Connectomics of the zebrafish's lateral-line neuromast reveals wiring and miswiring in a simple microcircuit
Figures
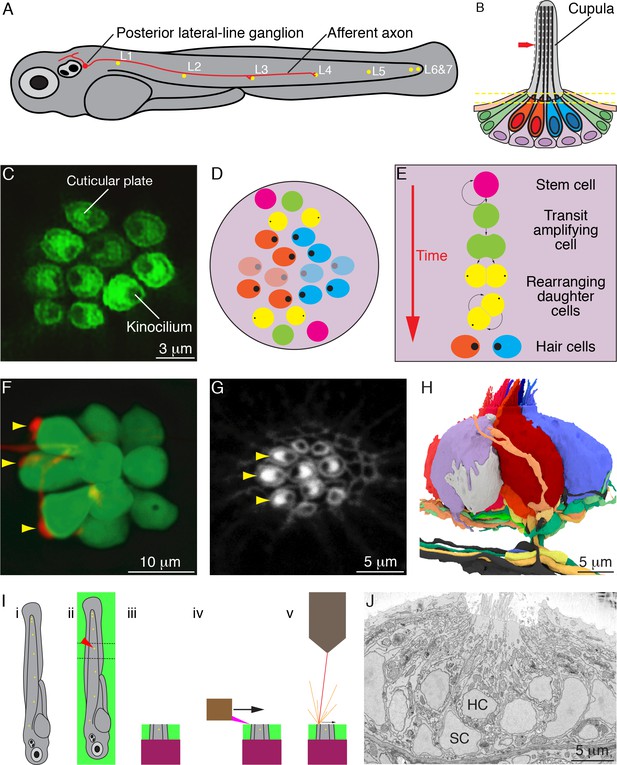
Organization of neuromasts and their innervation.
(A) A schematic diagram depicts the posterior lateral line of the larval zebrafish. While migrating from the ear to the tip of the tail, the first lateral-line primordium deposits about seven neuromasts (yellow, L1 through L6 and 7). The hair cells of these neuromasts are uniformly polarized along the anteroposterior axis; a second primordium subsequently deposits a few neuromasts (not shown) with a dorsoventral polarity. Individual neurons of the posterior lateral-line ganglion, one of which is portrayed, are innervated by hair cells in one to five successive neuromasts and convey information into the brainstem. (B) A schematic diagram of an individual neuromast shows a cluster of hair cells surmounted by a gelatinous cupula into which their hair bundles insert. When water movement (arrow) deflects the cupula, the long kinocilia are bent and their motion is communicated to and transduced by the stereocilia. Half of the hair cells (red) are polarized for sensitivity to caudad movement, the other half (blue) to rostrad motion. The hair cells are separated by supporting cells (lavender) and bounded by mantle cells (green). (C) A high-resolution, deconvolved fluorescence image shows the apical surfaces of hair cells in the plane of section indicated by parallel dashed lines in panel (B). The cuticular plate at the apex of each hair-cell soma is marked by actin-GFP. The dark spot within each cuticular plate represents the base of the kinocilium, which denotes the hair bundle's polarity. (D) A schematic diagram depicts the organization of hair cells in a neuromast of the posterior lateral line. There are roughly equal numbers of hair cells sensitive to caudad water motion (red) and to rostrad movement (blue). As hair cells migrate toward the neuromast's equator (pale cells), they senesce and die. (E) Uncharacterized stem cells located near the apical and basal poles of the neuromast produce transit-amplifying cells, each of which divides into daughter cells that undergo a rotatory rearrangement in about half the instances. After rearrangement has concluded, the nascent hair cells in wild-type larvae always adopt opposite polarities. (F) An individual afferent nerve fiber marked with mCherry forms postsynaptic endings (arrowheads) on three hair cells. (G) An image of the apical surface of the same neuromast shows phalloidin-labeled cuticular plates and hair bundles. Consistent with polarity-specific innervation, the three hair cells contacted by the fiber in panel (F) all display polarization to caudad stimuli. (H) A reconstruction of the innervation of a single neuromast includes afferent terminals, which receive synaptic input from sensory hair cells, and an efferent terminal that innervates hair cells. Details are provided in the caption of Video 2. (I) Steps in serial blockface scanning electron microscopy (SBFSEM) of a lateral-line neuromast. (i). A larva is preserved in aldehyde-based fixative and impregnated with heavy metal to enhance the specimen's contrast and electrical conductivity. (ii) The specimen is embedded in plastic and cut (dotted lines) to isolate the region containing the neuromast of interest (arrowhead). (iii) The trimmed specimen is secured to a mount and placed in the scanning electron microscope. (iv) A diamond knife makes hundreds to thousands of passes across the blockface, progressively scraping away tens of nanometers of the specimen. (v) After each passage of the knife, an electron beam scans the blockface; the emitted secondary electrons are collected to form an image of the sectioned specimen. (J) A typical serial blockface scanning electron micrograph shows several hair cells, whose nuclei (HC) occur in a layer above that encompassing supporting-cell nuclei (SC).
