Selective eradication of cancer displaying hyperactive Akt by exploiting the metabolic consequences of Akt activation
Figures
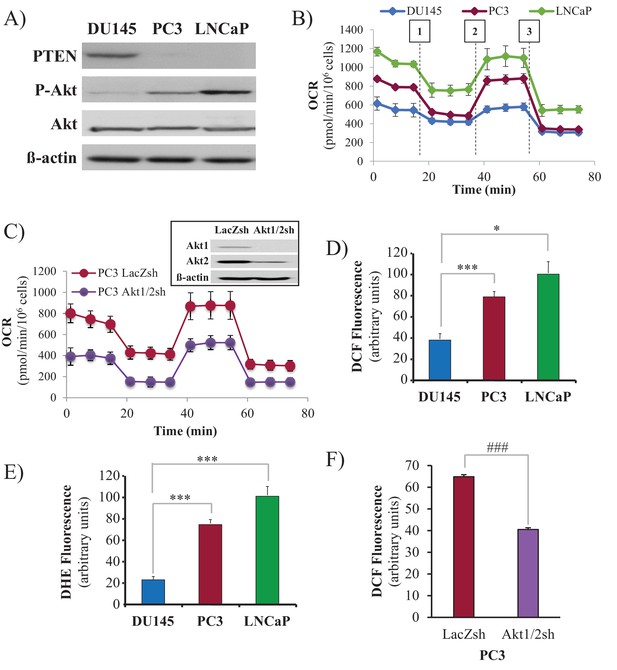
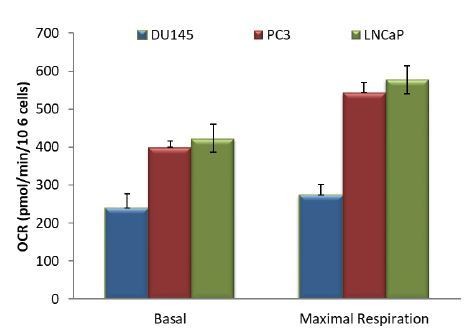
Akt activation in PTEN-deficient prostate cancer cells elevates oxygen consumption and intracellular ROS levels.
The human CaP cells DU145, PC3, and LNCaP were seeded in 10% FBS and harvested after two days to measure various parameters. (A) Immunoblot showing the expression levels of PTEN, P-Akt (ser 473), pan-Akt, and ß-actin as a loading control. (B) Oxygen consumption: OCR was measured using the Seahorse XF96e analyzer for all three CaP cell lines. After the OCR was established, oligomycin (1), FCCP (2), and rotenone/antimycin A (3) were added sequentially. The traces shown are representative of three independent experiments in which each data point represents technical replicates of four wells each ± SEM. (D, E) Relative ROS levels: CaP cells were incubated with H2DCFDA (D) or DHE (E), and the levels of fluorescence were analyzed by flow cytometry as an indicator of ROS levels. Data represent the mean ±SEM of three independent experiments performed in triplicate. *p<0.01, ***p<0.005 versus DU145. No significant differences between PC3 and LNCaP were observed. (C, F) Akt1 and Akt2 were knocked down in PC3 cells, and the OCR (C) and cytosolic ROS levels (F) were measured. The results are presented as the average of at least three independent experiments performed in triplicate ± SEM. ###p<0.0001 versus PC3 LacZsh. Insert in (C) shows the expression levels of Akt1, Akt2, and ß actin as a loading control in PC3 cells in which Akt1 and Akt2 were knocked down.
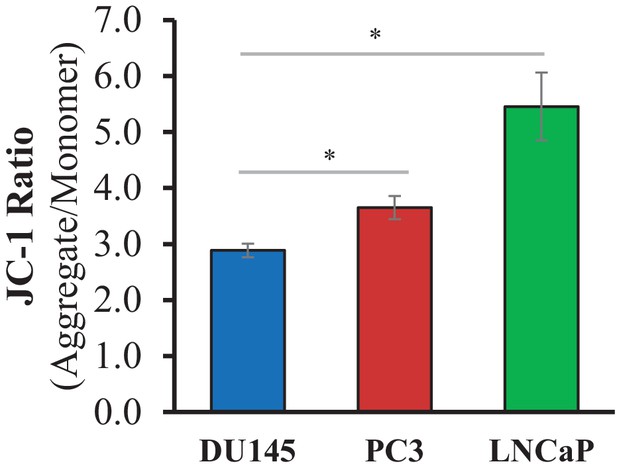
Mitochondrial membrane potential measured as JC-1 aggregate to monomer ratio.
The data represent the mean ±SEM of three independent quantification experiments performed in triplicate. *p<0.05 versus DU145.
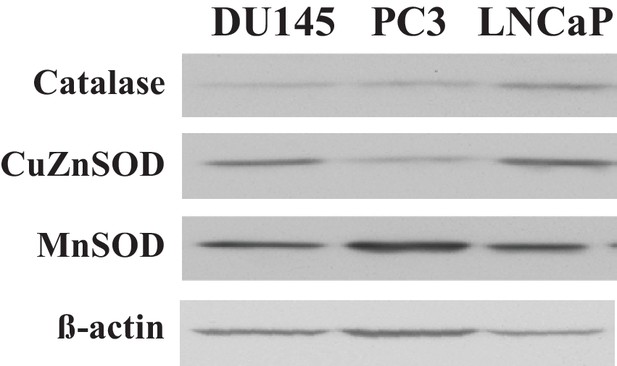
Immunoblot showing the expression levels of the detoxifying enzymes catalase, MnSOD, and Cu/ZnSOD (ß actin as a loading control) in all three CaP cell lines.
https://doi.org/10.7554/eLife.32213.004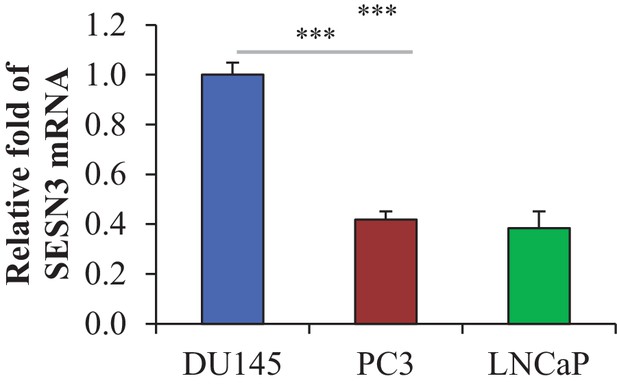
Level of Sesn3 mRNA relative to that of actin in CaP cells, as assessed by quantitative RT-PCR.
The data represent the mean ±SEM of three independent quantification experiments performed in triplicate. ***p<0.0001 versus DU145. (1s4-5) DU145 cells were transiently transfected with hSesn3 or control RNAi (Dharmacon), and PC3 cells were transiently transfected with lentivirus expressing hSesn3 or TOPO control 72 hr prior to the experiments.
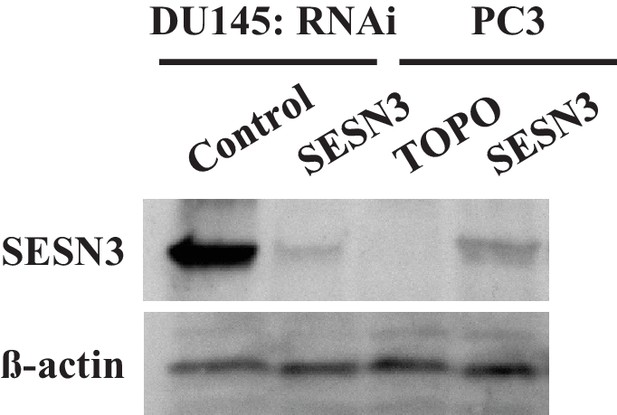
Immunoblot showing the expression levels of sestrin 3 (SESN3) and ß actin as a loading control.
https://doi.org/10.7554/eLife.32213.006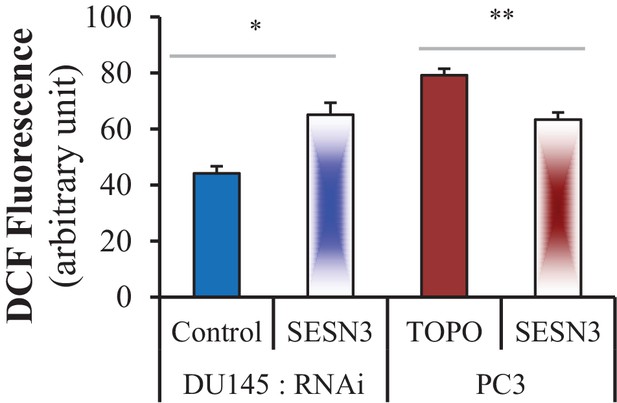
Level of ROS, as assessed by flow cytometry, after incubation with H2DCFDA.
