Activating the regenerative potential of Müller glia cells in a regeneration-deficient retina
Figures
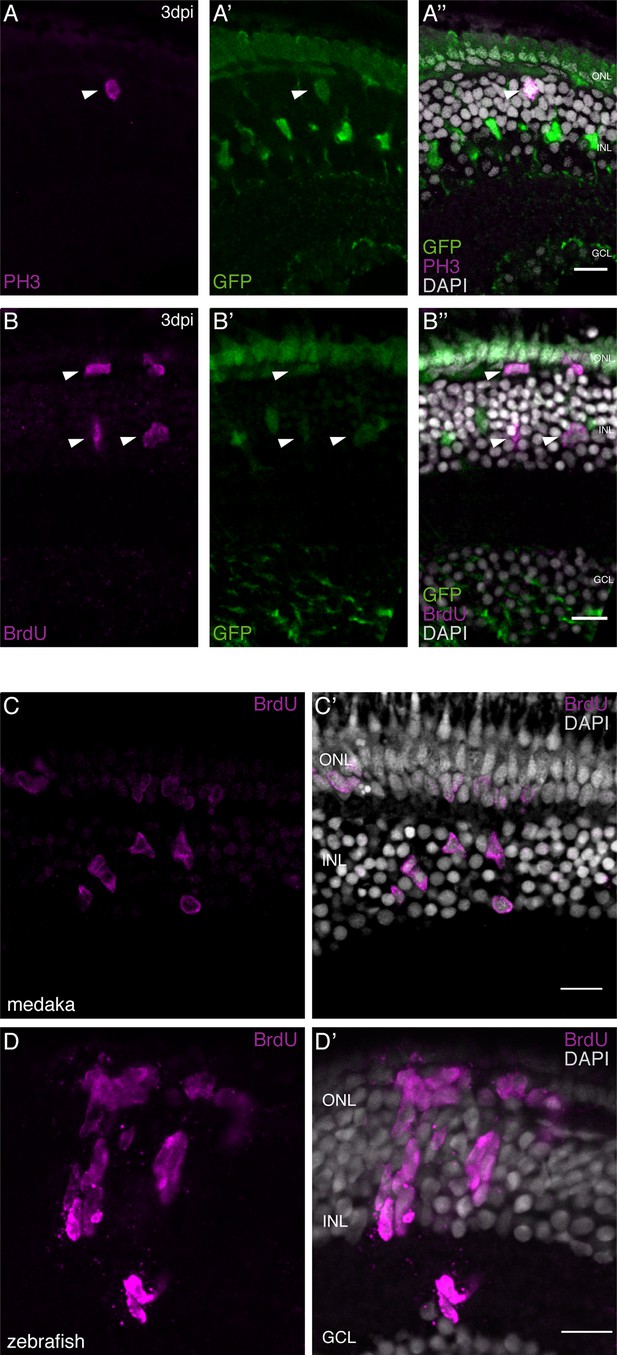
olMG cells re-enter the cell cycle after injury but do not generate neurogenic clusters.
(A–A’’) Cryosection of a needle-injured hatchling medaka retina of the transgenic line rx2::H2B-eGFP. PH3 stainings (magenta) on the hatchling medaka retinae 3 days post needle injury show mitotic cells present in the central retina (arrowhead), co-localizing with the rx2 nuclear reporter expression (green). (n = 4 fish, data obtained from two independent experiments). (B–B’’) Cryosection of a needle-injured hatchling medaka retina of the transgenic line rx2::H2B-eGFP. A 3-day pulse of BrdU marks proliferating cells in the central retina after needle injury (arrowheads). BrdU staining (magenta) co-localizes with rx2 nuclear reporter expression (green), indicating that olMG cells re-entered the cell cycle. (n = 6 fish, data obtained from three independent experiments). (C, C') Cryosection of a needle-injured hatchling medaka retina. BrdU-positive (magenta) single cells are present in the INL and ONL. (n = 6 fish, data obtained from two independent experiments). (D, D') Cryosection of a needle-injured zebrafish retina. BrdU-positive (magenta) neurogenic clusters are present in the INL. Additionally, BrdU-positive proliferating cells can be detected in the ONL (n = 3 fish, data obtained from two independent experiments). Scale bars are 10 μm.
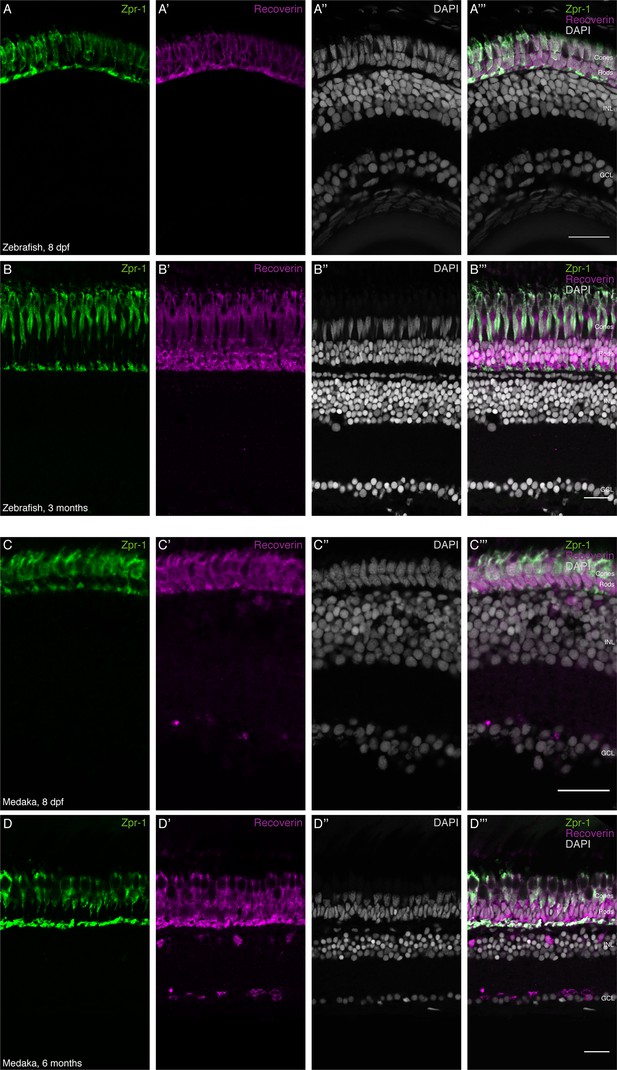
Rod photoreceptor density is increased during postembryonic growth of zebrafish but not medaka.
