Multivesicular bodies mediate long-range retrograde NGF-TrkA signaling
Figures
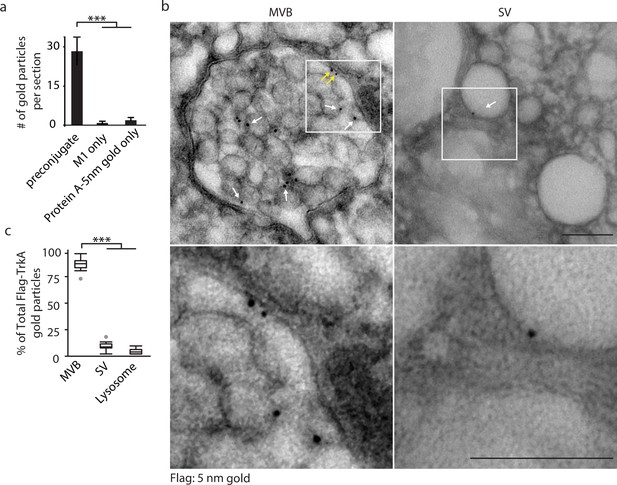
Retrograde TrkA+ endosomes are predominantly of multi-vesicular, not single-vesicular, ultrastructure.
(a,b) The Flag-TrkA transport assay was performed in compartmentalized sympathetic neurons using pre-conjugated anti-Flag antibody with Protein A-5 nm gold. Cells were fixed 1 hr post-NGF application and processed for EM. The percentage of Flag-TrkA gold particles localized to MVBs, single-membrane vesicles (SVs) or lysosomes was quantified (c). Note the presence of the Flag epitope on both the membrane of the intraluminal vesicles (white arrows) and the limiting membrane of the MVB (yellow arrows). High-magnification images of the boxed areas are shown in the bottom panels. (c) The Flag-TrkA assay was performed using pre-conjugated primary antibody and Protein A-5nm gold, or primary antibody or Protein A-5nm only. Cells were fixed 1 hr post-NGF stimulation and the number of gold particles per EM section was counted (n = 4). Scale bar: 100 nm. Data are represented as mean ± standard error of the mean (SEM) (a) or presented in box plot (c). In box plots, the top and the bottom of the central rectangle represents the 75th and 25th percentile value, respectively, and the line inside represents the median; the whisker on either side extends to the data point that is within the range of variation (1.5×(75th percentile – 25th percentile)) and data points beyond that range are plotted as individual dots. ***p<0.001 by one-way ANOVA with a Tukey’s post-hoc test. See also Figure 1—figure supplement 1.
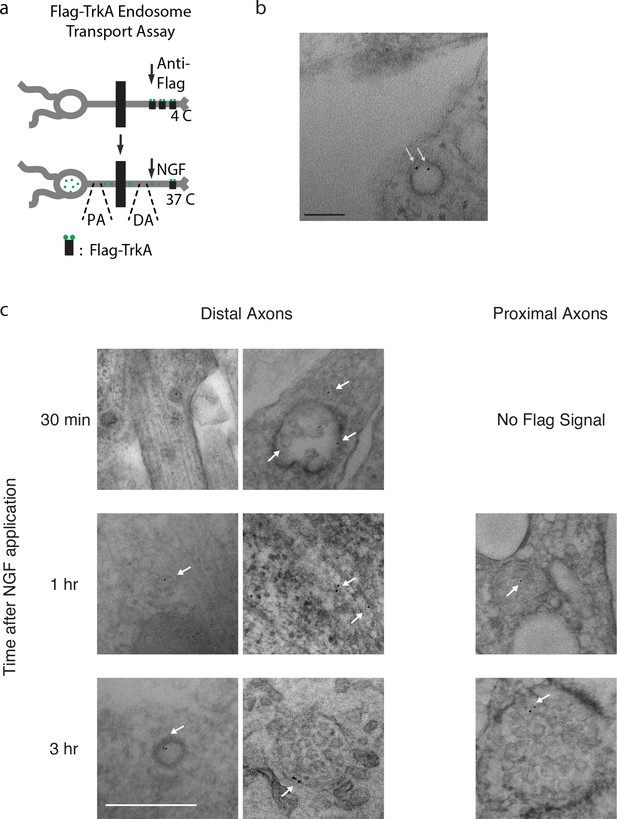
Retrogradely transported TrkA is associated with MVBs.
(a) Schematic of the Flag-TrkA endosome transport assay. DA: distal axons. PA: proximal axons. See also Materials and methods. (b) Newly internalized Flag-TrkA is sorted into early endosomes in distal axons. The Flag-TrkA assay was performed in compartmentalized sympathetic neurons using pre-conjugated anti-Flag antibody with Protein A-5 nm gold. Cells were fixed 5 min post-NGF application and processed for EM. White arrows denote Flag-TrkA gold particles within an endosome. n = 3. Scale bar: 100 nm. (c) Additional EM images of Flag-TrkA in axons. Sympathetic neurons grown in compartmentalized cultures were subjected to the pulse-block Flag transport assay and fixed at indicated time points. Flag-TrkA in distal and proximal axons were visualized by EM. Arrows denote individual Flag-TrkA complexes. Scale: 100 nm.
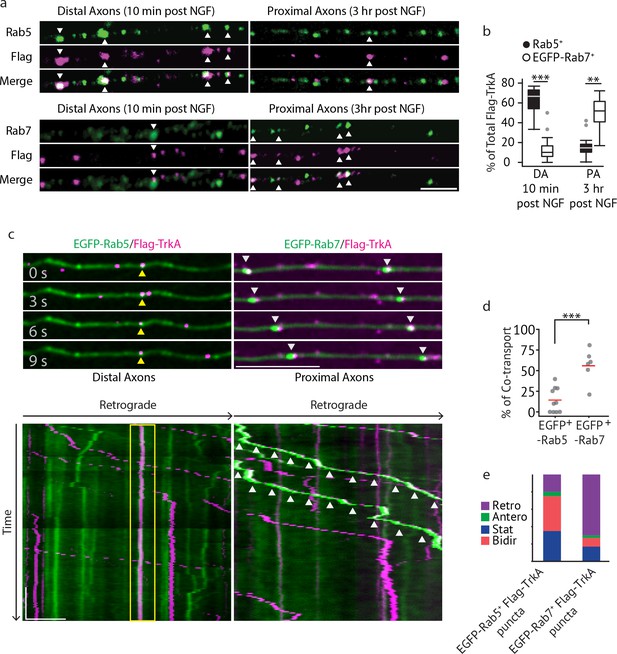
Multivesicular bodies, not early endosomes, are major carriers of retrograde TrkA signals in sympathetic neurons.