The data represent the mean ±SEM of three independent experiments performed in triplicate. *p=0.02, **p=0.01 versus the control for each cell line.
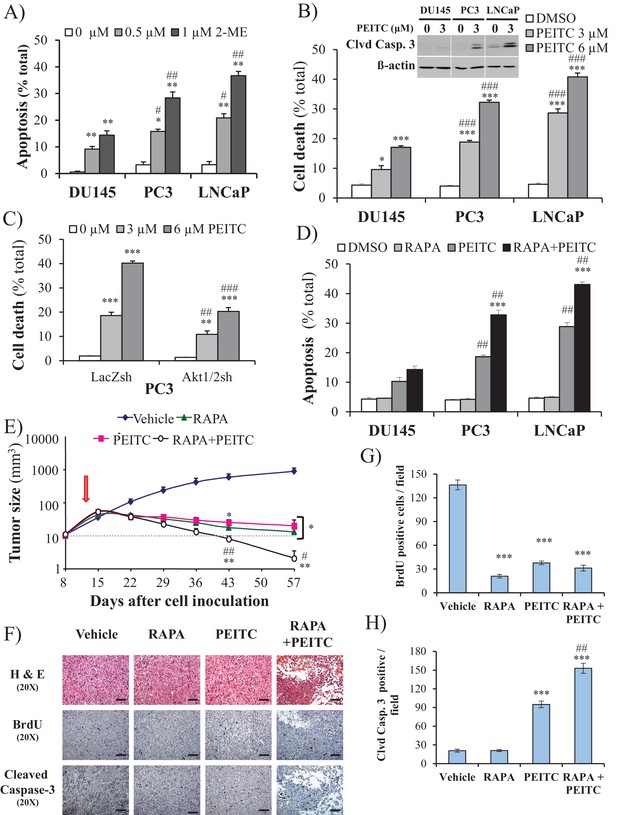
ROS inducers and the combination of a ROS inducer and rapamycin induce CaP PTEN-deficient cell death in vitro and eradicate their tumors in vivo.
(A) CaP cell lines were incubated with 2-ME for 24 hr, the cells were fixed and apoptosis was quantified by DAPI staining. The data represent the mean ±SEM of three independent experiments performed in triplicate. *p<0.005, **p<0.002 versus DMSO (0 µM) for each cell line. #p<0.02, ##p<0.01 versus DU145. (B) CaP cell lines were incubated with PEITC, collected and fixed for estimation of cell death by PI staining or lysed to extract total protein. They were then subjected to immunoblotting with cleaved caspase-3 and ß-actin as a loading control (insert). The data represent the mean ±SEM of three independent experiments performed in triplicate. *p<0.005, ***p<0.001 versus DMSO for each cell line. ###p<0.0005 versus DU145. (C) PC3 Akt1/2 knockdown cells were incubated with PEITC for 17 hr, and then cell death was estimated by PI staining as the percentage of apoptotic cells among total cells. The data represent the mean ±SEM of three independent experiments performed in triplicate. **p<0.001, ***p<0.0001 versus DMSO for each cell line. ##p<0.005, ###p<0.0001 versus PC3 LacZsh. (D) CaP cells were incubated for 8 hr with 20 nM rapamycin (RAPA) prior to the addition of PEITC (3 µM). After 17 hr of incubation with PEITC, the cells were fixed, and apoptosis was quantified by DAPI staining. The data represent the mean ±SEM of three independent experiments performed in triplicate. ***p<0.0001 versus PEITC for each cell line. ##p<0.0005 versus DU145. (E–H) In vivo therapeutic effect of rapamycin +PEITC in mice inoculated with PC3 prostate cancer cells. Thirty-two nude mice were subcutaneously injected with PC3 cells in both flanks and randomly divided into four groups (eight mice per group, 16 tumors per group) for treatment with PEITC, rapamycin (RAPA), a combination of RAPA +PEITC, or a solvent control (Vehicle). (E) Graph presenting the tumor growth rates in each group. Treatment began on day 13 (~15 mm3, red arrow) and stopped on day 43 after tumor cell inoculation. The data represent the average size ±SEM of 16 tumors up to day 43. The data collection from day 57 represent the average size of the eight remaining xenograft tumors only. *p<0.003, **p<0.002 versus vehicle. #p<0.03, ##p<0.01 versus PEITC or RAPA. (F) Cross-sections of tumors collected from the experiment described in (E). At day 50 after tumor cell inoculation, the tumor cross-sections were subjected to hematoxylin and eosin (H and E, top) staining, BrdU staining (middle), and anti-cleaved caspase-3 staining (bottom). Scale bars: 100 µm. (G, H) Histograms showing quantification of the positively stained cells in (F). The results are presented as the mean ±SEM of the positively stained cells of four sections from four treated mice. The stained cells were counted in four random fields of each section. ***p<0.0002 versus vehicle. ##p<0.001 versus PEITC.
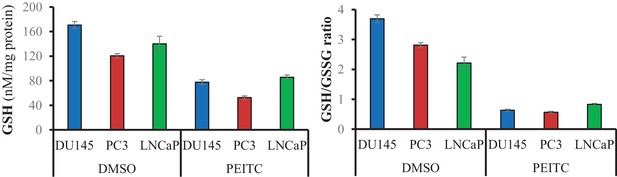
Glutathione levels (Left) and GSH/GSSG ratio (Right) in CaP cells after 8 hr incubation with DMSO or PEITC 6 μM.
The data represent the mean ±SEM of two independent experiments performed in duplicate.
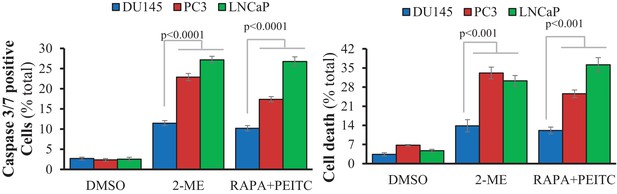
(Left) Apoptosis was measured on live cells by caspase 3/7 activity assay after drug treatment: 2-ME 1 µM (14 hr) or 20 nM rapamycin (5 hr) followed by 6 µM PEITC (8 hr).
The data represent the mean ±SEM of two independent experiments performed in quadruplicate. (Right) Cell death was assessed on fixed cells by DAPI staining after drug treatment: 2-ME 1 µM (20 hr) or 20 nM rapamycin (5 hr) followed by 6 µM PEITC (17 hr). The data represent the mean ±SEM of three independent experiments performed in triplicate.
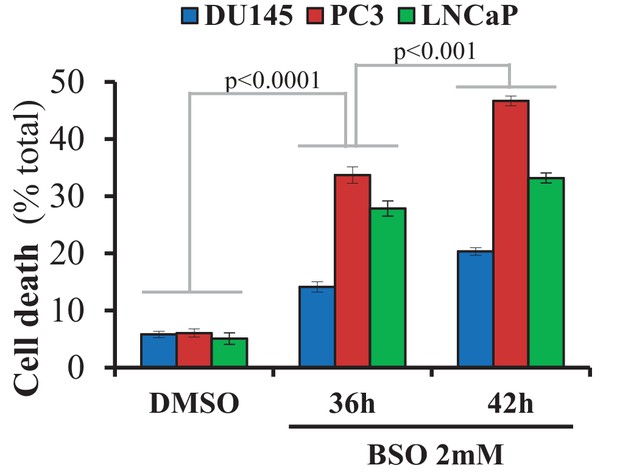
CaP cell lines were incubated with BSO (2 mM) for 36 and 42 hr, the cells were fixed and cell death was quantified by PI staining.
The data represent the mean ±SEM of three independent experiments performed in triplicate.
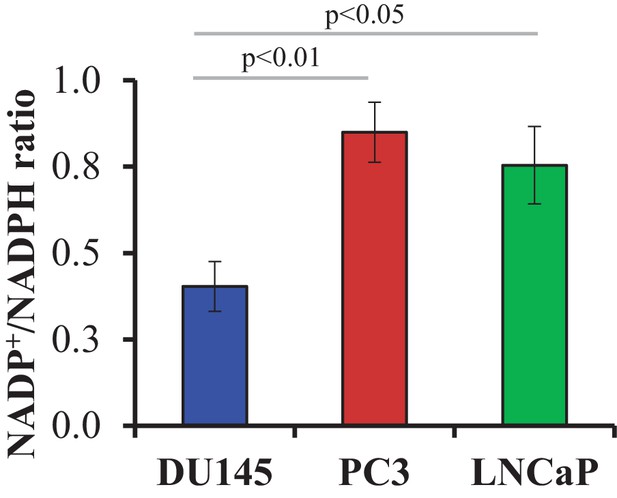
NADP+/NADPH ratio in CaP cells.