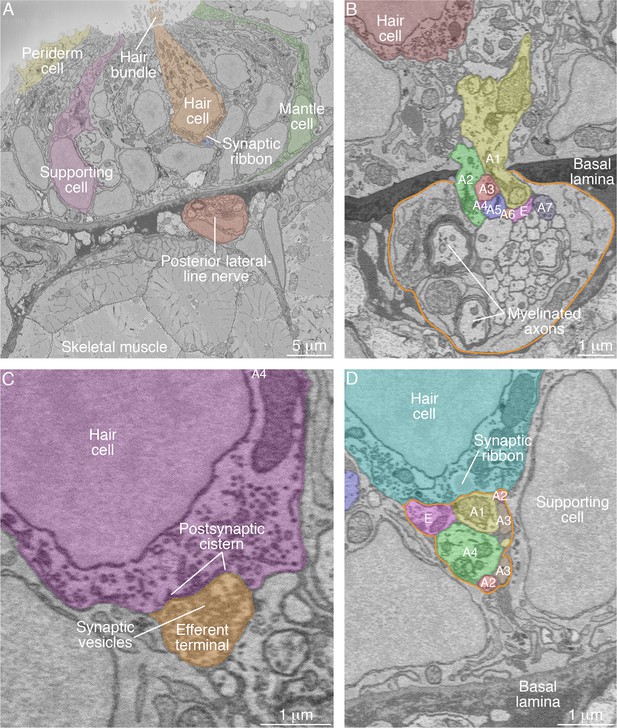
SBFSEM images of neuromast components.
(A) A low-power micrograph shows the general organization of a neuromast. The sensory organ lies between skeletal-muscle fibers, from which it is separated by the epithelial basal lamina, and the aqueous environment, into which the hair bundles protrude. The nuclei of hair cells form a layer above those of supporting cells. (B) The posterior lateral-line nerve (outlined in orange) contains two myelinated axons as well as numerous unmyelinated fibers. Seven afferent terminals, each denoted as ‘A’ and distinctively colored and numbered, arise as single branches of axons in the nerve and enter the neuromast through a pore in the epithelial basal lamina. One efferent axon labeled ‘E’ is also present. (C) The synaptic terminal of an efferent axon onto a hair cell displays a vesicle-filled bouton. Within the hair cell's cytoplasm lies a cistern that sequesters the Ca2+ that enters the cell during synaptic activity. (D) A perisynaptic compartment (outlined in orange) contains four afferent terminals, each denoted as ‘A’ and numbered, and a single efferent terminal labeled ‘E.’ The compartment is demarcated by two supporting cells. The hair cell makes an afferent synapse onto the axonal terminal A1. A synaptic ribbon in the hair cell is characterized by moderate electron density and a clear halo surrounded by synaptic vesicles. The ribbon is readily distinguished from the numerous mitochondria in all cell types. In each of the panels, the areas within some of the cellular contours delineated by annotators have been colored to emphasize specific cells or axonal terminals. In order to provide a broad color gamut, the scheme of coloration here is arbitrary and distinct from that in the other figures.