(A–B''') Cryosection of wild-type hatchling zebrafish and adult zebrafish retina. The ONL of the zebrafish retina is comprised of two nuclear layers, which contain only Zpr-1-positive (green) PRCs. The ONL of the adult zebrafish retina is comprised of four nuclear layers: one Zpr-1-positive (green) layer and three Recoverin-positive (magenta) layers. (C–D''') Cryosection of wild-type hatchling medaka and adult medaka retina. The ONL of the hatchling medaka retina is comprised of two nuclear layers: one Zpr-1-positive and one Recoverin-positive (magenta) layer. The ONL of the adult medaka retina is comprised of two nuclear layers: one Zpr-1-positive and one Recoverin-positive (magenta) layer. Scale bars are 20 μm.
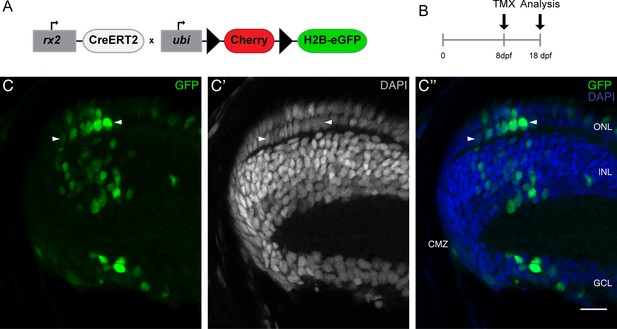
Rx2-positive CMZ cells generate rod photoreceptors, during post-embryonic growth.
(A–A') Cryosection of a retina of the transgenic line rx2::ERT2Cre, GaudíRSG. Hatchling fish were induced to recombine with tamoxifen and grown for 10 days. GFP-positive clones (green) close to the CMZ, which are derived from rx2-positive CMZ stem cells contain both rod (arrowheads) and cone PRCs. Scale bar is 10 µm.
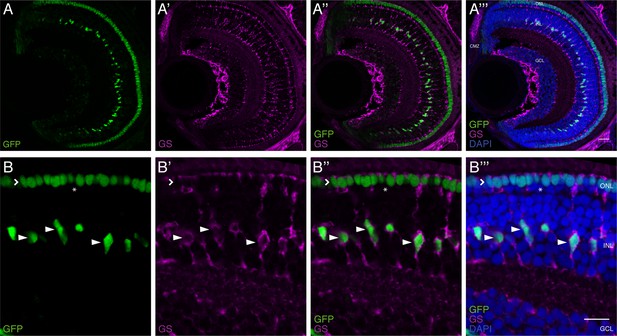
Rx2-reporter labels olMG cells, cone PRCs and CMZ cells in the hatchling medaka retina.
(A–B’’’) Cryosection of a hatchling medaka fish of the transgenic line rx2::H2B-eGFP. GFP-positive nuclei (green) are located in the INL, the ONL and the CMZ. GFP-positive nuclei in the INL overlap with Glutamine Synthetase (GS)-labeling (magenta, arrowheads), indicating that these cells are olMG cells. GFP-positive nuclei in the ONL are only present in the outer most nuclear layer (open arrowhead), indicating that these are cone PRCs. The inner layer of the ONL, where rod PRCs are located, is not labeled (asterisk). Scale bars are 20 μm (A–A''') and 10 μm (B–B''').
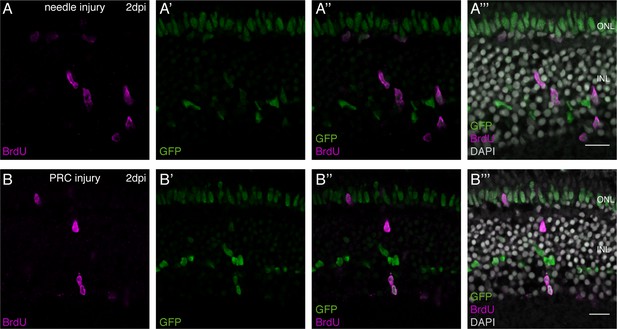
Injury-induced timing of olMG cell cycle re-entry.
(A–B’’’) Cryosection of either a needle-injured hatchling medaka retina (A–A''') or a PRC-injured hatchling medaka retina (B–B''') of the transgenic line rx2::H2B-eGFP. At 2 dpi, the first BrdU-positive (magenta) cells are detected in the central retina. BrdU co-localizes with rx2-driven GFP (green) in the INL and ONL (n = 3 fish each, data obtained from two independent experiments each). Scale bars are 10 μm.