The data represent the mean ±SEM of three measurements performed in duplicate.
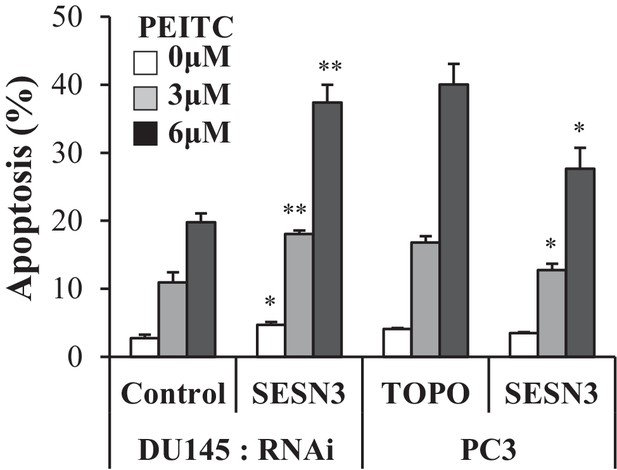
After modulation of SESN3 expression, PC3 and DU145 cells were treated with PEITC (0, 3 and 6 µM) for 17 hr, the cells were fixed and cell death was assessed by DAPI staining.
The data represent the mean ±SEM of three independent experiments performed in triplicate. *p<0.05, **p<0.01 versus the control for each cell line.
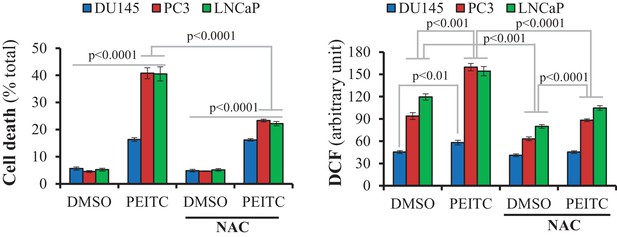
DU145, PC3, and LNCaP cells were incubated with N-acetylcysteine (100 µM NAC) for 2 hr prior to 17 hr of incubation with PEITC (6 µM) in the presence of NAC or not.
The graphs represent the cell death measured by PI staining (Left) or ROS levels after incubation with H2DCFDA (Right). The data represent the mean ±SEM of three independent experiments performed in triplicate.
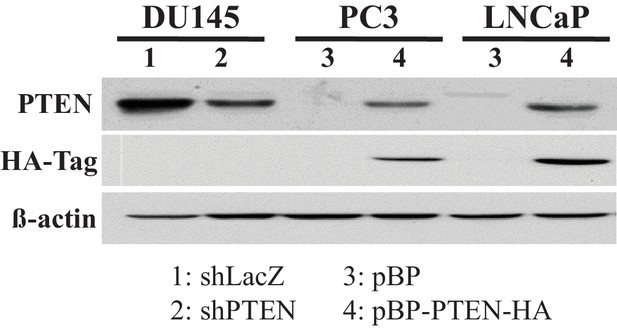
Immunoblot showing the expression of PTEN (and HA-Tag), and ß-actin as a loading control after PTEN was downregulated in DU145 cells (1: control shLacZ, 2: shPTEN) or overexpressed in PC3 and LNCaP cells (3: control pBP, 4: pBP-PTEN).
https://doi.org/10.7554/eLife.32213.015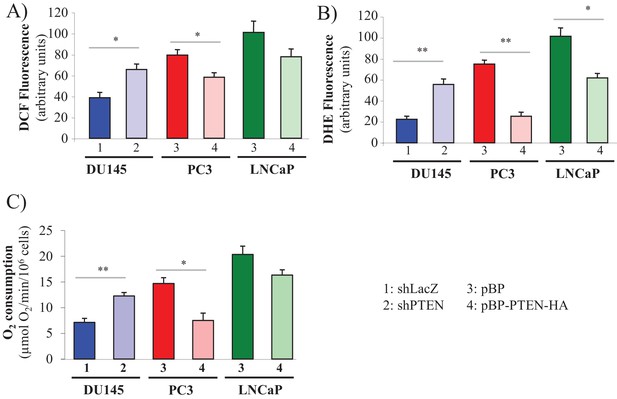
PTEN expression determines the levels of ROS and oxygen consumption.
PTEN was downregulated in DU145 cells (1: control shLacZ, 2: shPTEN) or overexpressed in PC3 and LNCaP cells (3: control pBP, 4: pBP-PTEN). (A, B) Relative ROS levels: cells were incubated with H2DCFDA (A) or DHE (B), and the levels of fluorescence were analyzed by flow cytometry as an indicator of ROS levels. (C) Basal oxygen consumption.
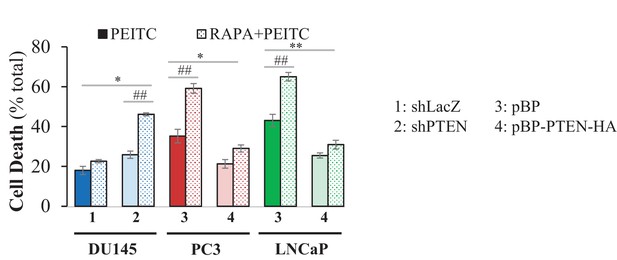
Cells were incubated with PEITC or rapamycin/PEITC for 17 hr and scored for apoptosis 17 hr later by DAPI staining.
The data represent the mean ±SEM of three independent experiments performed in triplicate. *p<0.05, **p<0.001 versus the control for each cell line. ##p<0.05 versus PEITC.
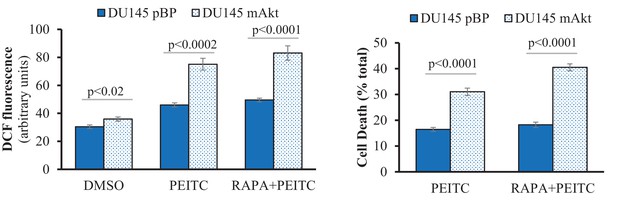
mAkt was stably overexpressed in DU145.
Cells were then incubated for 17 hr with PEITC or rapamycin/PEITC before measurement of relative cytosolic ROS level (Left) or cell death (Right).
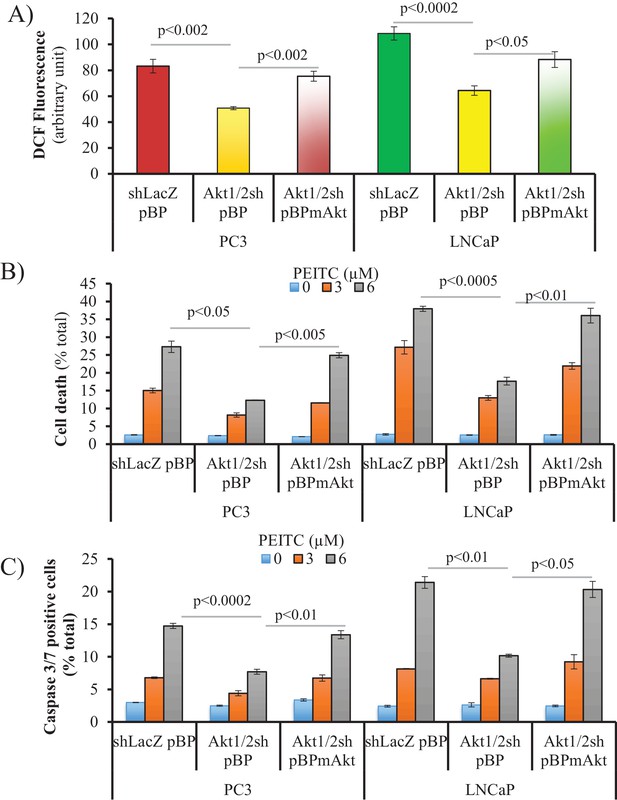
ROS levels and ROS-induced cell death are Akt-dependent.
Akt1 and Akt2 were knocked down in PC3 and LNCaP cells. Once cell lines were established, mAkt was re-expressed in these cells. Cells were incubated with PEITC for 17 hr, and then cytosolic ROS levels (A) and cell death estimated by PI staining were measured (B). Another set of cells was incubated with PEITC for 12 hr to estimate apoptosis by caspase 3/7 activity assay (C) as the percentage of positive cells over total cells. The data represent the mean ±SEM of three independent experiments performed in triplicate.