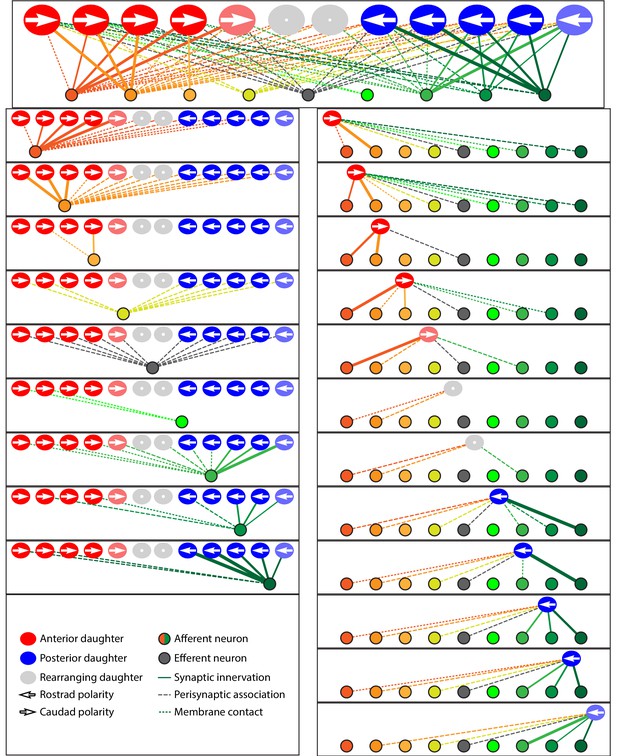
Microcircuit connectivity of the neuromast in wild-type zebrafish.
The diagram displays the complete set of associations between hair cells and axonal terminals within an individual neuromast (WT1), including ribbon synapses (solid lines), membrane contacts (coarsely dotted lines), and perisynaptic associations (finely dotted lines). Hair cells are divided into two groups according to their anteroposterior positions following rearrangement: more anterior hair cells are red, more posterior hair cells are blue. The gray cells shown here and in supplemental diagrams represent hair cells 0–5 hr post-mitosis, which have not developed a definite polarity. White arrows define the hair-bundle orientations. Both subpopulations are further ordered from left to right by age, with those greater than 15 hr post-mitosis lying to the left of those 5–15 hr post-mitosis and bearing a darker hue of red or blue. Sibling cells occupy the same left-to-right positions within their respective populations. Axonal terminals are ordered from left to right based upon whether they form ribbon synapses predominantly with the more anteriorly positioned or the more posteriorly positioned hair-cell subpopulation. The terminals of a single efferent neuron are colored dark gray, whereas the other terminals represent afferents. The top panel depicts the full set of associations constituting the neuromast's microcircuit. The left column depicts separately the set of associations for each axonal terminal, whereas the right column depicts the associations of each hair cell. The lines representing terminals making ribbon synapses are graded in width in uniform steps representing from one to six synapses.
-
Figure 3—source data 1
The tables provide a comprehensive set of measurements and calculations involving the data included in the main text and in Figure 3—figure supplement 1.
The data set includes eight wild-type neuromasts (WT1-WT8).
- https://doi.org/10.7554/eLife.33988.007
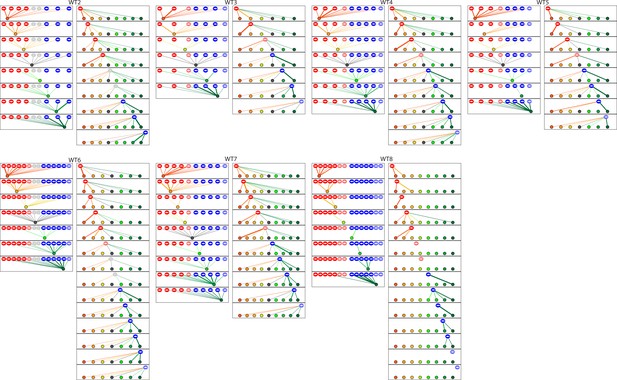
The diagrams depict the complete set of associations between hair cells and axonal terminals from seven additional wild-type neuromasts (WT2-8).
Owing to technical problems that precluded precise measurements, data set WT8 lacks information about membrane contacts and perisynaptic associations. The arrangement and symbols are identical to those of Figure 3.
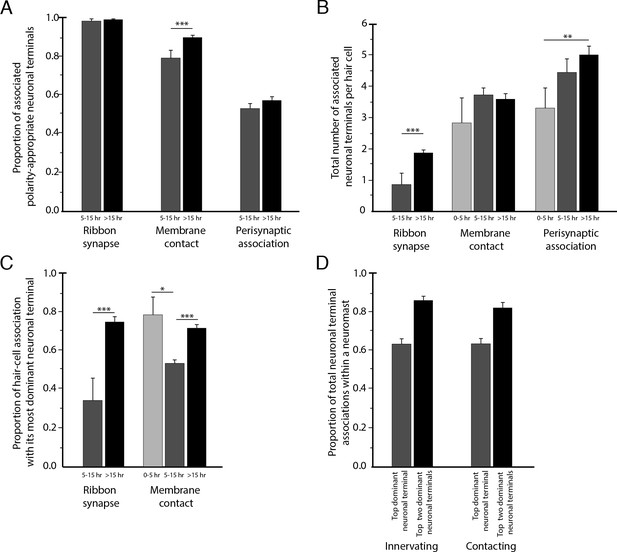
Statistical characteristics of the neuromast connectome.