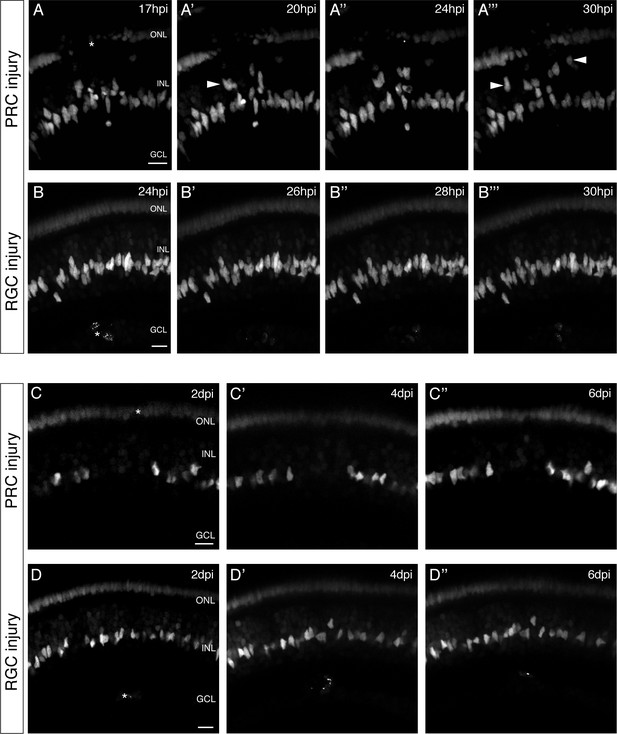
olMG cells react preferentially to PRC injuries by apical migration.
(A–B’’’) In vivo imaging of hatchling rx2::H2B-eGFP medaka retinae which were either injured in the ONL or the ganglion cell layer (GCL) (asterisks) using a two-photon laser and imaged consecutively until 30 hpi (n > 10 fish each, data obtained from >10 independent experiments each). (A–A’’’) After PRC injuries olMG nuclei (arrowheads) start migrating apically towards the ONL layer from 17 hpi on. The migration is not coordinated among different migrating nuclei. (B–B’’’) After RGC injuries no migration of olMG nuclei can be detected until 30 hpi. Scale bars are 10 μm. (C–D’’) In vivo imaging of hatchling rx2::H2B-eGFP medaka retinae which were either injured in the ONL or the GCL (asterisks) using a two-photon laser and imaged every second day after injury (n > 10 fish each, data obtained from >10 independent experiments each). (C–C’’) PRC injuries result in an apical migration of olMG nuclei into the injury site. The following days until 6 dpi the nuclei do not migrate back toward the INL resulting in a gap of olMG nuclei in the INL. (D–D’’) After RGC injuries no migration of olMG nuclei can be detected until 6 dpi. Scale bars are 10 μm.
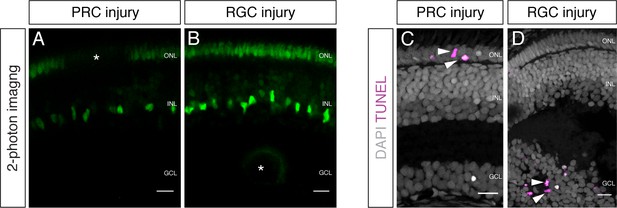
Two-photon mediated laser ablation enables targeted cell ablation in the retina resulting in specific cell death signatures.
(A–B) In vivo imaging of hatchling rx2::H2B-eGFP medaka retinae which were either injured in the ONL or the GCL (asterisks) using a two-photon laser. Targeted cell type ablation can be achieved; PRCs (A) as well as cells of the GCL (B) can be ablated. (C–D) Cryosections of hatchling medaka retinae which were either injured in the ONL or the GCL and fixed 16 hpi. TUNEL stainings (magenta) to detect programmed cell death show specific cell death of either ONL (C) or GCL (D). Scale bars are 10 μm.
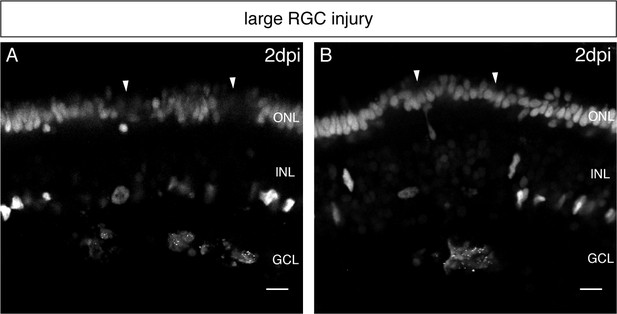
Increased RGC injuries lead to swelling and secondary cell death in the PRC layer.
(A–B) In vivo imaging of hatchling rx2::H2B-eGFP medaka retinae which were either injured in the GCL (asterisks) using a two-photon laser and imaged 2 days later (n = 10 fish, data obtained from five independent experiments). Large RGC injuries induce swelling and cell death in the PRC layer (arrowheads). olMG nuclei are largely depleted from the INL. Scale bars are 10 µm.
In vivo imaging of olMG nuclei reactions to a PRC injury
https://doi.org/10.7554/eLife.32319.011In vivo imaging of olMG nuclei reactions to a RGC injury
https://doi.org/10.7554/eLife.32319.012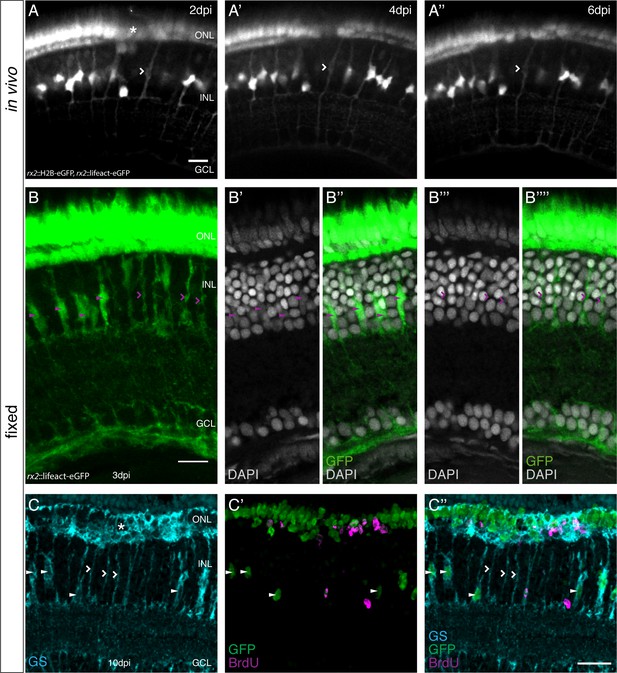
olMG nuclei are depleted after PRC injuries without cell body loss.