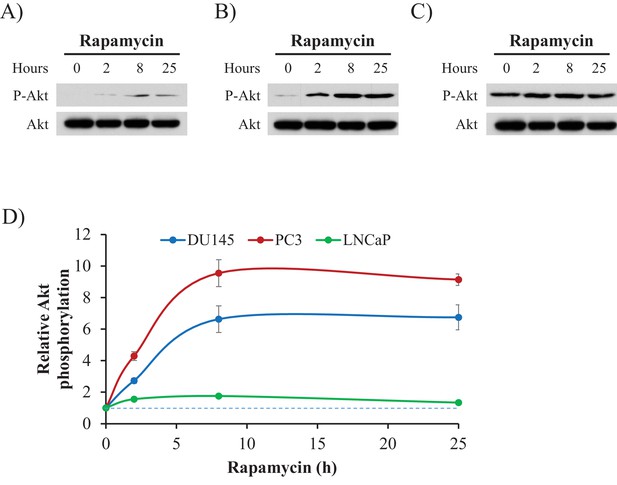
Rapamycin elevates Akt activity.
(A–C) DU145 (A), PC3 (B), and LNCaP cells (C) were treated with rapamycin (100 nM). Total cell extracts were prepared at different time points as indicated and subjected to immunoblotting with antibodies specific for Akt and p-Akt. (D) Quantification of immunoblots showing relative Akt phosphorylation, quantified using the NIH ImageJ software program, and normalized to the densitometric signal for total Akt as a control for protein expression. Values are expressed relative to time 0 and data represent the mean ±SEM of three independent experiments.
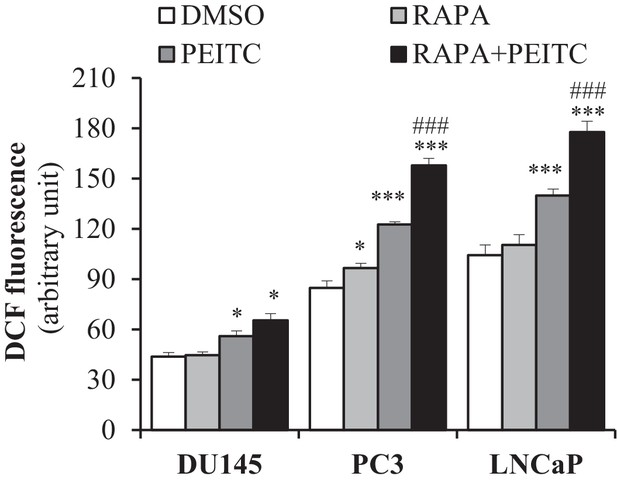
Rapamycin increases the ROS levels induced by PEITC.
When required, CaP cells were incubated with 20 nM rapamycin (RAPA) for 8 hr before the addition of PEITC (3 µM). After 17 hr of incubation with PEITC (±RAPA), the ROS levels in live cells after incubation with H2DCFDA were measured by flow cytometry. The data represent the mean ±SEM of three independent experiments performed in triplicate. *p<0.05, ***p<0.0001 versus DMSO for each cell line. ###p<0.0005 versus PEITC for each cell line.
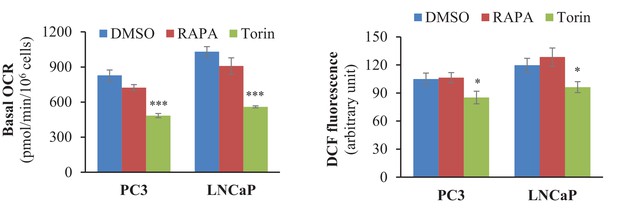
Torin, not rapamycin, decreases the OCR and ROS levels in PTEN-deficient CaP cells.
PC3 and LNCaP cells were incubated for 8 hr with rapamycin (RAPA, 20 nM) or torin (250 nM) before measurement of the OCR (Left) or cytoplasmic ROS levels (Right). The data represent the mean ±SEM of three independent experiments performed in triplicate. *p<0.05, ***p<0.0001 versus DMSO for each cell line.
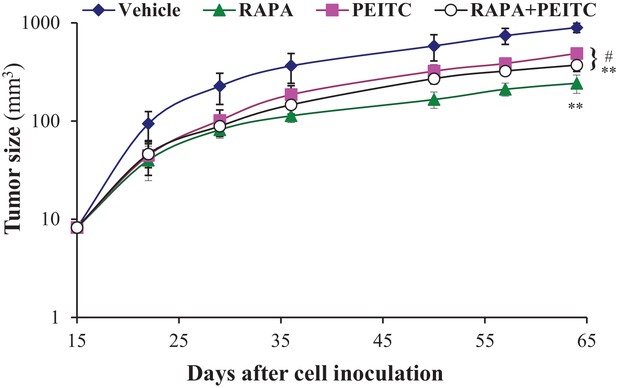
In vivo therapeutic effects of rapamycin +PEITC in mice inoculated with DU145 prostate cancer cells.
Twenty-four nude mice were injected subcutaneously with DU145 cells in both flanks and randomly divided into four groups (four mice per group, eight tumors per group) for treatment with PEITC, rapamycin (RAPA), a combination of RAPA +PEITC, or a solvent control (Vehicle). The graph represents the tumor growth rate in each group. Treatment began on day 18 (~15 mm3) and stopped on day 55 after tumor cell inoculation. The data represent the average size ±SEM of eight tumors up to day 57. Data collection on day 64 shows only the average sizes of the four remaining xenograft tumors. **p<0.02 versus vehicle, #p=0.02 versus RAPA.
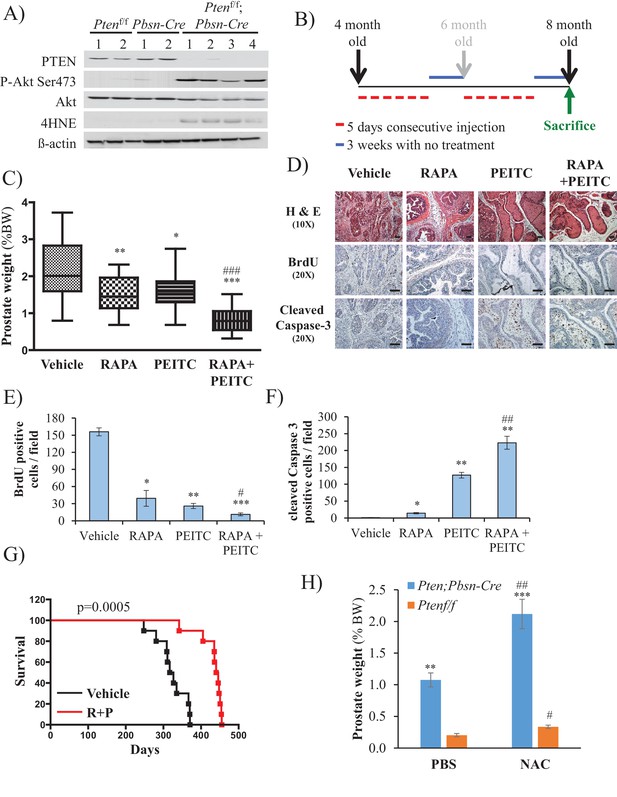
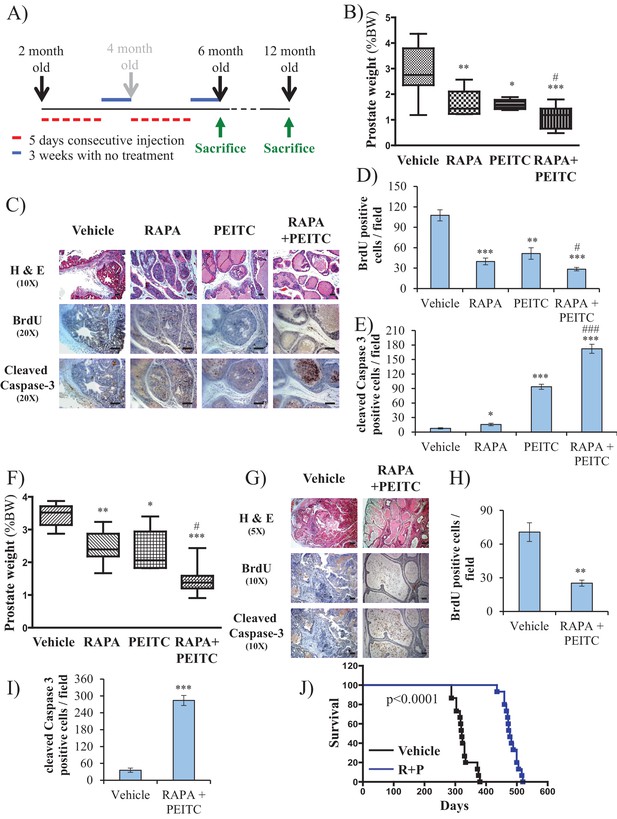
Effect of rapamycin, PEITC, and a combination of rapamycin and PEITC on cell proliferation, cell death, survival, and the tumors of Pbsn-Cre4;Ptenf/f mice.
