76%) | 20 (95%) | N/A | N/A |
| Atypical | 105 | 30% | 21 (20%) | 21 (30%) | 12 (11%) | 30 (29%) | N/A | N/A |
| Negative | 221 | 64% | 4 (2%) | 9 (4%) | 1 (0.4%) | 12 (5%) | N/A | N/A |
| Table 1b. Demographic, clinical and genetic features of the Surveillance cohort. | ||||||||
| Males without recurrence | 59 | 30% | 3 (5%) | 8 (14%) | 3 (5%) | 10 (17%) | 0 (0%) | 8 (14%) |
| Males with recurrence | 90 | 45% | 45 (50%) | 53 (59%) | 20 (22%) | 59 (66%) | 20 (22%) | 53 (59%) |
| Females without recurrence | 17 | 9% | 5 (29%) | 3 (18%) | 0 (0%) | 6 (35%) | 0 (0%) | 6 (35%) |
| Females with recurrence | 33 | 17% | 15 (45%) | 19 (58%) | 11 (33%) | 33 (100%) | 6 (18%) | 19 (58%) |
| Original Tumor Diagnosis | ||||||||
| PUNLMP | 12 | 4% | 5 (42%) | 2 (17%) | 1 (8%) | 6 (50%) | 0 (0%) | 2 (17%) |
| CIS | 25 | 8% | 11 (44%) | 13 (52%) | 6 (24%) | 14 (56%) | 5 (20%) | 10 (40%) |
| LGTCC | 107 | 35% | 27 (25%) | 34 (32%) | 8 (7%) | 41 (38%) | 0 (0%) | 59 (55%) |
| HGTCC | 62 | 20% | 22 (36%) | 24 (39%) | 10 (16%) | 30 (49%) | 4 (7%) | 16 (26%) |
| INTCC | 104 | 34% | 39 (38%) | 47 (45%) | 29 (28%) | 54 (52%) | 20 (19%) | 34 (33%) |
| Original Tumor Stage | ||||||||
| pTis | 25 | 8% | 11 (44%) | 13 (52%) | 6 (24%) | 14 (56%) | 5 (20%) | 10 (40%) |
| pTa | 181 | 58% | 54 (30%) | 60 (33%) | 19 (19%) | 77 (43%) | 4 (2%) | 77 (43%) |
| pT1 | 71 | 23% | 28 (39%) | 35 (49%) | 22 (31%) | 39 (55%) | 14 (20%) | 23 (32%) |
| pT2 | 23 | 7% | 9 (9%) | 9 (39%) | 7 (30%) | 12 (52%) | 5 (22%) | 10 (43%) |
| pT3 | 9 | 3% | 1 (11%) | 2 (22%) | 0 | 2 (22%) | 1 (11%) | 1 (11%) |
| pT4 | 1 | 0.3% | 1 (100%) | 1 (100%) | 0 | 1 (100%) | N/A | N/A |
| Routine cytology diagnosis* | ||||||||
| Positive | 30 | 15% | 21 (21%) | 25 (83%) | 20 (67%) | 27 (90%) | N/A | N/A |
| Atypical | 95 | 48% | 38 (40%) | 43 (45%) | 18 (19%) | 50 (53%) | N/A | N/A |
| Negative | 71 | 36% | 12 (17%) | 13 (18%) | 3 (4%) | 19 (27%) | N/A | N/A |
-
*Cytology was available on only a subset of cases.
N/A Not Available.
Demographic, clinical and genetic features of the UTUC cohort stratified by UroSEEK results.