(A) A histogram depicts the proportion of ribbon synapses, membrane contacts, and perisynaptic associations formed with axonal terminals of the appropriate polarity for hair cells of 5–15 hr post-mitosis and for more than 15 hr post-mitosis. (B) A histogram depicts the total number of axonal terminals per hair cell that form ribbon synapses, membrane contacts, or perisynaptic association for hair cells of 0–5 hr, 5–15 hr, or more than 15 hr post-mitosis. (C) For each hair cell, the axonal terminal receiving the greatest number of ribbon synapses and making the largest area of contact with hair cells was designated as the dominant neuronal arbor. The histogram displays the proportion of a hair cell’s ribbon synapses and membrane contacts associated with that terminal for hair cells of 0–5 hr, 5–15 hr, or more than 15 hr post-mitosis. (D) For each neuromast, neuronal arbors were ranked by the proportion of total ribbon synapses formed with the polarity-specific subpopulation. The histogram shows the proportion of ribbon synapses and afferent membrane contacts within a neuromast provided by the most dominant neuronal arbor and by the two less dominant arbors.
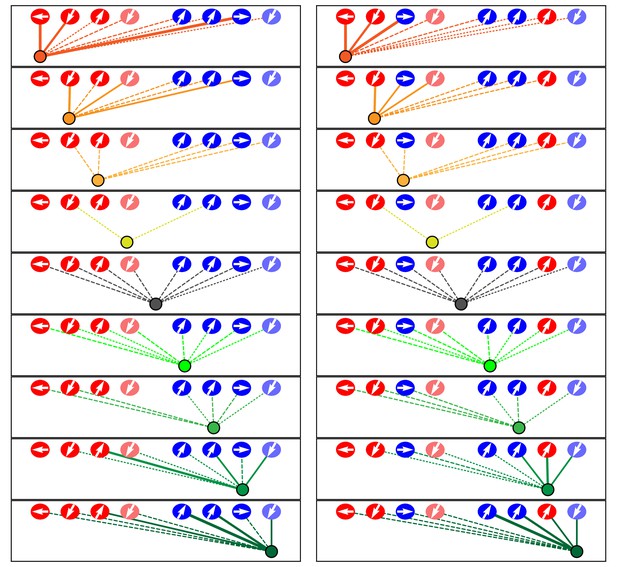
Microcircuit connectivity of a neuromast in a trilobite zebrafish.
The diagram displays the complete set of associations between neuronal arbors and hair cells in a trilobite neuromast (T1). Note the erratic orientations of the hair bundles' axes of mechanosensitivity in this mutant of the planar-cell-polarity pathway. In the left column, the arrangement and symbols are identical to those of Figure 3. Hair cells are divided into two groups according to their anteroposterior positions following rearrangement: more anterior hair cells are red, more posterior hair cells are blue. In the right column, the same data are shown after repositioning of a single pair of hair cells known to be siblings to show that the innervation pattern still consists of two mutually exclusive set of neurons, although hair-cell polarities are randomized. The lines representing terminals making ribbon synapses are graded in width to represent one or two synapses.
-
Figure 5—source data 1
The tables provide a comprehensive set of measurements and calculations involving the data included in the main text and in Figure 5—figure supplement 1.
The data set includes four trilobite neuromasts (T1–T4).
- https://doi.org/10.7554/eLife.33988.012
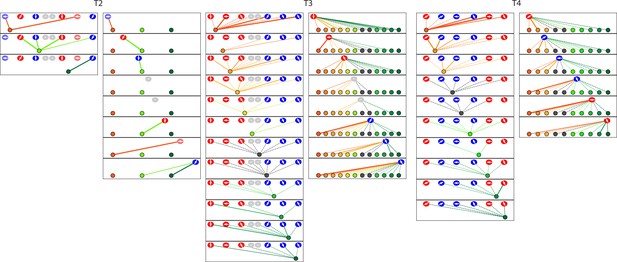
The diagrams depict the complete set of associations between hair cells and axonal terminals from three additional neuromasts (T2–T4) of trilobite mutants.