(A–A’’) In vivo imaging of a hatchling rx2::H2B-eGFP, rx2::lifeact-eGFP medaka retina which was injured in the ONL (asterisk) and imaged every second day after injury. Close to the injury site an olMG cell body without a nucleus can be detected at 2 dpi (A, empty arrowhead). The empty process remains until 6 dpi (A”) (n = 3 fish, data obtained from three independent experiments). Scale bar is 10 μm. (B–B’’’’) Maximum projection (B) and single planes of a cryosection of the injured hatchling medaka retina of the transgenic line rx2::lifeact-eGFP. The fish were injured, incubated in BrdU for 3 days and fixed at 3 dpi. Both GFP-positive cell bodies (green) which contain (arrowheads) and do not contain (empty arrowheads) a nucleus anymore are present. (n = 6 fish, data obtained from two independent experiments). Scale bar is 10 μm. (C–C’’) Maximum projection of a cryosection of the injured hatchling medaka retina of the transgenic line rx2::H2B-eGFP. The fish were injured in the ONL (asterisk), incubated in BrdU for 3 days and fixed at 10 dpi. Many GFP-positive nuclei (green) are located in the ONL, some co-localizing with BrdU (magenta). In the INL few GFP-positive nuclei are present. Many GS-positive (cyan) olMG cell bodies below the injury site do not contain a GFP-positive nucleus (empty arrowheads). Next to the empty cell bodies GFP-positive nuclei can be detected within GS-positive cell bodies (arrowheads) (n = 4 fish, data obtained from two independent experiments). Scale bar is 20 μm.
rx2::lifeact-eGFP retina at 3dpi
https://doi.org/10.7554/eLife.32319.014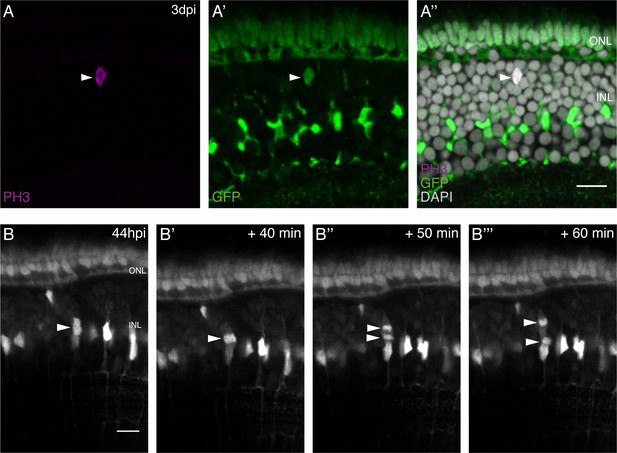
olMG cells divide in the INL with an apico-basal spindle orientation.
(A–A’’) Cryosection of an injured hatchling medaka retina of the transgenic line rx2::H2B-eGFP. PH3 stainings (magenta) on hatchling medaka retinae 3 days post PRC injury show mitotic olMG cells present in the INL (arrowhead), co-localizing with the rx2 nuclear reporter expression (green) (n = 4 fish, data obtained from three independent experiments). (B–B’’’) In vivo imaging of hatchling rx2::H2B-eGFP medaka retinae which were injured in the ONL and imaged starting at 44 hpi. OlMG nuclei which start to condense their chromatin can be detected in the INL (arrowheads). The divisions occur in an apico-basal manner (n = 6 fish, data obtained from six independent experiments, 5 out of 6 imaged divisions were apico-basal). Scale bars are 10 μm.
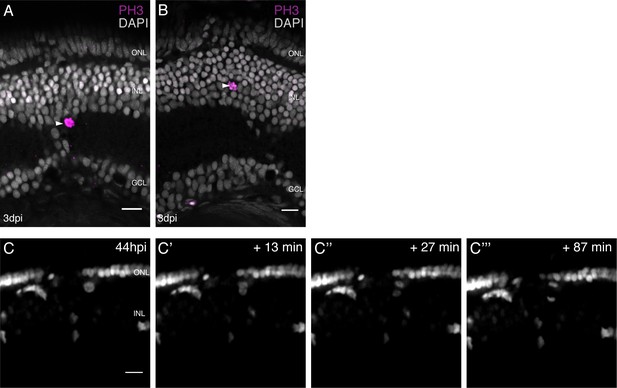
olMG cells divide in various positions in the the INL with an apico-basal spindle orientation.
(A–B) Cryosections of injured hatchling medaka retinae. PH3 stainings (magenta) at 3 days post needle injury show mitotic olMG cells present in different positions in the INL (arrowheads) (n = 4 fish, data obtained from two independent experiments). (C–C’’’) In vivo imaging of hatchling rx2::H2B-eGFP medaka retinae which were injured in the ONL and imaged starting at 44 hpi. An olMG nuclei which starts to condense its chromatin can be detected close to the ONL. The divisions occur in an apico-basal manner (n = 6 fish, data obtained from six independent experiments, 5 out of 6 imaged divisions were apico-basal). Scale bars are 10 μm.