(a,b) The Flag-TrkA endosome transport assay was performed in sympathetic neurons grown in compartmentalized microfluidic culture and infected with a lentivirus expressing EGFP-Rab7. The percentage of Flag-TrkA punctae (magenta) colocalized with the MVB marker EGFP-Rab7 or the EE marker Rab5 (green) in distal axons (DA) 10 min post NGF application or in axons proximal to cell bodies (PA) 3 hr post NGF application was quantified (n = 5). For DA experiments, cells were washed with NaCl/acetic acid to remove surface bound Flag signal prior to fixation. Scale bar: 5 μm. (c–e) Sympathetic neurons grown in compartmentalized culture were infected with lentivirus expressing EGFP-Rab7 or EGFP-Rab5. The Flag-TrkA assay was performed using pre-conjugated anti-Flag antibody and Alexa Fluoro secondary antibody during the 4◦C incubation step. Flag-TrkA (magenta) and EGFP (green) were then imaged consecutively in axons. Representative time-lapse images of each type of TrkA endosomes are shown (top panel). Kymographs of time-lapse images are shown in the bottom panel. Arrowheads denote individual endosomes. Scale bar: 10 μm; 1 min. The percentage of Flag-TrkA punctae co-transported with either marker was quantified (d). Note that not every axon expresses the endosomal marker fusion proteins and therefore Flag-TrkA in these axons was not always observed to be associated with fluorophore-tagged endosomal markers. The directionality of retrograde TrkA MVBs and EEs are shown in (e). A total of 168 (Rab5) and 207 (Rab7) endosomes were scored in four independent experiments for each condition. Data are presented in box plot (b) or dot plot (d). In dot plots, individual data points (dots) and the mean (red line) are shown. ***p<0.001 by two tailed unpaired Student’s t test. See also Figure 2—figure supplement 1 and Videos 1 and 2.
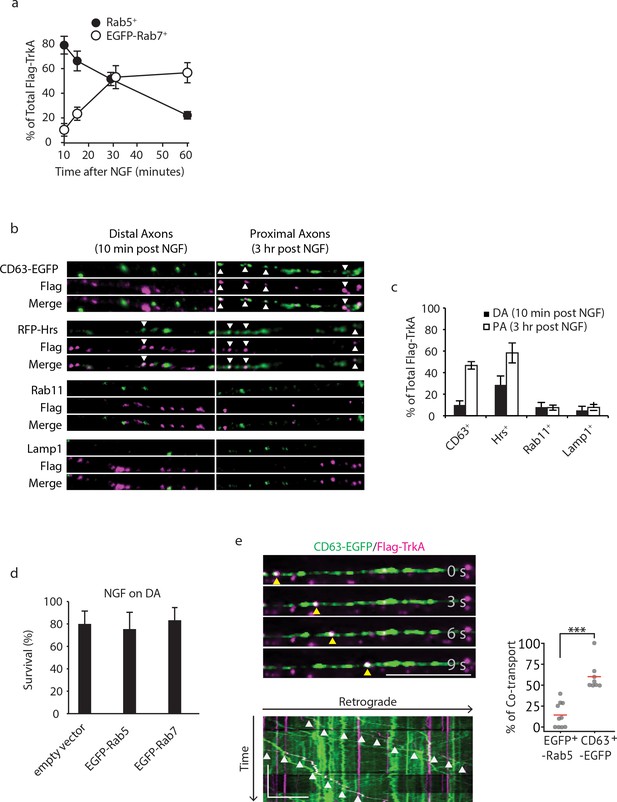
Retrogradely transported TrkA is associated with MVB markers.
(a) Colocalization of Flag-TrkA endosomes with EGFP-Rab5 or EGFP-Rab7 in distal axons post internalization over time was quantified (n = 3). (b,c) Colocalization between Flag-TrkA (magenta) and CD63-EGFP, RFP-Hrs, Rab11 and Lamp1 (green) in distal and proximal axons of compartmentalized sympathetic neurons. For distal axon experiments, cells were fixed 10 mins post-NGF application and were stripped with NaCl/acetic acid to remove surface Flag antibody. For proximal axon experiments, cells were incubated for 3 hr post-NGF application (n = 3). Scale bar: 10 μm. (d) Compartmentalized sympathetic neurons were infected with a virus expressing an empty vector, EGFP-Rab5 or EGFP-Rab7, and neuronal survival was assessed. Expression of EGFP-Rab5 or EGFP-Rab7 did not affect retrograde NGF-dependent survival of sympathetic neurons (n = 3). (e) Sympathetic neurons grown in compartmentalized culture were infected with lentivirus expressing CD63-EGFP. The Flag-TrkA assay was performed using pre-conjugated anti-Flag antibody and Alexa Fluoro secondary antibody during the 4◦C incubation step. Flag-TrkA (magenta) and EGFP (green) were then imaged consecutively in axons. Representative time-lapse images of each type of TrkA endosomes are shown (top panel). The kymograph of time-lapse images are shown in the bottom panel. Arrowheads denote individual endosomes. Scale bar: 10 μm; 1 min. The percentage of Flag-TrkA punctae co-transported with CD63-EGFP was quantified. A total of 173 endosomes were scored in four independent experiments for each condition. ***p<0.001 by two tailed unpaired Student’s t test. Data are represented as mean ± SEM (a,c,d) or presented in dot plot (e). See also Video 3.
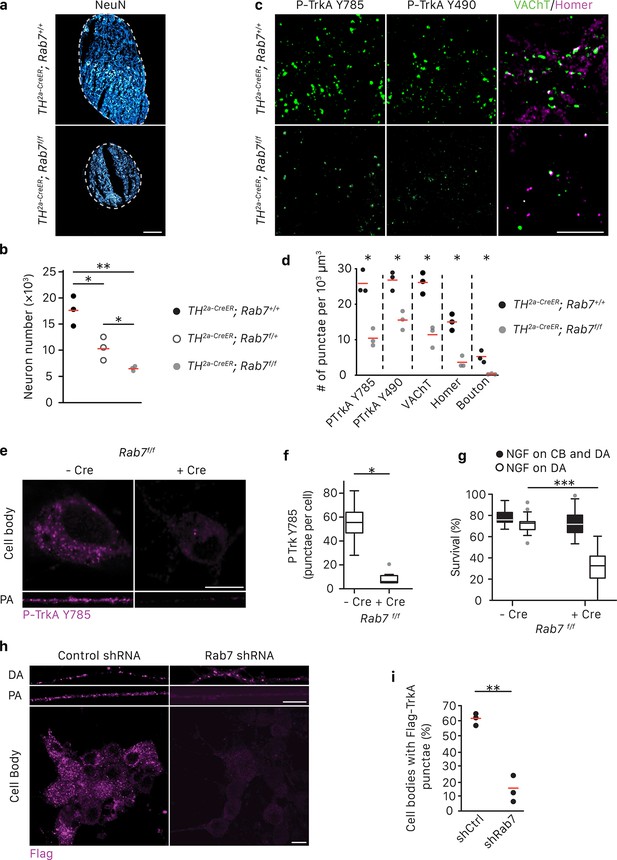
Rab7 mediates survival and synaptogenesis of sympathetic ganglia in vivo and retrograde NGF/TrkA transport, signaling and survival in vitro.