(A) Tissue lysates were prepared from prostates isolated from four control mice (Ptenf/f or Pbsn-Cre4) and four Pbsn-Cre4;Ptenf/f mice. Immunoblot analysis shows the expression levels of PTEN, Akt-P (ser 473), total-Akt, p21, 4HNE, and ß-actin as a loading control. (B) Schematic of mouse treatment: control (Ptenf/f or Pbsn-Cre4) and Pbsn-Cre4;Ptenf/f mice were randomly divided into four groups of 9 to 16 mice at 4 months of age, and they received a daily (5 days a week) intraperitoneal injection of drugs, PEITC (35 mg/kg BW), rapamycin (2 mg/kg BW), rapamycin in combination with PEITC (1:1), or solvent control, for 6 consecutive weeks. Treatment was then interrupted for 3 weeks and resumed at 6 months of age for another 6 weeks. The mice were sacrificed at 8 months of age and examined for the presence of prostate hyperplasia. (C) Graphs showing the relative prostate weight to total body weight (% body weight) of Pbsn-Cre4;Ptenf/f mice treated with vehicle (n = 15 mice), rapamycin (RAPA, n = 11), PEITC (n = 9), or RAPA +PEITC (n = 16). The box plots represent the 25th to 75th percentiles (boxes) with the median, and the whiskers represent the maximum and minimum values. *p=0.05, **p=002, ***p<0.0001 versus vehicle. ###p<0.0005 versus PEITC. (D) Cross-sections of prostate tissues collected at 8 months from Pbsn-Cre4;Ptenf/f mice treated with different drugs were subjected to H and E staining (top), BrdU staining (middle), and anti-cleaved caspase-3 staining (bottom). Scale bars: 100 µm (E, F) Histograms showing quantification of the positively stained cell cross-sections shown in Figure 3D for BrdU (E) and cleaved caspase-3 (F). The results are presented as the mean ±SEM of positively stained cells of four sections from four treated mice. The stained cells were counted in four random fields of each section. *p<0.002, **p<0.005, ***p<0.0002 versus vehicle. #p=0.04, ##p=0.01 versus PEITC. (G) A cohort of 20 Pbsn-Cre4;Ptenf/f mice treated with vehicle (n = 10) or rapamycin in combination with PEITC (R + P; n = 10) were kept alive, and Kaplan-Meier curves of the percentage of mice survival are shown. The vehicle-treated mice have a medium survival age of 322 days versus 443 days for the ‘R + P’ treated mice. The p-values and median survival were calculated by log-rank tests. (H) Graph showing the relative prostate weights of Pbsn-Cre4;Ptenf/f mice (n = 15) treated with N-acetyl-cysteine (NAC, n = 9) or PBS (n = 6) at 8 months of age and 11 Ptenf/f mice (NAC, n = 7 and PBS, n = 4). The data represent the mean ±SEM. **p=0.0006, ***p=0.0001 versus Ptenf/f. #p=0.01, ##p=0.003 versus PBS for each mice genotype.
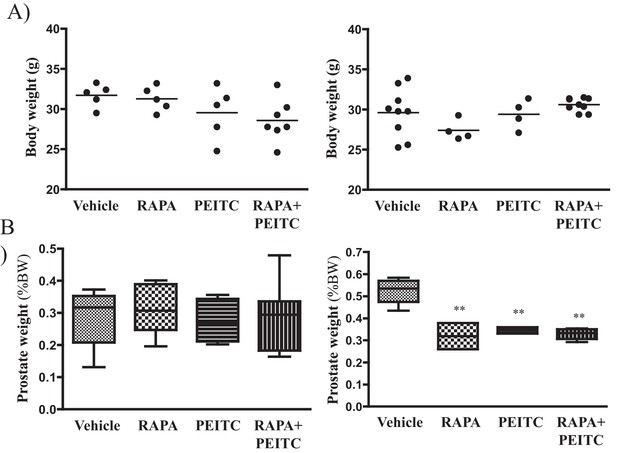
Graphs showing the body weights of control (left) and Pbsn-Cre4;Ptenf/f (right) mice at the end-point (8 months).
The numbers of treated mice in the control group were vehicle (n = 6), rapamycin (RAPA, n = 12), PEITC (n = 8), and RAPA +PEITC (n = 8), and the numbers of treated mice in the Pbsn-Cre4;Ptenf/f group were vehicle (n = 15), RAPA (n = 11), PEITC (n = 9), and RAPA +PEITC (n = 16). No significant differences were detected.
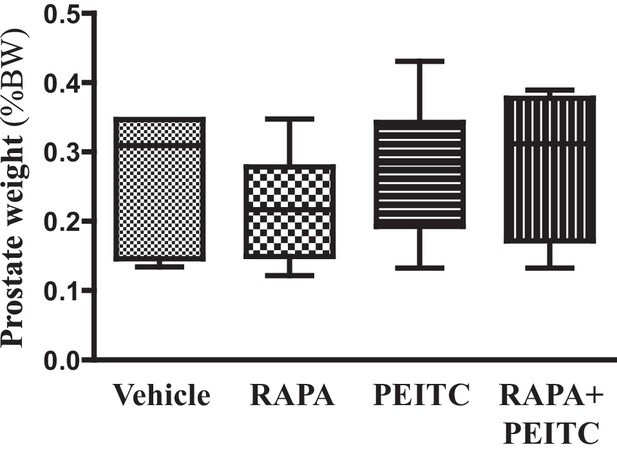
Graphs showing the relative prostate weights of the control mice sacrificed at 8 months.
The box plots represent the 25th to 75th percentiles (boxes) with the median, and the whiskers represent the maximum and minimum values. No significant differences were detected.
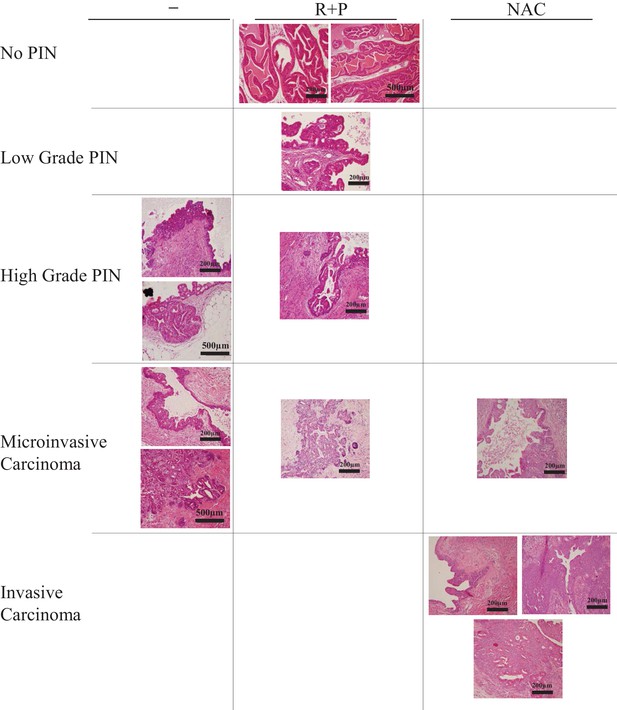
Representative histopathological images.
Representative images of different prostate tumor grades in the anterior lobe of the prostate of untreated mice (-), rapamycin +PEITC-, and NAC-treated mice. The individual images were derived from different individual mice. Scale bars = 200 µm for 20× magnification, 500 µm for 10× magnification.
Early treatment of Pbsn-Cre4;Ptenf/f mice with rapamycin +PEITC inhibits tumor growth and increases survival, even after treatment was halted for 6 months.