In vivo imaging of an olMG division after PRC injury
https://doi.org/10.7554/eLife.32319.017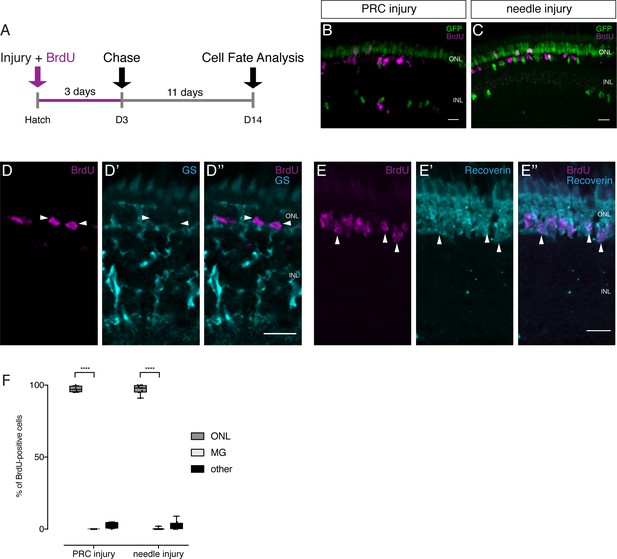
Lineage tracing after injuries reveals the preferential regeneration of PRCs.
(A) Scheme outlining the experimental procedure. Hatchling medaka were injured in the retina with a two-photon laser ablating either PRCs or RGCs or with a needle ablating all cell types. The fish were incubated in BrdU for 3 days and analyzed at 14 dpi. (B–C) PRC injuries result in BrdU-positive cells in the ONL, mostly in the rod layer. No BrdU-positive olMG cells are present and fewer GFP-positive olMG cells are found in the INL (n = 4 fish, data obtained from two independent experiments). Needle injuries result in BrdU-positive cells in the ONL, mostly in the rod layer. Except for 1 BrdU-positive olMG cell in one fish, no BrdU-positive olMG cells are detected. GFP-positive olMG nuclei are largely depleted from the INL (n = 10 fish, data obtained from three independent experiments). (D–D'') After needle injuries BrdU-positive cells (magenta) in the ONL are not co-labeled with GS (cyan), indicating that they are not olMG cells (n = 8 fish, data obtained from two independent experiments). (E–E'') After needle injuries BrdU-positive cells (magenta) in the ONL are co-labeled with Recoverin (cyan), indicating that they are PRCs (n = 5 fish, data obtained from one experiment). Scale bars are 10 μm. (F) Quantification of the location of BrdU-positive cells reveals that in all injury types BrdU-positive cells are predominantly located in the ONL (PRC injury: 54 cells in four retinae, needle injury: 550 cells in 10 retinae). ****p<0.0001. Box plots: median, 25th and 75th percentiles; whiskers show maximum and minimum data points.
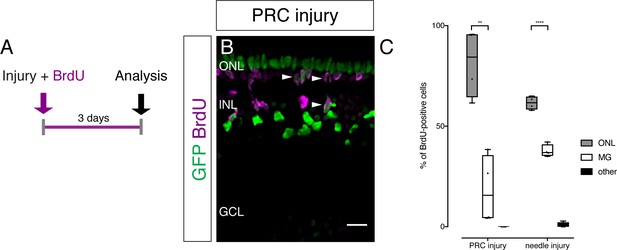
PRC and needle injuries trigger proliferation of olMG cells.
(A) Scheme outlining the experimental procedure. Hatchling medaka were injured in the retina with a two-photon laser, ablating either PRCs or RGCs. The fish were incubated in. BrdU for 3 days and analyzed subsequently. (B) PRC injuries induce cell cycle re-entry of olMG cells detected by BrdU uptake. BrdU-positive nuclei are located in the INL (arrowheads) and below or in the ONL (arrowheads). Scale bar is 10 μm. (C) Counting of BrdU-positive nuclei and assigning them to different categories (ONL, olMG cells or other cell types) reveals individual profiles of the different injury types. BrdU-positive cells are mostly located in the ONL after PRC injuries (80%) and needle injuries (60%) (PRC injury: 215 cells in four retinae, needle injury: 114 cells in four retinae), whereas no BrdU-positive cells are detected after RGC injuries. **p=0.0019, ****p<0.0001. Box plots: median, 25th and 75th percentiles; whiskers show maximum and minimum data points. (n = 4 fish each, data obtained from three independent experiments each).
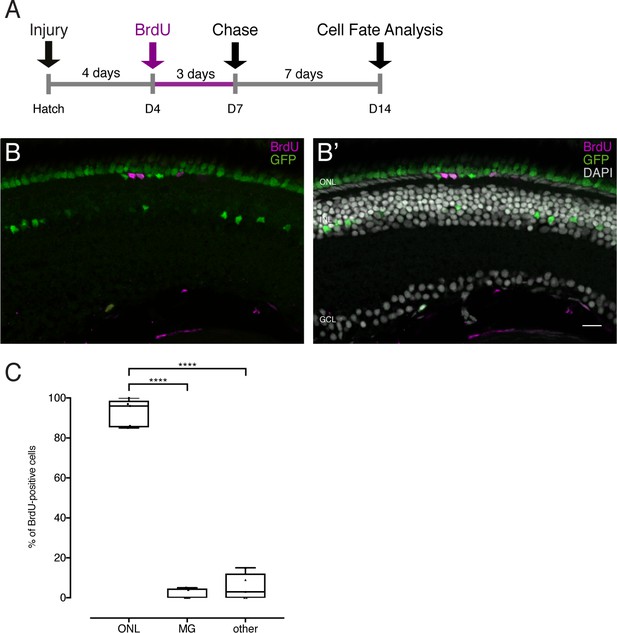
Late BrdU application after injury labels the same cell population as early BrdU application.