(a,b) Neuronal cell counts of SCGs from Rab7+/+; Th2a-CreER, Rab7f/+; Th2a-CreER and Rab7f/f; Th2a-CreERmice at P7. Tamoxifen was administered at E14 (0.5 mg) to induce Cre expression (n = 3). Scale bar: 100 μm. (c,d) Rab7+/+; Th2a-CreER and Rab7f/f; Th2a-CreERmice were treated with 1 mg tamoxifen at P7 and SCGs were harvested at P14. TrkA signaling was assessed by P-Trk Y490 and Y785 staining. Synaptic organization was assessed by VAChT (green) and Homer (magenta) staining, and VAChT/Homer colocalization (n = 3). (e–g) Sympathetic neurons harvested from P0 Rab7f/f pups were grown in microfluidic chambers and infected with a lentivirus expressing the Cre recombinase. Cells were incubated with NGF in the distal axon compartment and anti-NGF and the caspase inhibitor, BAF, in the cell body compartment for 48 hr, and TrkA signaling was assessed in axons and cell soma was assessed by P-Trk Y785 immunostaining (e,f). Alternatively, retrograde NGF-dependent neuronal survival was assessed (g) (n = 3). (h,i) The Flag-TrkA transport assay was performed in sympathetic neurons infected with lentivirus expressing either a control shRNA or an shRNA against Rab7. The accumulation of Flag-TrkA punctae in cell bodies, which represent retrogradely transported TrkA, was assessed (n = 3). Scale bar: 10 μm (c,e,h). Data are presented in dot plots (b,d,i) or box plots (f,g). *p<0.05, **p<0.01 and ***p<0.001 by one-way ANOVA with a Tukey’s post-hoc test (b,g) or a two tailed unpaired Student’s t test (d,f,i). See also Figure 3—figure supplement 1.
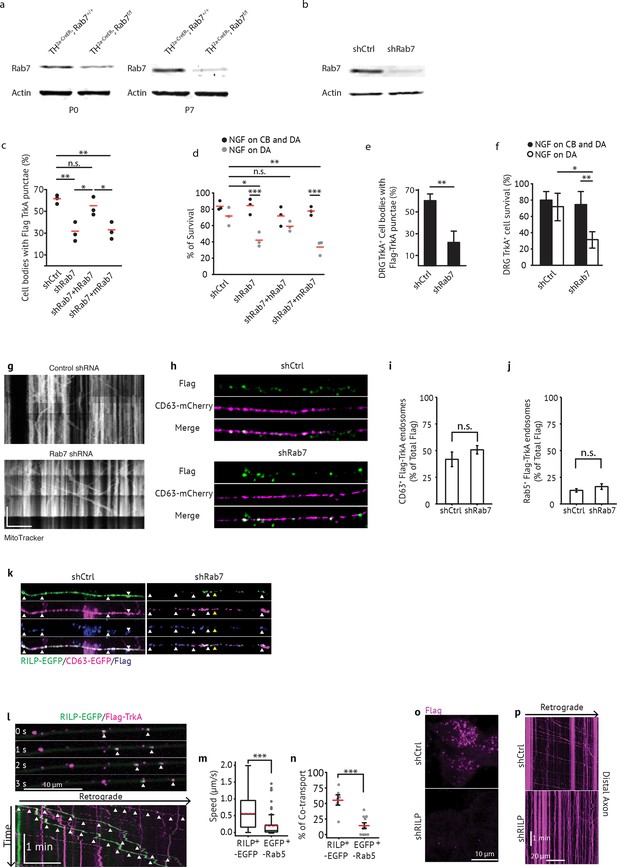
Rab7 is required for retrograde TrkA transport and survival in sympathetic and sensory neurons.
(a) Rab7+/+; Th2a-CreER and Rab7f/f; Th2a-CreERmice were treated with tamoxifen at E14 to induce Cre expression. SCGs were harvested at P0 or P7 and levels of Rab7 protein were assessed by immunoblot. (b) Sympathetic neurons grown in mass culture were infected with a virus expressing either a control shRNA or an shRNA against Rab7. Cells were harvested 72 hr post-infection and levels of Rab7 were assessed by immunoblot. Rab7 shRNA expression led to ~80% decrease in Rab7 protein levels. Rab7+/+; Th2a-CreER and Rab7f/f; Th2a-CreERmice were treated with tamoxifen at E14 to induce Cre expression. SCGs were harvested at P0 or P7 and levels of Rab7 protein were assessed by immunoblot. (c) Sympathetic neurons grown in compartmentalized chambers were infected with lentivirus expressing: a control shRNA, an shRNA against Rab7, Rab7 shRNA and human Rab7, or Rab7 shRNA and mouse Rab7. Cells were then grown either in the presence of NGF only in the distal axon compartment, or with NGF in both the distal axon and the cell body compartments. 72 hr later, the Flag-TrkA endosome transport assay was performed and accumulation of retrogradely transported Flag-TrkA punctae within cell bodies was assessed (n = 3). (d) Compartmentalized sympathetic neurons were infected with the same four combinations of virus as in (c). Cell survival was assessed 36–48 hr later (n = 3). (e,f) DRG primary sensory neurons grown in compartmentalized cultures were infected with lentivirus expressing either a control shRNA or an shRNA against Rab7. 72 hr later, retrograde TrkA transport (e) or survival (f) was assessed in TrkA+ neurons (n = 3). (g) Mitochondrial movement is not perturbed by Rab7 knockdown. Sympathetic neurons grown in compartmentalized culture were infected with either control or Rab7 shRNA. Movement of mitochondria in axons was monitored in real time using mitotracker. Scale: 10 um; 1 min. (h,i) Colocalizaton of Flag-TrkA (green) and CD63-mCherry (magenta) in distal axons 60 min post-NGF stimulation is comparable between neurons expressing control shRNA or Rab7 shRNA. Quantification is shown in (g). (j) Colocalizaton of Flag-TrkA and Rab5 in distal axons 60 min post-NGF stimulation is comparable between neurons expressing control shRNA or Rab7 shRNA. (k) Colocalization between RILP-EGFP and CD63-mCherry+ Flag-TrkA MVBs in distal axons (60 min post-NGF application) in compartmentalized neurons expressing a control shRNA or an shRNA against Rab7. White arrowheads denote RILP/CD63/Flag triple positive endosomes in control axons (left) or CD63/Flag double-positive endosomes that are RILP negative (right). Yellow arrowheads show one RILP/CD63/Flag triple positive endosome in Rab7 knockdown axons. (l) RILP-EGFP (green) co-transport with retrograde Flag-TrkA (magenta) endosomes. Arrowheads denote individual double-labeled endosomes. (m) Rate of movement of RILP-EGFP+ TrkA or EGFP-Rab5+ TrkA endosomes. (n) Percentage of co-transport of RILP-EGFP and TrkA or EGFP-Rab5 and TrkA. (o) Accumulation of retrograde Flag-TrkA endosomes in cell soma in compartmentalized neurons expressing a control shRNA or shRNA against RILP. (p) Kymograph of Flag-TrkA movement in distal axons in compartmentalized neurons expressing a control shRNA or shRNA against RILP. Data are presented in dot plot (c,d) or represented as mean ± SEM (e,f). *p<0.05, **p<0.01 and ***p<0.001 by one-way ANOVA with a Tukey’s post-hoc test.