(A) Schematic of mice treatment: control and Pbsn-Cre4;Ptenf/f mice were randomly divided into four groups of four to 10 mice at 2 months of age, and they received IP drug injections as indicated in the schematic. A pool of mice was sacrificed at 6 or 12 months of age and examined for the presence of prostate hyperplasia. (B) Graphs showing the relative prostate weights of Pbsn-Cre4;Ptenf/f mice sacrificed at 6 months and treated with vehicle (n = 9), RAPA (n = 4), PEITC (n = 4), or RAPA +PEITC (n = 8). The box plots represent the 25th to 75th percentiles (boxes) with the median, and the whiskers represent the maximum and minimum values. *p=0.03, **p=0.05, ***p<0.0001 versus vehicle. #p=0.05 versus PEITC. (C) Representative cross-sections of prostate tissues were treated as described in Figure 4A and collected from Pbsn-Cre4;Ptenf/f mice treated with different drugs at 6 months. The sections were subjected to H and E staining (top), BrdU staining (middle), and anti-cleaved caspase-3 staining (bottom). Scale bars: 100 µm. (D, E) Histograms showing quantification of the positively stained cell cross-sections for BrdU (D) and cleaved caspase-3 (E). The results are presented as the mean ±SEM of the positively stained cells of four sections from four treated mice. The stained cells were counted in four random fields of each section. *p=0.03, **p<0.001, ***p<0.0001 versus vehicle. #p<0.05, ###p<0.0001 versus PEITC. (F) Graphs representing the relative prostate weights of Pbsn-Cre4;Ptenf/f mice sacrificed at 12 months and treated with vehicle (n = 5), RAPA (n = 7), PEITC (n = 6), or RAPA +PEITC (n = 10). The box plots represent the 25th to 75th percentiles (boxes) with the median, and the whiskers represent the maximum and minimum values. *p=0.03, **p=0.05, ***p<0.0001 versus vehicle. #p<0.05 versus PEITC. (G) Representative cross-sections of prostate tissues were treated with vehicle or RAPA +PEITC and collected at 12 months from Pbsn-Cre4;Ptenf/f mice left untreated for 6 months after the initial treatment. The sections were subjected to H and E staining (top), BrdU staining (middle), and anti-cleaved caspase-3 staining (bottom). Scale bars: 50 µm for 5× objective (H and E), 100 µm for 10× objective. (H, I) Histograms showing quantification of the positively stained cell cross-sections for BrdU (H) and cleaved caspase-3 (I). The results are presented as the mean ±SEM of the positively stained cells of four sections from four treated mice. The stained cells were counted in four random fields from each section. **p=0.003, ***p<0.0001 versus vehicle. (H) A cohort of 30 Pbsn-Cre4;Ptenf/f mice treated with vehicle (n = 15) or rapamycin in combination with PEITC (R + P; n = 15) were kept alive, and Kaplan-Meier curves of the percentage of survival of these mice is shown. The vehicle-treated mice have a median survival age of 321 days versus 477 days for the ‘R + P’ treated mice. The p-values and median survival for the indicated treatments were calculated by log-rank tests.
(A) Graphs showing the body weights of control (left) and Pbsn-Cre4;Ptenf/f (right) mice at 6 months.
The numbers of treated mice in the control group were vehicle (n = 5), RAPA (n = 5), PEITC (n = 5), and RAPA +PEITC (n = 7), and the numbers of treated mice in the Pbsn-Cre4;Ptenf/f group were vehicle (n = 9), RAPA (n = 4), PEITC (n = 4), and RAPA +PEITC (n = 8). No significant differences were detected. (B) Graphs showing the relative prostate weights of control mice sacrificed at 6 months (left) and 12 months (right). The box plots represent the 25th to 75th percentiles (boxes) with the median, and the whiskers represent the maximum and minimum values. The numbers of control-treated mice at 12 months were vehicle (n = 4), RAPA (n = 4), PEITC (n = 4), and RAPA +PEITC (n = 8). **p=0.005 versus vehicle.
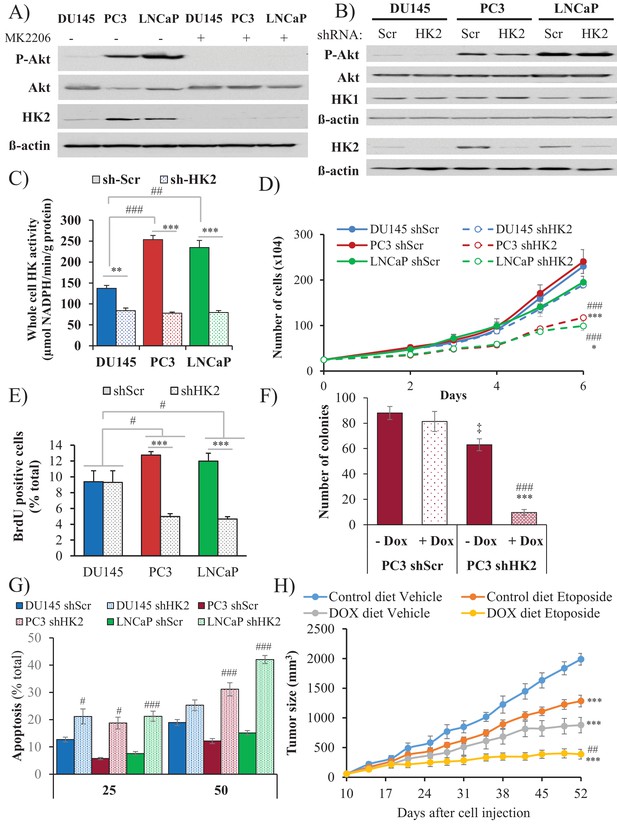
Depletion of HK2 in PTEN-deficient CaP cells inhibits proliferation, oncogenesis, and tumorigenesis while overcoming chemoresistance.
(A) DU145, PC3, and LNCaP cells were treated with MK-2206 (0.5 μM - 24 hr) to inhibit Akt. The immunoblot shows the protein levels of P-Akt, total Akt, HK2, and ß-actin as a loading control. (B–G) DU145, PC3, and LNCaP cells expressing an inducible control (Scr) or HK2 shRNA were exposed to 900 ng/ml doxycycline for 5 days for HK2 deletion prior to analysis. (B) Immunoblot showing the protein levels of P-Akt, total Akt, HK2, HK1, and ß-actin as a loading control. (C) Graphs depicting the total hexokinase activity in these cells. The data represent the mean ±SEM of three independent experiments performed in triplicate. **p<0.002, ***p<0.001 versus shScr for each cell line. ##p<0.001, ###p<0.0001 versus DU145. (D) Cell proliferation after HK2 deletion in the CaP cell lines. The data represent the mean ±SEM of three independent experiments performed in triplicate. *p=0.02, ***p<0.001 versus shScr for each cell line on day 6. ###p<0.0005 versus DU145 shHK2 on day 6. (E) BrdU incorporation after HK2 deletion. The data represent the mean ±SEM of three independent experiments performed in triplicate. ***p<0.0001 versus shScr for each cell line. #p<0.05 versus DU145. (F) Anchorage-independent growth (soft-agar): PC3 Tet-ON control (SCR) and HK2-sh cells were plated in 0.35% agarose-containing medium before and after HK2 knockdown with doxycycline as described in the experimental procedures, and they were allowed to grow for approximately 3 weeks with bi-weekly media changes. The bar graphs represent the average quantification of the soft agarose colonies in PC3 cells ± SEM of three independent experiments performed in triplicate. ***p<0.0005 versus PC3 shScr +doxycycline. ‡p=0.02 versus PC3 shScr – doxycycline. ###p<0.0001 versus PC3 shHK2 – doxycycline. (G) After HK2 knockdown with doxycycline, cells were treated with etoposide for 24 hr before apoptosis was assessed by DAPI staining, which is presented as the percentage of apoptotic cells among total cells. The data represent the mean ±SEM of three independent experiments performed in triplicate. **p<0.001, ***p<0.0002 versus DMSO (0 µM) for each cell line. #p<0.001, ###p<0.0003 versus shScr. (H) In vivo therapeutic effect of etoposide in mice inoculated with PC3 prostate cancer cells. Twenty-four nude mice were injected subcutaneously with PC3 Tet-ON HK2sh cells in both flanks and randomly divided into four groups (six mice per group, 12 tumors per group) for treatment with etoposide or solvent control (Vehicle). When the tumors were palpable, two groups were provided a doxycycline diet, while the two other groups remained on the control diet. Etoposide (or vehicle) treatment was started 3 days after the diet was changed (day 13), and treatment was stopped on day 48 after tumor cell inoculation. The data represent the average size ±SEM of 12 xenograft tumors per group. Statistical analysis from day 52 (end-point): ***p<0.0001 versus the control diet vehicle. ##p<0.005 versus the doxycycline diet vehicle.