(A) Scheme outlining the experimental procedure. Hatchling medaka were injured in the retina with a needle ablating all cell types. The fish were incubated in BrdU from 4 dpi until 7 dpi and analyzed at 14 dpi. (B–C) BrdU-positive cells are located in the ONL, 1 out of 5 fish contained 1 BrdU-positive olMG cell. GFP-positive olMG nuclei are depleted from the INL (139 cells in 5 retinae). ****p<0.0001. Box plots: median, 25th and 75th percentiles; whiskers show maximum and minimum data points (n = 5 fish, data obtained from two independent experiments). Scale bar is 10 μm.
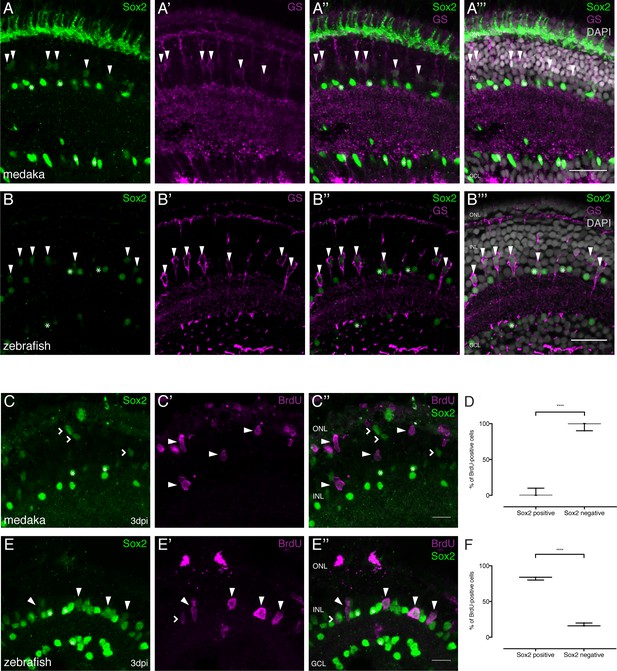
Sox2 is present in MG cells of the hatchling medaka and zebrafish retina but not maintained after injury in medaka.
(A–A’’’) Cryosection of an uninjured hatchling medaka retina. Sox2 (green) labeled cells with round nuclei are present in the INL and the GCL. Sox2-labeled cells with round nuclei are ACs present in the INL and the GCL (asterisks). Sox2-positive cells with elongated nuclei are present in the INL (arrowheads). Co-labeling with GS (magenta) proves that cells with elongated nuclei are olMG cells. Additional staining, which is likely unspecific staining since sox2 mRNA cannot be detected there (Reinhardt et al., 2015), can be detected in the ONL. (B–B’’’) Cryosection of an uninjured zebrafish retina at 9 dpf. Sox2 (green)-positive cells with round nuclei are present in the INL and the GCL. Sox2-labeled cells with round nuclei are ACs present in the INL and the GCL (asterisks). Sox2-positive cells with elongated nuclei are present in the INL (arrowheads). Co-labeling with GS (magenta) proves that cells with elongated nuclei are drMG cells. Scale bars are 20 μm. (C–C’’) Cryosection of an injured hatchling medaka retina at 3 dpi. BrdU (magenta, arrowheads) labeled cells are not co-labeled with Sox2 (green, arrowheads). Sox2-positive cells with elongated nuclei, indicating non-proliferative olMG cells, are found in the INL (open arrowheads). Sox2-positive cells with round nuclei are ACs present in the INL and the GCL (asterisks) (n = 3 fish, data obtained from two independent experiments). (D) Quantification of the amount of Sox2-positive and negative proliferating cells of BrdU-positive cells at 3 dpi in medaka (74 cells in 3 retinae). ****p<0.0001. Box plots: median, 25th and 75th percentiles; whiskers show maximum and minimum data points. (E–E’’) Cryosection of an injured zebrafish retina at 3 dpi. BrdU-(magenta) and Sox2-(green) double positive cells can be detected in the INL (arrowheads). BrdU-positive Sox2-negative cells can rarely be detected (open arrowhead). Sox2-labeled cells with round nuclei are ACs present in the INL and the GCL (asterisks) (n = 3 fish, data obtained from two independent experiments). Scale bars are 10 μm. (F) Quantification of the amount of Sox-positive and negative proliferating cells of BrdU-positive cells at 3 dpi in zebrafish (68 cells in 3 retinae). ****p<0.0001. Box plots: median, 25th and 75th percentiles; whiskers show maximum and minimum data points.