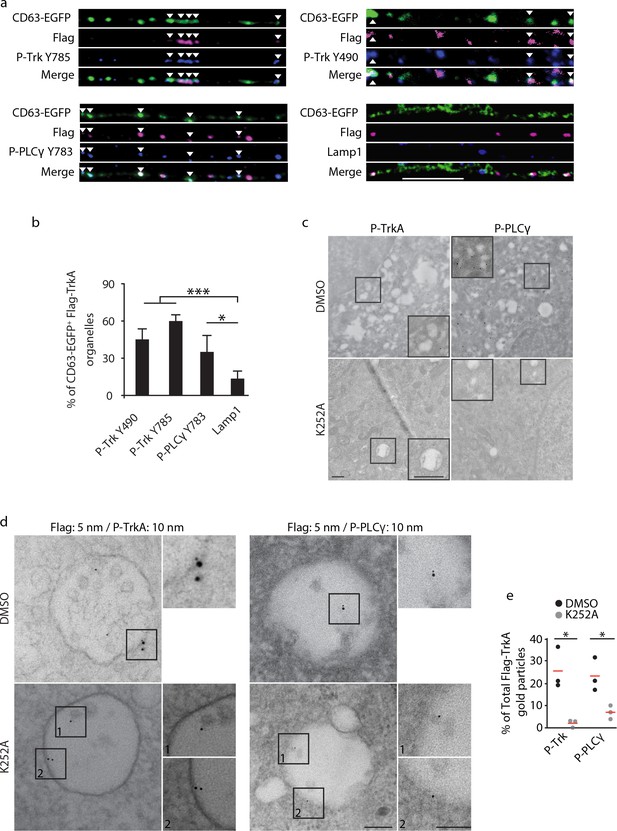
Retrograde TrkA+ MVBs associate with key effectors of the NGF/TrkA signaling pathway.
(a,b) The Flag-TrkA endosome transport assay was performed in compartmentalized sympathetic neurons expressing CD63-EGFP. The extent of CD63-EGFP+ Flag-TrkA (green/magenta) punctae in proximal axons colocalized with P-Trk Y490 and Y785, P- PLCγ Y783 and Lamp1 (blue) was quantified (b) (n = 3). Scale bar: 10 μm. (c) Sympathetic neurons grown in mass culture were NGF-deprived for 16 hr and were then stimulated with NGF for 1 hr in the presence of DMSO (vehicle control) or K252a (200 nM), a Trk kinase inhibitor. P-TrkA and P-PLCγ signals in cell bodies were assessed by pre-embed immunogold labeling (n = 3). Insets: high-magnification images of the boxed areas. (d,e) The Flag-TrkA assay was performed in compartmentalized sympathetic neurons using pre-conjugated anti-Flag antibody with a 5-nm gold secondary antibody in the presence of DMSO or K252a in the cell body compartment. Neurons were fixed 1 hr post NGF stimulation and P-TrkA and P-Plcγ signals were revealed by immunogold labeling (10 nm). The extent of Flag-TrkA gold particles associated with P-TrkA or P-PLCγ in cell bodies was assessed (n = 3). Scale bar: 100 nm. Data are represented as mean ± SEM (b) or presented in dot plot (e). *p<0.05 and **p<0.01 by one-way ANOVA with a Tukey’s post-hoc test (b) or a two tailed unpaired Student’s t test (e). See also Figure 4—figure supplement 1.
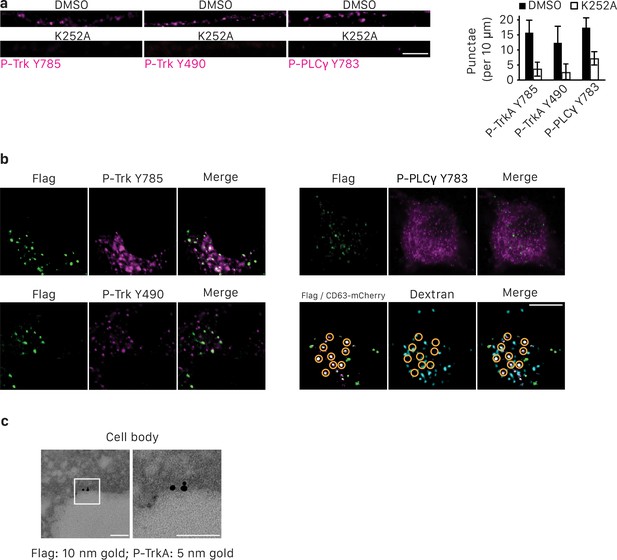
Retrograde TrkA+ endosomes associate with key effectors of NGF/TrkA signaling pathway.
(a) Compartmentalized sympathetic neurons were NGF- and serum-deprived for 6 hr and then stimulated with NGF in the presence of either DMSO or K252a for 20 min in distal axons. Cells were then immunostained for P-Trk Y490, P-Trk Y785 and P-PLCγ (n = 3). (b) The Flag-TrkA assay was performed in compartmentalized sympathetic neurons. Cells were fixed 3 hr post-NGF application and colocalization between Flag-TrkA (green) with P-Trk Y490, P-Trk Y785, P-PLCγ Y783 (Magenta) or lysosomes (blue) in cell bodies was assessed (n = 3). Lysosomes were labeled by pre-loading the cells with Dextran (blue) for 2 hr prior to the Flag assay. Yellow circles denote CD63-mCherry+ Flag-TrkA endosomes. Scale bar: 10 μm. (c) The Flag-TrkA assay was performed in sympathetic neurons in mass culture. Cells were fixed 30 s post-NGF application and colocalization between Flag-TrkA (10 nm gold) with P-Trk Y785 (5 nm gold) on plasma membrane was assessed (n = 3). 63.4 ± 3.5% Flag-TrkA were associated with P-TrkA. Scale bar: 100 nm. Data are represented as mean ± SEM.
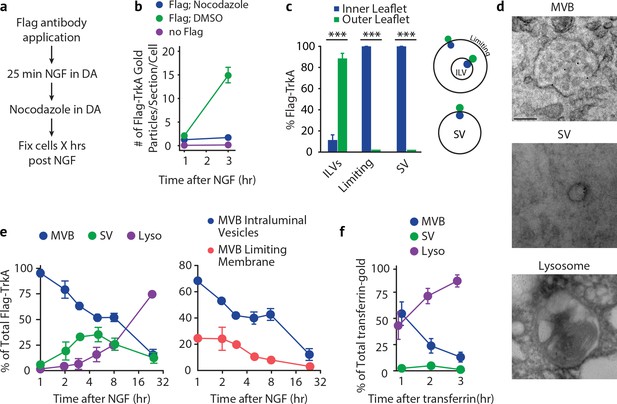
Retrogradely transported TrkA+ endosomes within cell bodies evolve from MVBs into simple, single-membrane vesicle structures.