Owing to technical problems that precluded precise measurements, data set T2 lacks information about membrane contacts and perisynaptic associations. The arrangement and symbols are identical to those of Figure 3.
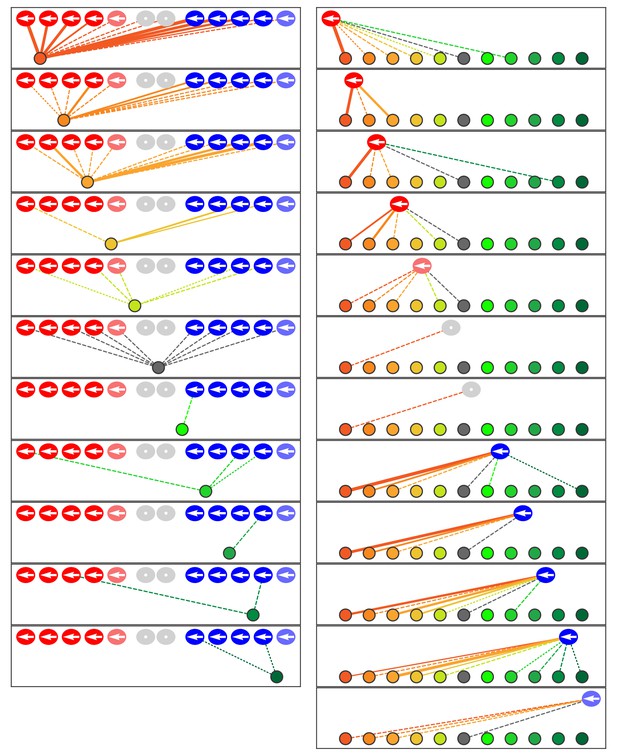
Microcircuit connectivity of the neuromast in Notch-overexpression mutant zebrafish.
The diagram portrays the complete set of associations between axonal terminals and hair cells in a neuromast (N1) from a Notch-overexpression transgenic larva. Note the uniform polarization of the hair bundles for mechanosensitivity to rostrad stimuli. The arrangement and symbols are identical to those of Figure 3. Hair cells are divided into two groups according to their anteroposterior positions following rearrangement: more anterior hair cells colored in reddish shades while more posterior hair cells are blue. Because nascent hair cells 0–5 hr post-mitosis can be assigned neither a stable anteroposterior position nor a distinct hair-bundle orientation, they are marked as grey and located between the two hair-cell subpopulations. The lines representing the terminals making ribbon synapses are graded in width to represent from one to three synapses.
-
Figure 6—source data 1
The tables provide a comprehensive set of measurements and calculations involving the data included in the main text and in Figure 6—figure supplement 1.
The data set includes two Notch-overexpression neuromasts (N1 and N2).
- https://doi.org/10.7554/eLife.33988.016
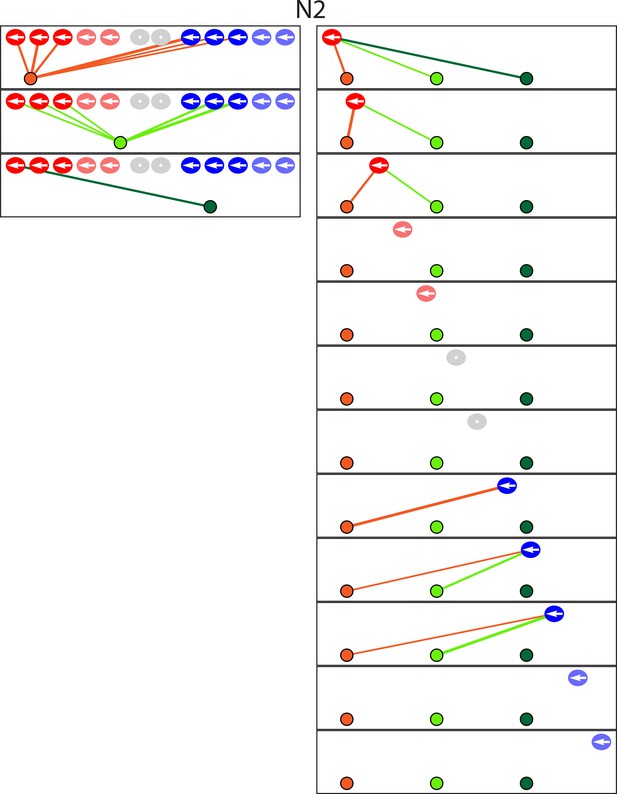
The diagram depicts the complete set of associations between hair cells and axonal terminals from one additional neuromast (N2) of a Notch-overexpression transgenic animal.