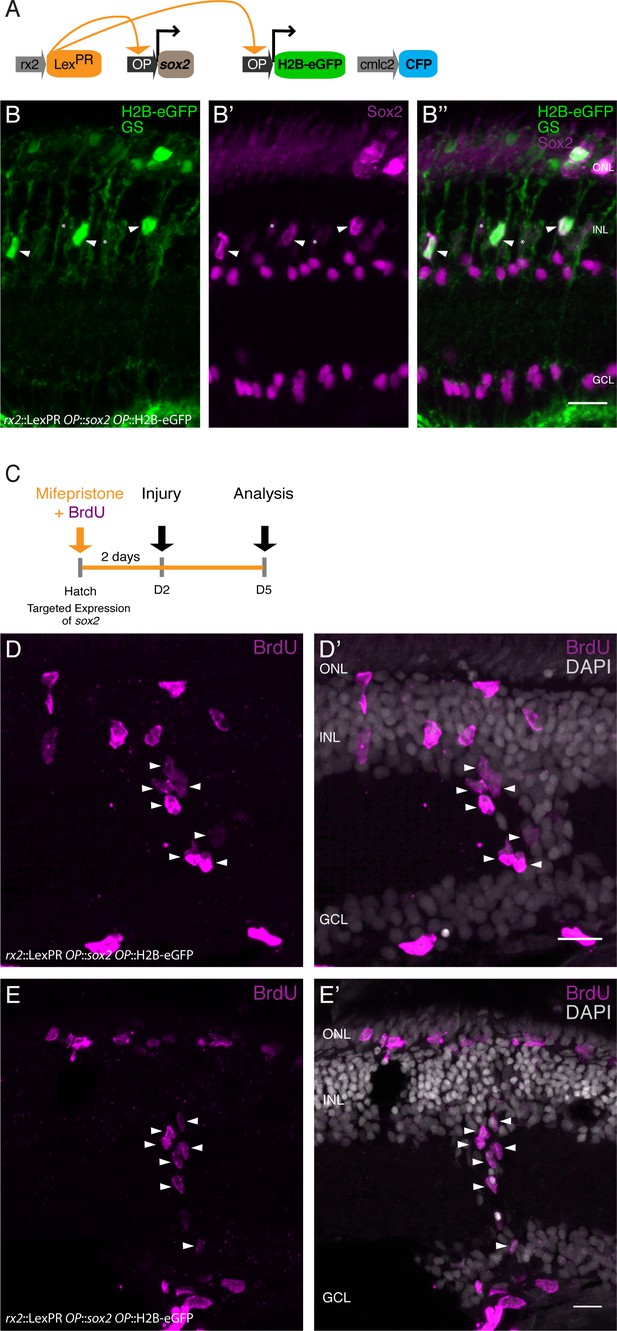
Expression of sox2 via the LexPR system increases Sox2 protein levels in olMG cells and triggers proliferating cluster formation after injury in medaka.
(A) Genetic construct used for sox2 induction. (B–B'') Cryosection of a retina of a mifepristone-induced rx2::LexPR OP::sox2, OP::H2B-eGFP transgenic fish at 5 days after induction. Nuclear-localized GFP (green) labels positively induced cells, which contain an increased amount of Sox2 protein (magenta, arrowheads) in comparison to non-induced cells (asterisks) (n = 6 fish, data obtained from two independent experiments). (C) Induction scheme for sox2 induction. (D–E') Cryosections of mifepristone-induced rx2::LexPR OP::sox2, OP::H2B-eGFP transgenic fish at 3 days after needle injury. BrdU-positive (magenta) cells can be detected in all retinal layers and some BrdU-positive clusters are present in the INL and between INL and GCL (n = 4 fish, data obtained from one experiment). Scale bars are 10 μm.
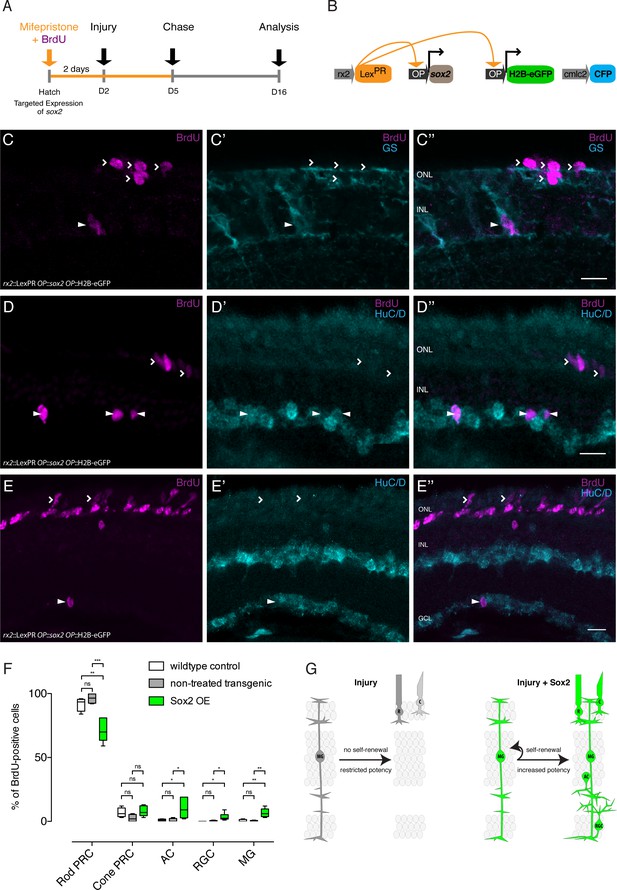
Sox2 induces a regeneration response in olMG cells.