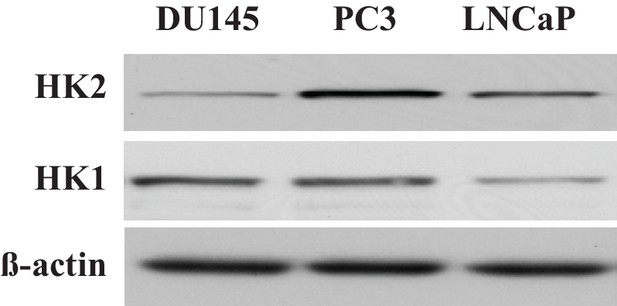
Total protein was extracted from CaP cells and subjected to immunoblotting with HK1, HK2, and ß-actin as a loading control.
https://doi.org/10.7554/eLife.32213.032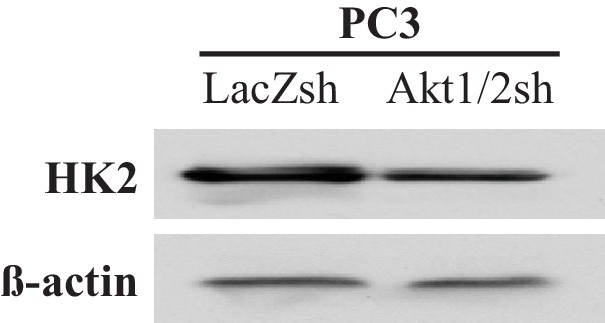
Expression levels of HK2 and ß-actin as a loading control in PC3 cells in which Akt1 and Akt2 were stably knocked down.
https://doi.org/10.7554/eLife.32213.033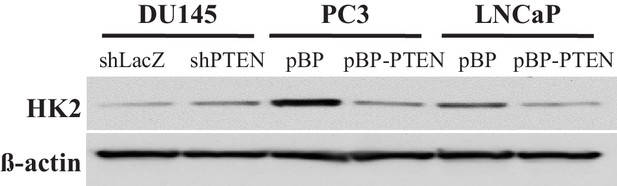
Immunoblot showing expression of HK2 (and ß-actin as loading control) in CaP cells where PTEN is either downregulated (DU145) or overexpressed (PC3 and LNCaP).
https://doi.org/10.7554/eLife.32213.034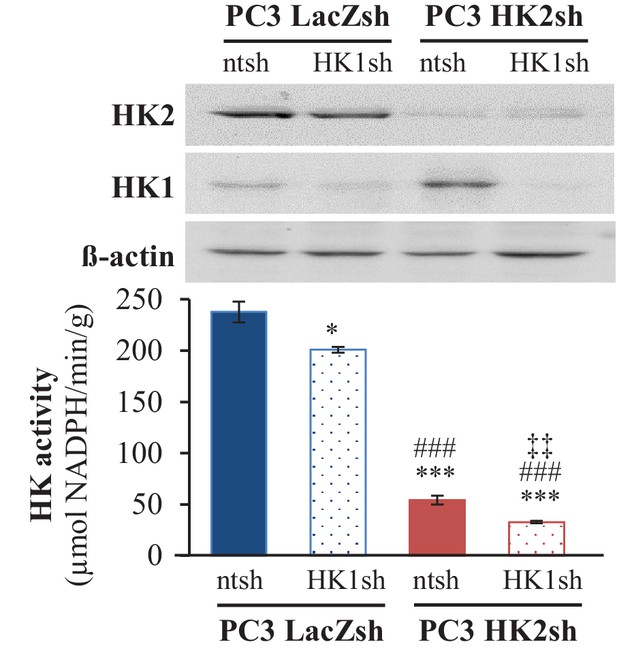
HK1 was stably knocked down in PC3 cells after HK2 knockdown.
The immunoblot shows the expression levels of HK1, HK2, and actin as a loading control in PC3 control, HK1 knockdown, HK2 knockdown, and double HK1 and HK2 knockdown cells. The graph shows the total HK activity in the same cells. The data represent the mean ±SEM of three independent experiments performed in duplicate. *p=0.005, ***p<0.0001 versus PC3 LacZsh ntsh. ###p<0.0001 versus PC3 LacZsh HK1sh. ‡p=0.01 versus PC3 HK2sh ntsh.
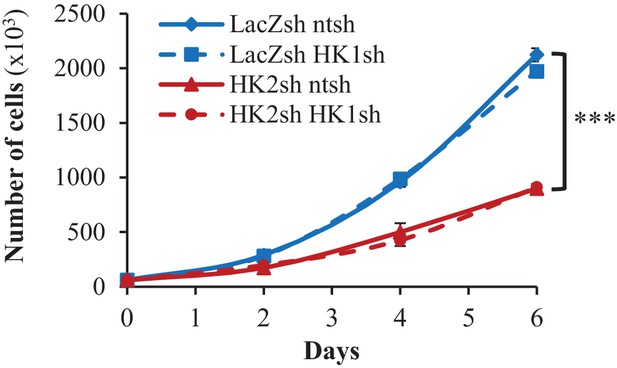
Cell proliferation after HK1 and/or HK2 deletion in PC3 cells.
The data represent the mean ±SEM of three independent experiments performed in triplicate. ***p<0.0001 versus LacZsh cells on day 6.
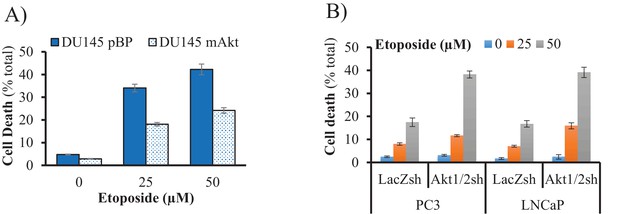
Etoposide-induced cell death is Akt-dependent.
(A) After mAkt overexpression, DU145 cells were treated with etoposide for 24 hr before cell death was assessed by PI staining on live cells with Celigo Image cytometer. (B) Akt1 and Akt2 were knocked down in PC3 and LNCaP cells. Cells were then incubated for 24 hr with etoposide before measurement of cell death by PI staining on live cells with Celigo Image cytometer. Data are expressed as the percentage of dead cells among total cells and represent the mean ±SEM of two independent experiments performed in triplicate.
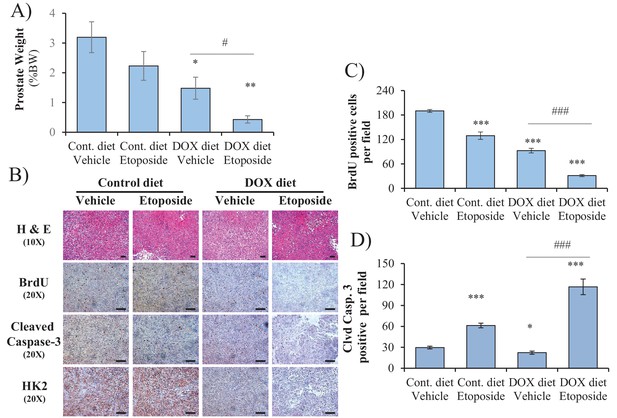
Data analysis for in vivo therapeutic study described in Figure 5H.
(A) Graphs showing the relative xenograft tumor weights of mice treated with control diet/vehicle, control diet/etoposide, DOX diet/vehicle, and DOX diet/etoposide. The data represent the average size ±SEM of 12 xenograft tumors per group. *p<0.05, **p<0.001 versus control diet vehicle. #p<0.05 versus DOX diet vehicle. (B) The cross-sections of xenograft tumors collected at end-point (day 52) were subjected to H and E staining, BrdU staining, anti-cleaved caspase-3 staining, and HK2 staining (from top to bottom). Scale bars: 100 µm. (C, D) Histograms showing quantification of the positively stained cells in (B). The results are presented as the mean ±SEM of the positively stained cells of two sections from six xenograft tumors. The stained cells were counted in three random fields of each section. *p<0.05, ***p<0.0005 versus the control diet vehicle. ###p<0.0005 versus DOX diet vehicle.

Effect of HK2 knockdown on ECAR.
PC3 cells expressing an inducible control (Scr) or HK2 shRNA were exposed to 900 ng/ml DOX for 5 days for HK2 deletion before analysis. ECAR was measured after HK2 deletion using the Seahorse XF96e analyzer.
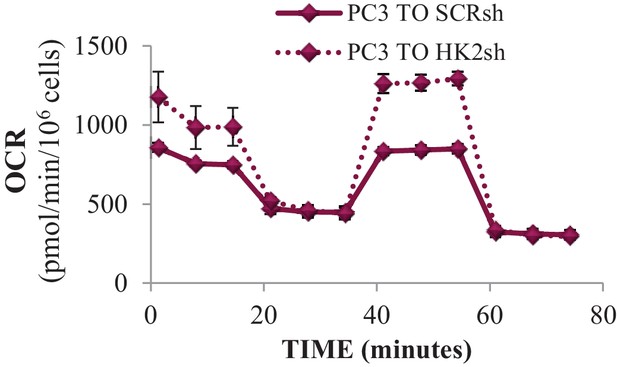
Effect of HK2 knockdown on oxygen consumption.
PC3 cells expressing an inducible control (Scr) or HK2 shRNA were exposed to 900 ng/ml DOX for 5 days for HK2 deletion before analysis. OCR was measured after HK2 deletion using the Seahorse XF96e analyzer.
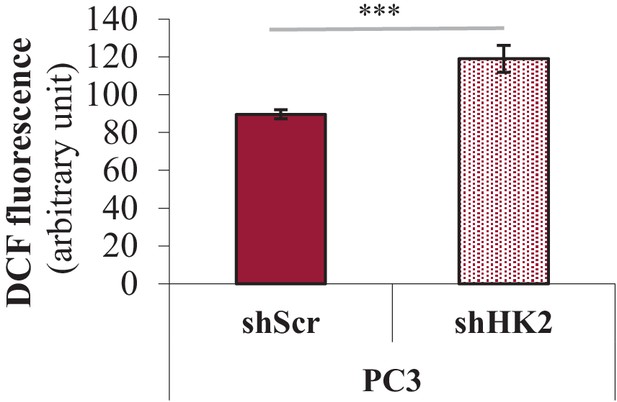
Effect of HK2 knockdown on ROS levels.