(a) Schematic of the pulse-block assay. The Flag-TrkA assay is performed in Ntrk1Flag sympathetic neurons cultured in compartmentalized microfluidic chambers as in Figure 1—figure supplement 1A using pre-conjugated anti-Flag antibody and Protein A-5 nm gold. Nocodazole is applied to distal axons 25 min post-NGF application to block retrograde transport. Neurons are fixed at indicated time points and processed for EM. (b) The Flag-TrkA assay was performed in WT neurons, Ntrk1Flag neurons treated with either DMSO or nocodazole in distal axons (25 min post NGF). Cells were fixed at indicated time points and processed for EM. The number of gold particles per section per cell was quantified (n = 3, Results were pooled from over 150 Flag-gold particles). (c) The pulse-block assay was performed as in (a), and membrane topology of the Flag epitope was assessed. A schematic of the inner- or outer-leaflet position for membrane of ILVs, the limiting membrane and SVs is shown on the right. (d,e) The pulse-block assay was performed and the distribution of retrogradely transported Flag-TrkA gold particles in MVBs, single-membrane vesicle structures (SVs) and lysosomes within the cell body over time was assessed (e) Results were pooled from over 150 Flag-gold particles from four samples for each time point. Representative images of retrograde Flag-TrkA in each of the three membrane compartments are shown in (d). (f) Sympathetic neurons in compartmentalized cultures were incubated with transferrin-gold (6 nm) in distal axons and the pulse-block assay was performed. The distribution of retrograde transferrin-gold in MVBs, SVs and lysosomes was assessed by EM. For (d–f), over 150 endosomes were counted for each condition at each time point in four independent experiments. Scale bar: 100 nm. Data are represented as mean ± SEM. ***p<0.001 by two-way ANOVA with a Tukey’s post-hoc test (e) or a two-tailed unpaired Student’s t test (c). See also Figure 5—figure supplement 1.
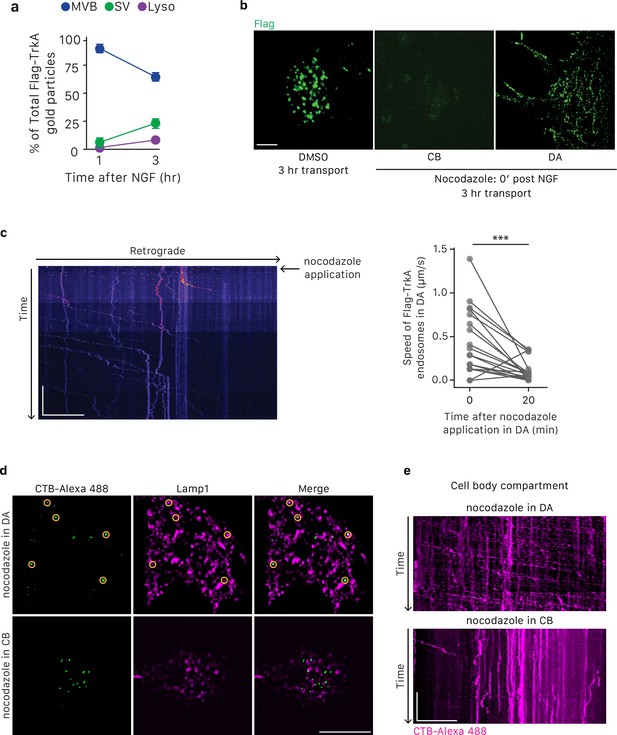
Nocodazole treatment effectively blocks microtubule-dependent axonal trafficking and retrograde Flag-TrkA transport.
(a) Quantification of retrograde Flag-TrkA localization in MVB, SV and lysosome in cell bodies over time (n = 4,>200 gold particles scored for each time point). Data for the 1 hr time point is the same as in Figure 1C. (b) The Flag-TrkA assay was performed in compartmentalized sympathetic neurons with DMSO or nocodazole (10 μM) applied at the time of NGF application in DA. Accumulation of Flag-TrkA punctae in cell bodies and distal axons was assessed 3 hr post-NGF application (n = 3). Compared to the vehicle control, very few, if any, Flag punctae were observed in nocodazole treated cells. Scale bar: 20 μm. (c) The Flag-TrkA assay was performed in compartmentalized sympathetic neurons with nocodazole (10 μM) applied 25 min post-NGF application in DA. Movement of retrograde Flag-TrkA endosomes was monitored by live imaging in the middle grooves of microfluidic chambers (n = 3). A representative kymograph is shown (left panel). The rate of movement of individual Flag-TrkA endosomes in distal axons at the time of nocodazole application and 20 min afterwards was measured (Right panel). Retrograde movement halted ~15 min post-nocodazole application. Scale bar: 20 μm; 5 min. (d,e) Alexa-488-labeled CTB was applied to the cell body compartment of compartmentalized neuronal culture in the presence of nocodazole either in the cell body or distal axon compartment. The endocytic trafficking of CTB-Alexa488 to lysosomes (c) and axonal movement of CTB (d) were assessed. Scale bar: 10 μm (c). 10 μm; 1 min (d). **p<0.01 using a two-tailed paired Student’s t test.
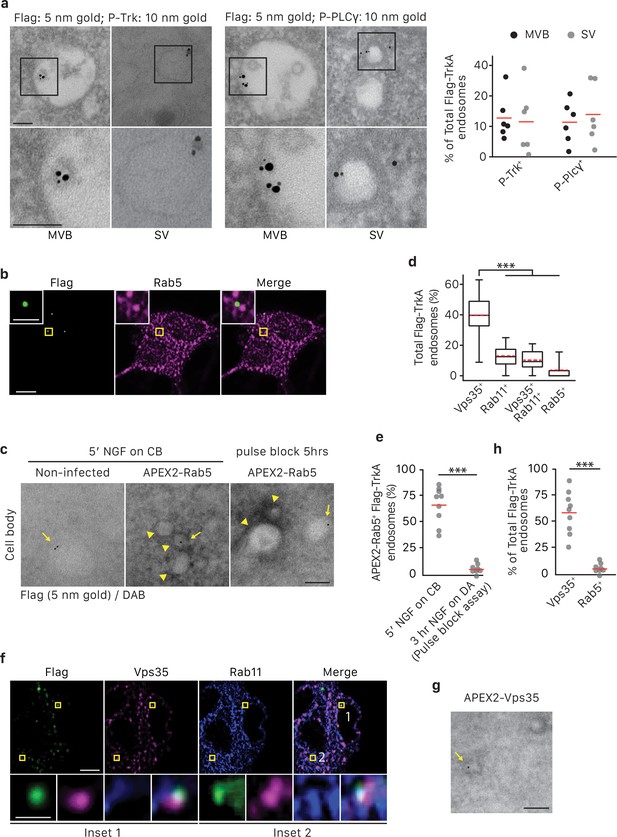
TrkA+ single vesicles formed de novo in cell bodies after retrograde transport are signaling competent and are not Rab5+early endosomes.