https://doi.org/10.7554/eLife.32143.008| N | % | Ten-gene multiplex positive | TERT positive | Aneuploidy positive | UroSEEK positive | |
|---|---|---|---|---|---|---|
| All subjects | 56 | 100% | 64% | 29% | 39% | 75% |
| Gender | ||||||
| Males | 24 | 43% | 71% | 33% | 54% | 83% |
| Females | 32 | 57% | 59% | 25% | 28% | 69% |
| CKD stage | ||||||
| 0–2 | 25 | 45% | 68% | 36% | 44% | 76% |
| 3A | 14 | 25% | 50% | 21% | 43% | 71% |
| 3B | 10 | 18% | 80% | 20% | 40% | 80% |
| 4 | 4 | 7% | 25% | 50% | 0% | 50% |
| 5 | 3 | 5% | 100% | 0% | 33% | 100% |
| Tumor grade | ||||||
| Low | 6 | 11% | 67% | 50% | 17% | 67% |
| High | 50 | 89% | 64% | 26% | 42% | 76% |
| Tumor stage | ||||||
| Ta | 11 | 20% | 73% | 55% | 45% | 82% |
| T1 | 8 | 14% | 50% | 0% | 38% | 75% |
| T2 | 10 | 18% | 80% | 20% | 10% | 80% |
| T3 | 24 | 43% | 67% | 33% | 54% | 79% |
| T4 | 3 | 5% | 0% | 0% | 0% | 0% |
| Upper urinary tract tumor site | ||||||
| Lower ureter | 17 | 30% | 76% | 18% | 35% | 76% |
| Upper ureter | 1 | 2% | 100% | 0% | 0% | 100% |
| Ureterovesical junction | 2 | 4% | 0% | 0% | 0% | 0% |
| Lower ureter and upper ureter | 2 | 4% | 100% | 50% | 50% | 100% |
| Renal pelvis | 21 | 38% | 57% | 38% | 38% | 76% |
| Renal pelvis and lower ureter | 4 | 7% | 75% | 25% | 50% | 100% |
| Renal pelvis and upper ureter | 5 | 9% | 40% | 40% | 60% | 60% |
| Renal pelvis, lower ureter, upper ureter | 4 | 7% | 75% | 25% | 50% | 75% |
| Synchronous bladder cancer | ||||||
| Present | 21 | 38% | 52% | 29% | 33% | 62% |
| Absent | 35 | 63% | 71% | 29% | 43% | 83% |
| UTUC risk factors | ||||||
| Aristolactam-DNA adducts present | 54 | 96% | 65% | 30% | 39% | 74% |
| Smoking history | 10 | 18% | 70% | 30% | 60% | 70% |
| CKD, chronic kidney disease. |
Additional files
-
Supplementary file 1
Development of PCR-based assays to identify tumor-specific mutations in urinary cells.
- https://doi.org/10.7554/eLife.32143.010
-
Supplementary file 2
Demographic and clinical features of the BC early detection cohort.
- https://doi.org/10.7554/eLife.32143.011
-
Supplementary file 3
Mutations detected by Multiplex Assay in primary tumor tissues from the early detection cohort.
- https://doi.org/10.7554/eLife.32143.012
-
Supplementary file 4
Primers used to detect mutations in the 10-gene multiplex panel and TERT.
- https://doi.org/10.7554/eLife.32143.013
-
Supplementary file 5
Mutations detected by the 10-gene Multiplex Assay in urine samples from the early detection cohort.
- https://doi.org/10.7554/eLife.32143.014
-
Supplementray file 6
Mutations detected by the TERT Assay in urine samples from the early detection cohort.
- https://doi.org/10.7554/eLife.32143.015
-
Supplementary file 7
Chromosome arm gains and losses detected by the Aneuploidy Assay in urine samples from early detection cohort.
- https://doi.org/10.7554/eLife.32143.016
-
Supplementary file 8
Mutations detected by TERT Assay in primary tumor tissues from the early detection cohort.
- https://doi.org/10.7554/eLife.32143.017
-
Supplementary file 9
Individual clinical data for the UTUC cohort.
- https://doi.org/10.7554/eLife.32143.018
-
Supplementary file 10
Mutations detected in urinary cell DNA from UTUC patients by the 10-gene multiplex assay.
- https://doi.org/10.7554/eLife.32143.019
-
Supplementary file 11
TERT promoter mutations identified in urinary cell DNA from UTUC patients.
- https://doi.org/10.7554/eLife.32143.020
-
Supplementary file 12
Urinary cell DNA samples from UTUC patients that scored positive for aneuploidy.
- https://doi.org/10.7554/eLife.32143.021
-
Supplementary file 13
Comparison of copy number variations in matched tumor and urinary cell DNA samples from the UTUC cohort.
- https://doi.org/10.7554/eLife.32143.022
-
Supplementary file 14
Mutations detected in primary tumor DNA from UTUC patients by the 10-gene multiplex assay.
- https://doi.org/10.7554/eLife.32143.023
-
Supplementary file 15
TERT promoter mutations identified in primary tumor DNA from UTUC patients.
- https://doi.org/10.7554/eLife.32143.024
-
Supplementary file 16
Demographic and clinical features of the Surveillance Cohort.
- https://doi.org/10.7554/eLife.32143.025
-
Supplementary file 17
Mutations detected by the 10-gene Multiplex Assay on urine samples from the BC Surveillance cohort.
- https://doi.org/10.7554/eLife.32143.026
-
Supplementary file 18
Mutations detected by the TERT Assay on urine samples from the BC Surveillance cohort.
- https://doi.org/10.7554/eLife.32143.027
-
Supplementary file 19
Chromosome arm gains and losses detected by the Aneuploidy Assay in urine samples from the BC Surveillance cohort.
- https://doi.org/10.7554/eLife.32143.028
-
Supplementary file 20
Summary of the performance of Cytology vs.UroSEEK in both BC cohort tumors.
- https://doi.org/10.7554/eLife.32143.029
-
Supplementary file 21
Primers used for identity matching of tumor and urinary cell DNA samples from UTUC patients.
- https://doi.org/10.7554/eLife.32143.030
-
Transparent reporting form
- https://doi.org/10.7554/eLife.32143.031