PC3 cells expressing an inducible control (Scr) or HK2 shRNA were exposed to 900 ng/ml DOX for 5 days for HK2 deletion before analysis. Cells were incubated with H2DCFDA, and the level of fluorescence was analyzed by flow cytometry as an indicator of ROS levels after HK2 deletion. The data represent the mean ±SEM of three independent experiments performed in triplicate. ***p<0.005 versus PC3 shScr.
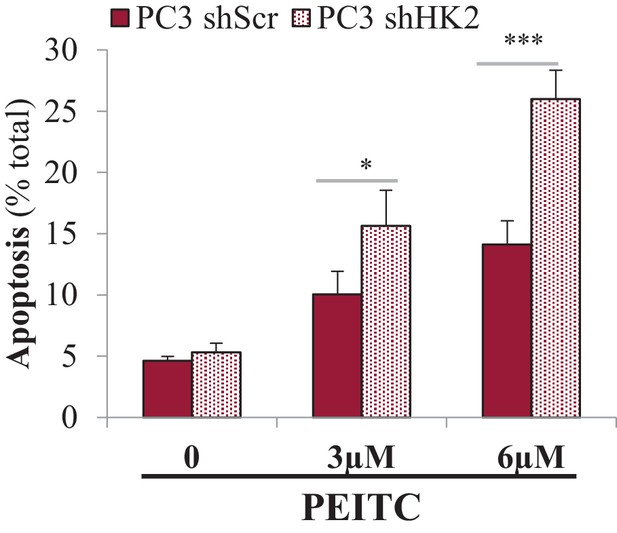
Effect of HK2 knockdown on PEITC-induced cell death.
PC3 cells expressing an inducible control (Scr) or HK2 shRNA were exposed to 900 ng/ml DOX for 5 days for HK2 deletion before analysis. After HK2 knockdown with DOX, cells were treated with PEITC (0, 3 and 6 µM) for 17 hr before apoptosis was assessed by DAPI staining, which is presented as the percentage of apoptotics among total cells. The data represent the mean ±SEM of three independent experiments performed in triplicate. *p<0.02, ***p<0.0001 versus PC3 shScr.
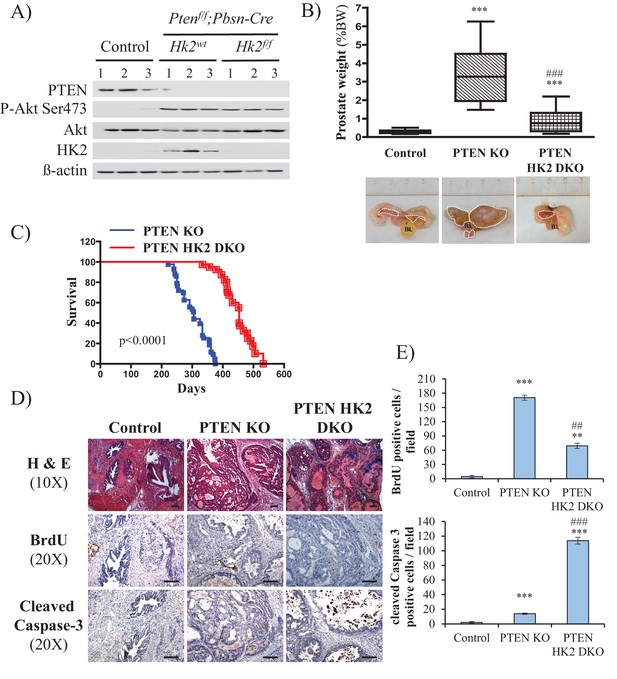
Deletion of HK2 in the prostates of Pbsn-Cre4;Ptenf/f mice extends survival and inhibits tumor growth by inhibiting proliferation and increasing cell death.
(A) Tissue lysates were prepared from prostates isolated from three control mice (Ptenf/f;HK2f/f), three Pbsn-Cre4;Ptenf/f mice and three Pbsn-Cre4;Ptenf/f;HK2f/f/ mice. The immunoblot shows the expression levels of PTEN, Akt-P (ser 473), total-Akt, HK2, and ß-actin as a loading control. (B) Graphs showing the relative prostate weights of control (n = 23), Pbsn-Cre4;Ptenf/f (PTEN KO, n = 21), and Pbsn-Cre4;Ptenf/f;HK2f/f (PTEN-HK2 DKO, n = 29) mice. The box plots represent the 25th to 75th percentiles (boxes) with the median, and the whiskers represent the maximum and minimum values. ***p<0.0001 versus control. ### p<0.0001 versus PTEN KO. The pictures are representative of macroscopic views of the prostates (delineated by a white dash line) of control (left panel), PTEN KO (middle panel), and PTEN-HK2 DKO (right panel) mice. (C) A cohort of 43 PTEN KO and 40 PTEN-HK2 DKO mice were kept alive, and Kaplan-Meier curves of the percentage of survival of these mice are shown. The PTEN KO mice have a median survival age of 305 days versus 453 days for the PTEN HK2 DKO mice. The p-values and median survival for the indicated treatments were calculated by log-rank tests. (D) The cross-sections of prostate tissues collected at 8 months from control, PTEN KO, and PTEN-HK2 DKO mice were subjected to hematoxylin and eosin (H and E) staining (top), BrdU staining (middle), and anti-cleaved caspase-3 staining (bottom). (E) Histograms showing quantification of the positively stained cells in (D). The results are presented as the mean ±SEM of the positively stained cells of four sections from four treated mice. The stained cells were counted in four random fields of each section. **p<0.0005, ***p<0.0001 versus control. ##p<0.0005, ###p<0.0001 versus PTEN KO.
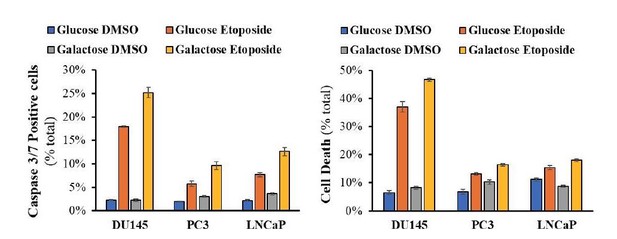
Cells were allowed to plate in 48-well plates (1x104 cells/well) for 16h and then media was switched to media with 11mM glucose or 11mM Galactose for 24h prior to addition of DMSO or Etoposide (50μM) for 14h (caspase 3/7 assay) or 24h (PI staining on unfixed cells).
https://doi.org/10.7554/eLife.32213.047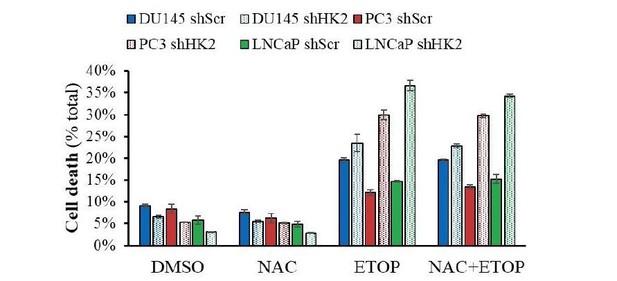
Cells were allowed to plate in 48-well plates (1.5X104 cells/wells) for 16h and then pre-treated with NAC (100μM) for 2h prior to addition of Etoposide (50μM) for 24h.
At end-point, PI and Hoechst 33342 were added to wells for 30min and plates were visualized with Celigo Image Cytometer, and cell death was calculated.
Tables
Histopathologic analysis relative to Figure 3.
https://doi.org/10.7554/eLife.32213.030| Grade | |||||
|---|---|---|---|---|---|
| No PIN | Low grade PIN | High grade PIN | Microinvasive carcinoma | Invasive carcinoma | |
| Pbsn-Cre4;Ptenf/f* | 66% | 33% | |||
| Pbsn-Cre4;Ptenf/f R + P† | 33% | 16% | 33% | 16% | |
| Pbsn-Cre4;Ptenf/f + NAC‡ | 25% | 75% | |||
-
*The anterior lobes of prostates from untreated mice were analyzed by histopathology at 8 months (percentage of mice with highest grade is indicated).
†The anterior lobes of prostates from mice treated at 4 months with rapamycin and PEITC (R + P) were analyzed by histopathology at 8 months (percentage of mice with highest grade is indicated).
-
‡The anterior lobes of prostates from mice treated at 4 months with NAC were analyzed by histopathology at 8 months (percentage of mice with highest grade is indicated).
Additional files
-
Transparent reporting form
- https://doi.org/10.7554/eLife.32213.044