(a) The pulse-block assay was performed in compartmentalized sympathetic neurons using pre-conjugated anti-Flag antibody with 5 nm gold secondary antibody. Cells were fixed 5 hr post-NGF application and immunogold-labeled for P-Trk or P-PLCγ (10 nm). Shown are representative images of an MVB and SV containing retrograde Flag-TrkA gold particles that are juxtaposed to P-Trk or P-PLCγ. High magnification images of the boxed areas are shown in the bottom panel. The percentage of these signaling competent MVBs and SVs in cell bodies was quantified (n = 6). Scale bar: 100 nm. (b,d) The pulse-block assay was performed in compartmentalized sympathetic neurons and colocalization between Flag-TrkA (green) and Rab5 (magenta) in cell bodies was assessed 5 hr post-NGF application. Insets show magnification of the boxed areas. Quantification is shown in (d) (n = 4). Scale bar: 5 μm and 1 μm (inset). (c,e) Sympathetic neurons grown in mass culture were infected with a virus expressing APEX2-Rab5. The Flag-TrkA assay was performed using pre-conjugated anti-Flag antibody with Protein A-5 nm gold secondary antibody and cells were fixed 5 min post-NGF stimulation. DAB staining was performed and cells were processed for EM. The extent of gold particles associated with APEX2+ endosomes in cell bodies was assessed. APEX2+ endosomes were identified based on the dark staining associated with endosomal membranes (arrowheads), compared to the lack of contrast in non-infected cells (left panel). In the right panel, the pulse-block assay was performed in compartmentalized sympathetic neurons expressing APEX2-Rab5. Cells were fixed 5 hr post-NGF stimulation and the extent of gold particles resided in APEX2+ endosomes was assessed. Arrows denote endosomes containing Flag-TrkA and arrowheads denote APEX2+ endosomes. Quantification is shown in (e) (n = 3). Scale bar: 100 nm. (f) The pulse-block assay was performed in compartmentalized sympathetic neurons and colocalization between Flag-TrkA (green), Vps35 (magenta) and Rab11 (blue) in cell bodies was assessed at 5 hr post-NGF application. Insets show magnification of the boxed areas. Quantification is shown in (d) (n = 4). Scale bar: 5 μm and 1 μm (inset). (g) The pulse-block assay was performed in compartmentalized sympathetic neurons expressing APEX2-Vps35. Cells were fixed 5 hr post-NGF stimulation and the extent of gold particles resided in APEX2+ endosomes was assessed (n = 3). Shown is a representative EM image. Scale bar: 100 nm. Data are presented in dot plot (a,e,h) or box plot (d). ***p<0.001 by one-way ANOVA with a Tukey’s post-hoc test (d) or a two-tailed unpaired Student’s t test (e,g). See also Figure 6—figure supplement 1.
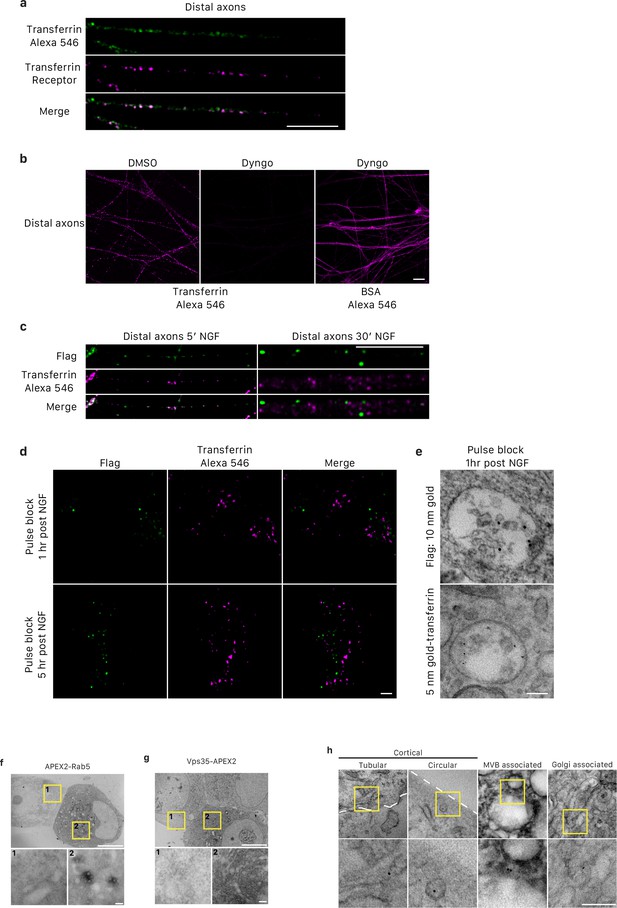
Retrogradely transported transferrin and Flag-TrkA are sorted in distinct MVBs and characterization of TrkA+ single vesicles.
(a) Internalized transferrin-Alexa 546 was co-localized with transferrin receptor in distal axons of compartmentalized sympathetic neurons. Scale: 10 μm. (b) Internalization of transferrin-Alexa 546, but not BSA, in distal axons of compartmentalized sympathetic neurons was abolished with treatment of Dyngo, a dynamin inhibitor, exclusively to distal axons. Scale: 10 μm. (c) Internalized transferrin-Alexa 546 and Flag-TrkA were co-localized in distal axons of compartmentalized sympathetic neurons after 5 min, but not 30 min, NGF stimulation. Scale: 10 μm. (d) Retrogradely transported Flag-TrkA and transferrin-Alexa 546 were not co-localized in cell bodies. Distal axons of compartmentalized sympathetic neurons were incubated with Flag antibody and transferrin-Alexa 546. The pulse-block assay was performed. Cells were fixed 1 hr or 5 hr post-NGF application and assessed by immunostaining. (e) Distal axons of compartmentalized sympathetic neurons were incubated with Flag antibody conjugated to Protein A-10nm gold and transferrin-5nm gold. The pulse-block assay was performed. Cells were fixed 1 hr post-NGF application assessed by EM. (e,f) Characterization of APEX2-Rab5 and APEX2-Vps35. Sympathetic neurons in mass culture were infected with a virus expressing APEX2-Rab5 or APEX2-Vps35. 48 hr post-infection, cells were fixed, subjected to DAB staining and processed for EM. For each construct, a representative EM micrograph is shown with an infected cell and an adjacent non-infected cell. Insets show higher magnification of the boxed areas. No overt abnormality with respect to cellular organization and vesicle morphology and localization was observed. Notice the overall higher contrast exhibited in cells expressing APEX2. APEX2 signals were enriched around endosomal membranes as seen from the images with higher magnification. Scale bar: 5 μm; 100 nm (inset). (g) The pulse-block assay was performed in compartmentalized sympathetic neurons. Cells were fixed 5 hr post-NGF application and processed for EM. Shown are examples of Flag-TrkA+ single vesicles that have distinct morphology, subcellular localization and organelle association. Left to right: a tubular shaped TrkA SV that is close to the plasma membrane (white dashed contour); a circular shaped TrkA SV that is close to the plasma membrane (white dashed contour); a circular shaped TrkA SV that is associated with an MVB; and a tubular shaped TrkA SV that is associated with a Golgi apparatus. Magnified images of boxed areas are shown in the lower panel. Scale bar: 100 nm.
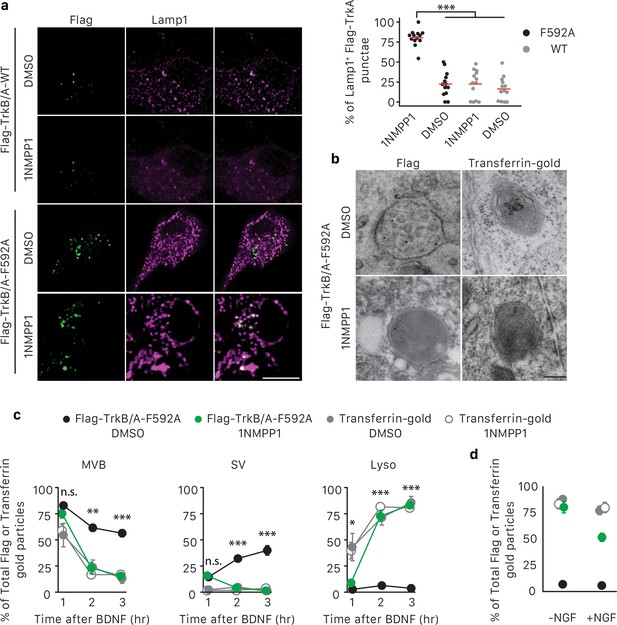
TrkA kinase activity within endosomes regulates maturation and fate of retrograde TrkA+ endosomes.