Owing to technical problems that precluded precise measurements, this data set lacks information about membrane contacts and perisynaptic associations. Terminals that do not form synaptic associations are excluded from the diagram. The arrangement and symbols are identical to those of Figure 3.
Videos
Relation of preimaging by confocal fluorescence microscopy to SBFSEM images.
The initial portion of the video depicts 31 hr of preimaging by confocal fluorescence microscopy of a wild-type neuromast (WT4). Each hair cell expresses green-fluorescent protein and is denoted by a uniquely colored dot. Note the extension of dynamic processes from the newly formed hair cells at the upper and lower poles of the neuromast, an activity associated with the formation of ribbon synapses. At the conclusion of preimaging, the specimen was placed in fixative solution prior to subsequent SBFSEM preparation and imaging. As shown at the conclusion of the video, there is clear correspondence between the preimaged hair cells and their representations in the resultant SBFSEM data. Dynamic processes are apparent on the bases of the white and especially of the gray hair cell. In order to provide a broad color gamut, the scheme of coloration here is arbitrary and distinct from that in the other figures.
Animated reconstruction of a wild-type neuromast.
This neuromast (WT1) contains ten mature hair cells with hair bundles as well as a pair of nascent hair cells (lavender and gray) that have completed their rearrangement but not yet separated and formed hair bundles. Lateral views demonstrate the beveled hair bundles with stout kinocilia at their tall edges. Hair cells are colored according to their anteroposterior positions following rearrangement: more anterior hair cells in reddish shades and more posterior hair cells in blue. The terminals that form ribbon synapses with the former are delineated in various hues of yellow and orange and those associated with the latter are shaded green and blue. The single efferent terminal is black. All the axons extend their terminal processes though a single orifice in the basal lamina, which is colored purple. The spaces between the hair cells, and those around which the axonal terminals are arrayed, represent the positions of unseen supporting cells. In the second half of the sequence all the hair cells save one are removed to provide a view of the pattern of innervation, which is then further restricted to show the single dominant terminal.
Animated reconstruction of a trilobite mutant neuromast.
This neuromast from a trilobite mutant specimen (T1) contains eight hair cells and eleven axonal terminals. The upper portions of the hair bundles were lost owing to fracture of the specimen block, but preimaging by confocal fluorescence microscopy sufficed for the assignment of bundle polarities. Hair cells are colored according to their anteroposterior positions following rearrangement: more anterior hair cells in reddish shades and more posterior hair cells in blue. The single efferent terminal is black. The basal lamina is colored purple. The spaces between the hair cells, and those around which the axonal terminals are arrayed, represent the positions of unseen supporting cells. In the second half of the sequence all the hair cells save one are removed to provide a view of the pattern of innervation, which is then further restricted to show the single dominant terminal. Occasional discontinuities in the terminals are an artifact of three-dimensional rendering rather than signifying an absence of data.
Animated reconstruction of a neuromast from a Notch-overexpression transgenic animal.
This neuromast from a Notch-overexpresion larva (N1) contains ten mature hair cells and one pair of immature hair cells along with eleven axonal terminals. Hair cells are colored according to their anteroposterior positions following rearrangement: more anterior hair cells in reddish shades and more posterior hair cells in blue. The terminals that form ribbon synapses with the former are delineated in various hues of orange and those associated with the latter are shaded green. The single efferent terminal is black. The spaces between the hair cells, and those around which the axonal terminals are arrayed, represent the positions of unseen supporting cells. In the second half of the sequence all the hair cells save one are removed to provide a view of the pattern of innervation, which is then further restricted to show the single dominant terminal. Occasional discontinuities in the terminals are an artifact of three-dimensional rendering rather than signifying an absence of data.
Additional files
-
Transparent reporting form
- https://doi.org/10.7554/eLife.33988.018