(A–B) Induction scheme and construct (B) used for sox2 induction. (C–C’’) Cryosection of a sox2-induced hatchling medaka retina. BrdU-positive (magenta) olMG cells, which are labeled by GS (cyan) can be detected in the INL (arrowhead). Additional BrdU-positive cells are located in the ONL, in the location of both rods and cones (open arrowheads). (D–E'') Cryosections of sox2-induced hatchling medaka retinae. BrdU-positive (magenta) ACs, which are labeled by HuC/D (cyan) can be detected in the INL (D-D'', arrowheads) and GCL (E-E'', arrowheads). Additional BrdU-positive cells are located in the ONL, in the location of both rods and cones (open arrowheads) (n = 7 sox2 OE fish and n = 8 control fish, data obtained from two independent experiments each). Scale bars are 10 μm. (F) Quantification of the location of BrdU-positive cells in sox2-induced fish (607 cells in 14 retinae) versus wild-type control fish treated with mifepristone (341 cells in eight retinae) and transgenic rx2::LexPR OP::sox2, OP::H2B-eGFP fish not treated with mifepristone (218 cells in four retinae) reveals an increase in BrdU-positive olMG cells, ACs and RGCs as well as a decrease in rod PRCs in sox2-induced fish. Wild-type control vs Sox2 OE: Rod PRC **p=0.0031, Cone PRC ns p=0.678, AC *p=0.0434, RGC *p=0.0445, MG **p=0.0083. Non-treated transgenic vs Sox2 OE: Rod PRC ***p=0.0004, Cone PRC ns p=0.528, AC *p=0.0445, RGC *p=0.0445, MG **p=0.0061. Box plots: median, 25th and 75th percentiles; whiskers show maximum and minimum data points. (G) olMG cells respond to injuries by proliferation without self-renewal and restriction toward PRC fate. Targeted expression of sox2 induces self-renewal and increased potency of olMG cells.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Strain (Oryzias latipes) | Cab | other | medaka Southern wild type population | |
| Strain (O. latipes) | rx2::H2B-eGFP | this paper | ||
| Strain (O. latipes) | rx2::lifeact-eGFP | this paper | ||
| Strain (O. latipes) | rx2::H2B-eGFP QuiH | this paper | ||
| Strain (O. latipes) | rx2::LexPR OP::sox2 OP::H2B-eGFPcmlc2::CFP | this paper | ||
| Strain (O. latipes) | GaudíRSG | PMID: 25142461 | ||
| Strain (O. latipes) | rx2::CreERT2 | PMID: 25908840 | ||
| Strain (Danio rerio) | AB | other | Wildtype zebrafish strain | |
| Strain (D. rerio) | Albino | other | ||
| Antibody | anti-BrdU (rat) | Bio-Rad/AbD Serotec (Germany) | BU1/75, RRID: AB_609566 | 1: 200 |
| Antibody | anti-eGFP (chicken/IgY, polyclonal) | Thermo Fisher (Waltham, Massachusetts, USA) | A10262, RRID: AB_2534023 | 1: 500 |
| Antibody | anti-HuC/D (mouse, monoclonal) | Thermo Fisher | A21271, RRID: AB_221448 | 1: 200 |
| Antibody | anti-GS (mouse, monoclonal, clone GS-6) | Milipore (Burlington, Massachusetts, USA) | MAB302, RRID: AB_2110656 | 1: 500 |
| Antibody | anti-pH3 (Ser10) (rabbit, polyclonal) | Millipore | 06–570, RRID: AB_310177 | 1: 500 |
| Antibody | anti-Recoverin (rabbit, polyclonal) | Millipore | AB5585, RRID: AB_2253622 | 1: 200 |
| Antibody | anti-Sox2 (rabbit, polyclonal) | Genetex (Irvine, California, USA) | GTX101506, RRID: AB_2037810 | 1: 100 |
| Antibody | anti-Zpr-1 (mouse, Imonoclonal) | ZIRC (Eugene, Oregon, USA) | RRID: AB_10013803 | 1: 200 |
| Antibody | anti-chicken Alexa Fluor 488 (donkey) | Jackson (West Grove, Pennsylvania, USA) | 703-545-155, RRID: AB_2340375 | 1: 750 |
| Antibody | anti-mouse Alexa Fluor 546 (goat) | Thermo Fisher | A-11030, RRID: AB_2534089 | 1: 750 |
| Antibody | anti-mouse Cy5 (donkey) | Jackson | 715-175-151, RRID: AB_2340820 | 1: 750 |
| Antibody | anti-rabbit DyLight 549 (goat) | Jackson | 112-505-144 | 1: 750 |
| Antibody | anti-rabbit Alexa Fluor 647 (goat) | Thermo Fisher | A-21245, RRID: AB_2535813 | 1: 750 |
| Antibody | anti-rat DyLight 549 (goat) | Jackson | 112-505-143 | 1: 750 |
| Antibody | anti-rat Alexa Fluor 633 (goat) | Thermo Fisher | A-21094, RRID: AB_2535749 | 1: 750 |
| Recombinant DNA reagent | rx2::H2B-eGFP (plasmid) | this paper | Vector with I-SceI meganuclease sites | |
| Recombinant DNA reagent | rx2::lifeact-eGFP (plasmid) | this paper | Vector with I-SceI meganuclease sites | |
| Recombinant DNA reagent | rx2::LexPR OP::sox2 OP (plasmid) | this paper | Vector with I-SceI meganuclease sites | |
| Recombinant DNA reagent | OP::H2B-eGFP cmlc2::CFP (plasmid) | this paper | Vector with I-SceI meganuclease sites | |
| Sequence-based reagent | PRC primer for medaka sox2 | fwd with BamHI site: TAATGGATCCATGTATAACATGATGGAGACTGAAC, rev with NotI site: TAATGCGGCCGCTTACATGTGTGTTAACGGCAGCGTGC | ||
| Chemical compund, drug | 5-Bromo-2′-deoxyuridine (BrdU) | Sigma Aldrich (St. Louis, Missouri, USA) | B5002 | |
| Chemical compund, drug | Mifepristone | Cayman (Ann Arbor, Michigan, USA) | 84371-65-3 | |
| Chemical compund, drug | 1-phenyl-2-thiourea (PTU) | Sigma Aldrich | P7629 | |
| Chemical compund, drug | Tamoxifen | Sigma Aldrich | T5648 | |
| Chemical compund, drug | Tricaine | Sigma Aldrich | A5040 | |
| Other | DAPI | Roth (Germany) | 28718-90-3 | 1:500 dilution in 1xPTW of 5 mg/ml stock |
Additional files
-
Transparent reporting form
- https://doi.org/10.7554/eLife.32319.024