(a) Compartmentalized WT sympathetic neurons were infected with a virus expressing either Flag-TrkB/A-WT or Flag-TrkB/A-F592A. The pulse-block kinase assay was performed as in Figure 5a, with BDNF instead of NGF stimulation in distal axons and with the cell body compartment treated with DMSO or 500 nM 1NMPP1 during the course of the experiments. Cells were fixed 5 hr post-BDNF application, and colocalization between Flag-TrkA (green) and the lysosome marker Lamp1 (magenta) was assessed (n = 3). Scale bar: 10 μm. (b,c) The pulse-block kinase assay was performed in sympathetic neurons expressing Flag-TrkB/A-F592A using pre-conjugated anti-Flag antibody with Protein A-5 nm gold or a transferrin-gold tracer (6 nm). Cells were fixed at indicated time points and the number of Flag-TrkB/A-F592A or transferrin-gold particles associated with MVBs, SVs and lysosomes was scored (c) (n = 3). Shown in (b) are representative EM micrographs of retrogradely transported Flag-TrkA and transferrin-gold in MVBs or lysosomes at the 3 hr time point. Scale bar: 100 nm. (d) The pulse-block kinase assay was performed as in (b) with either the presence or absence of NGF in the cell body compartment during the course of experiments. The extent of lysosomal Flag-TrkA or transferrin-gold was assessed 3 hr post-BDNF application (n = 3). Data are presented in dot plot (a) or represented as mean ± SEM (c,d). *p<0.05, **p<0.01 and ***p<0.001 by two-way ANOVA with Tukey’s post-hoc test (a) or three-way ANOVA with Tukey’s post-hoc test (c,d). See also Figure 7—figure supplement 1.
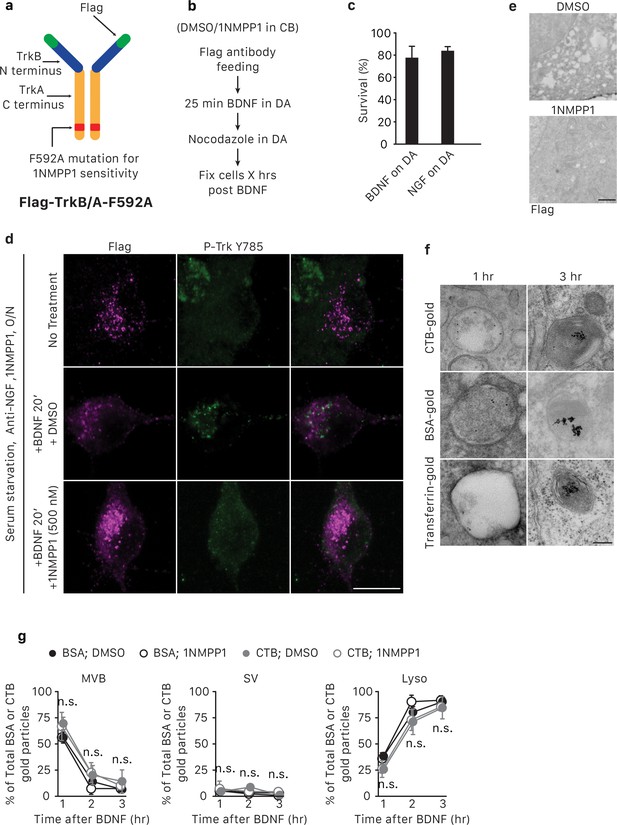
Expression of Flag-TrkB/A-F592A allows specific activation and inhibition of TrkA kinase activity within endosomes.
(a) Schematic of the Flag-TrkB/A-F592A construct. See also Materials and methods. (b) Schematic of the pulse-block kinase assay. See also Materials and methods. (c) Compartmentalized sympathetic neurons expressing Flag-TrkB/A-F592A were cultured in conditions in which distal axons were exposed to BDNF or NGF while the cell body compartment was neurotrophin-deprived. Neuronal survival was assessed 48 hr later. Neurons expressing Flag-TrkB/A-F592A can rely on BDNF applied to distal axons as the sole source of survival signal, indicating that the modified receptor can be activated by BDNF and the retrograde survival signal can be transmitted to cell bodies (n = 3). (d) WT neurons in mass culture expressing Flag-TrkB/A-F592A virus were serum- and neurotrophin-starved overnight and then incubated with no neurotrophin, BDNF and DMSO, or BDNF and 500 nM 1NMPP1. 20 min later, cells were salt/acid stripped and Flag-TrkB/A-F592A internalization and activation was assessed by Flag (magenta) and P-Trk Y785 (green) immunostaining (n = 3). The lack of P-Trk signals in the BDNF/1NMPP1-treated cells suggest that 1NMPP1 treatment is efficacious in inhibiting TrkA F592A kinase activity and BDNF stimulation does not lead to activation of endogenous TrkA receptors, whose kinase activity is 1NMPP1 insensitive. Scale bar: 10 μm. (e) WT neurons in mass culture expressing Flag-TrkB/A-592A were serum- and neurotrophin-starved overnight and subsequently stimulated with BDNF in the presence of DMSO or 1NMPP1 for 20 min. Cells were then fixed and processed for P-Trk Y785 immunogold labeling. 1NMPP1 treatment eliminated the majority of P-Trk signal as seen in the EM micrograph. Scale bar: 1 μm. (f,g) The pulse-block kinase assay was performed in sympathetic neurons expressing Flag-TrkB/A-F592A using CTB-gold, BSA-gold or transferrin-gold (6 nm). Cells were fixed at indicated time points and the number of gold particles associated with MVBs, SVs and lysosomes was scored for each condition (g) (n = 3). Shown in (f) are representative EM micrographs of retrogradely transported CTB-gold, BSA-gold and transferrin-gold in MVBs or lysosomes. Scale bar: 100 nm. Data are represented as mean ± SEM. Statistical analysis was done using one-way ANOVA with a Tukey’s post-hoc test.
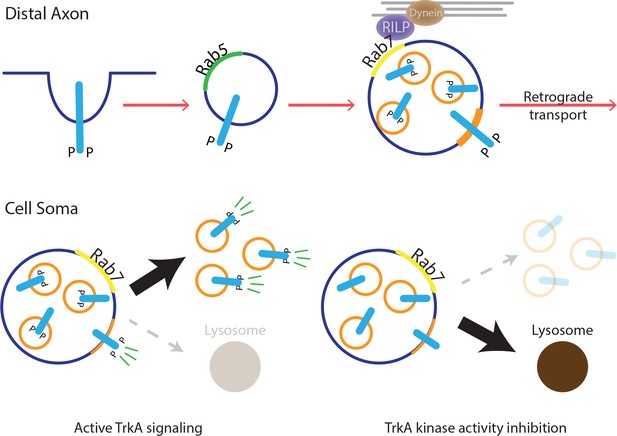
Model for MVB-mediated retrograde TrkA transport and signaling.
This schematic illustrates a model for how multivesicular bodies control propagation of retrograde NGF-TrkA signals. In distal axons, newly internalized NGF/TrkA complexes are sorted into Rab5+ early endosomes, and these TrkA+ early endosomes mature to form multivesicular bodies. Rab7, which localizes to TrkA+ MVBs, facilitates rapid long-range retrograde axonal transport, and in turn, neuronal survival and synapse formation. In neuronal soma, active endosomal TrkA signaling induces de novo formation of single-membrane vesicles from retrogradely transported MVBs and prevents TrkA+ MVBs fusion with lysosomes. Together, these TrkA activity-dependent MVB dynamics promote and sustain transduction of retrograde NGF signals.
Videos
EGFP-Rab5+ TrkA+ endosomes in distal axons are stationary/oscillatory.
Time lapses of EGFP-Rab5 (cyan, bottom panel) and Flag-TrkA (magenta, middle panel) trafficking (merged channel, top panel) in distal axons of sympathetic neurons grown in compartmentalized cultures. Notice a stationary EGFP-Rab5+ Flag-TrkA endosome (#1) and three retrogradely transported EGFP-Rab5- Flag-TrkA endosomes (#2–4). Retrograde is to the right. The video was acquired at 2 fps (frames per second) and played at 15 fps.
EGFP-Rab7+ TrkA+ endosomes display consistent and processive retrograde movement in axons.
Time lapses of EGFP-Rab7 (cyan, bottom) and Flag-TrkA (magenta, middle) trafficking (merged channel, top) in axons in the middle grooves of microfluidic chambers adjacent to the distal axon compartment. Shown are three retrogradely transported EGFP-Rab7+ Flag-TrkA endosome (#1–3). Retrograde is to the right. The video was acquired at 2 fps (frames per second) and played at 15 fps.
CD63-EGFP+ TrkA+ endosomes display consistent and processive retrograde movement in axons.
Time lapses of CD63-EGFP (cyan, bottom) and Flag-TrkA (magenta, middle) trafficking in axons (merged channel, top) in the middle grooves of microfluidic chambers adjacent to the distal axon compartment. Shown are two retrogradely transported CD63-EGFP+ Flag-TrkA endosome (#1–2). Retrograde is to the right. The video was acquired at two fps (frames per second) and played at 15 fps.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Genetic reagent (Mus musculus) | Ntrk1Flag | (Sharma et al., 2010) PMID: 20696380 | Mice were handled and housed in accordance with Harvard Medical School and Johns Hopkins University IACUC guidelines | |
| Genetic reagent (M. musculus) | Th2a-CreER | (Abraira et al., 2017) PMID: 28041852 | ||
| Genetic reagent (M. musculus) | Rab7flox | (Roy et al., 2013) PMID: 23615463 | ||
| Antibody | NeuN | Abcam (Cambridge, MA) | 1:1000 | |
| Antibody | Rab5 | 1:500 | ||
| Antibody | Rab7 | 1:200 for ICC; 1:1000 for immunoblot | ||
| Antibody | Lamp1 | 1:1000 | ||
| Antibody | P-Trk Y490 | Cell signaling(Danvers, MA) | 1:2000; 1:100 for immunoEM | |
| Antibody | P-Trk Y785 | |||
| Antibody | P-PLCγ | 1:500; 1:50 for immunoEM | ||
| Antibody | VAChT | Enzo Life Sciences (Farmingdale, NY) | 1:1000 | |
| Antibody | Homer1 | Synaptic Systems (Germany) | 1:500 | |
| Antibody | Flag | Sigma (St. Louis, MO) | 1 ug/ml | |
| Antibody | Alexa conjugated secondary antibodies (488, 555, 647) | Thermo Fisher Scientific (Waltham, MA) | 1:1000 | |
| Antibody | IgG F(ab’)2–6 nm/10 nm gold secondary antibodies | Aurion (Netherlands) | 1:50 | |
| Antibody | Protein A-5nm/10 nm gold | Made by The Harvard Medical School EM Facility | 1:50 | |
| Recombinant DNA reagent | FUW | (Lois et al., 2002) PMID: 11786607 | Addgene 14882 | |
| Recombinant DNA reagent | APEX2 | (Lam et al., 2015) PMID: 25419960 | Addgene 49385 | |
| Recombinant DNA reagent | FUW-EGFP-Rab5 | This paper | ||
| Recombinant DNA reagent | FUW-EGFP-Rab7 | This paper | ||
| Recombinant DNA reagent | FUW-CD63-EGFP | This paper | ||
| Recombinant DNA reagent | FUW-CD63-mCherry | This paper | ||
| Recombinant DNA reagent | FUW-RILP-EGFP | This paper | ||
| Recombinant DNA reagent | FUW-APEX2-Rab5 | This paper | ||
| Recombinant DNA reagent | FUW-APEX2-Vps35 | This paper | ||
| Recombinant DNA reagent | FUW-Flag-TrkB/A-WT | This paper | The TrkB/A chimeric receptor comprises the extracellular domain of TrkB (nucleotide 1–1242) and the transmembrane and intracellular domains of TrkA (nucleotide 1156–2400) | |
| Recombinant DNA reagent | FUW-Flag-TrkB/A-F592A | This paper | ||
| Sequence-based reagent | Primers for genotypingTh2a-CreER | CATGCCCATATCCAATCTCC and CTGGAGCGCATGCAGTAGTA | ||
| Sequence-based reagent | Primers for genotyping Rab7flox | CTCACTCACTCCTAAATGG and TTAGGCTGTATGTATGTGC | ||
| Sequence-based reagent | shRNAs for Rab7 | GAAGTTCAGTAACCAGTACAA; GCGGCAGTATTCTGTACAGTA; GCCCTTAAACAGGAAACAGAA; TGAACCCATCAAACTGGACAA; TGCTGTGTTCTGGTGTTTGAT | ||
| Sequence-based reagent | shRNAs for RILP | CAGCTATGCAGGAGGCTTAAC; AGATCAAGGCCAAGATGTTAG; CCAGAATTTCTTTGGCTTATG; TTCAGCAGGGAAGAGCTTAAG; AGGAGCGGAATGAGCTCAAAG | ||
| Sequence-based reagent | Scrambled shRNA | CCTAAGGTTAAGTCGCCCTCG | ||
| Chemical compound, drug | K252a | EMD Millipore (Billerica, MA) | ||
| Chemical compound, drug | 1NMPP1 | |||
| Chemical compound, drug | Nocodazole | |||
| Chemical compound, drug | Saponin | MP Biomedicals (Santa Ana, CA) | ||
| Chemical compound, drug | 3.3’-Diaminobenzidine (DAB) | |||
| Chemical compound, drug | 6 nm gold-conjugated transferrin, CTB, BSA | Electron Microscopy Sciences (Hatfield, PA) |
Additional files
-
Transparent reporting form
- https://doi.org/10.7554/eLife.33012.020





