Glucocorticoid receptor-mediated amygdalar metaplasticity underlies adaptive modulation of fear memory by stress
Figures
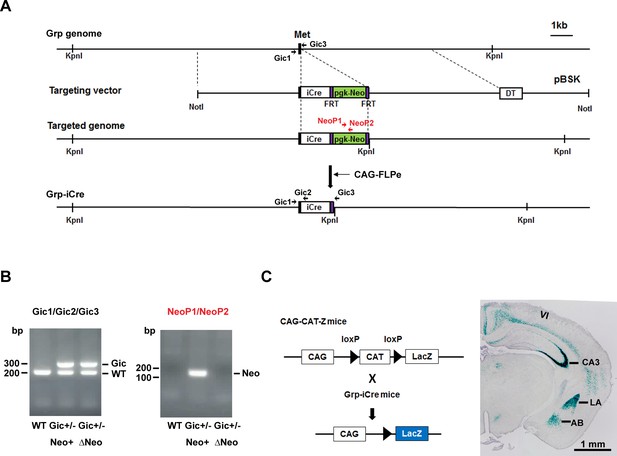
Generation and characterization of Grp-iCre mice.
(A) Schematic diagram of the gene targeting strategy. iCre and pgk-neo cassettes flanked by two FRT sites were inserted into the gastrin-releasing peptide gene (Grp) locus. Met is the translation initiation site of Grp. The location of PCR primers (Gic1, Gic2, Gic3, NeoP1, and NeoP2) used for genotyping are indicated. DT, diphtheria toxin gene; pBSK, pBluescriptII SK. The chimeric mouse obtained was crossed with a CAG-FLPe mouse to delete the pgk-neo cassette and establish the Grp-iCre (Gic) mouse line. (B) Genotyping PCR of genomic DNA prepared from WT; Gic+/−, Neo+; Gic+/−, and ΔNeo mice. (C) Cre activity in Grp-iCre mice was examined by crossing Grp-iCre mice with lacZ reporter mice (CAG-CAT-Z) mice. β-galactosidase expression in a Grp-iCre/CAG-CAT-Z mouse brain stained with X-gal. X-gal staining revealed robust Cre-loxP recombination in the lateral nucleus of the amygdala (LA) and the hippocampal CA3 region, with sparser recombination in the accessary basal nucleus of the amygdala (AB), and in layer 6 of the cerebral cortex (VI).
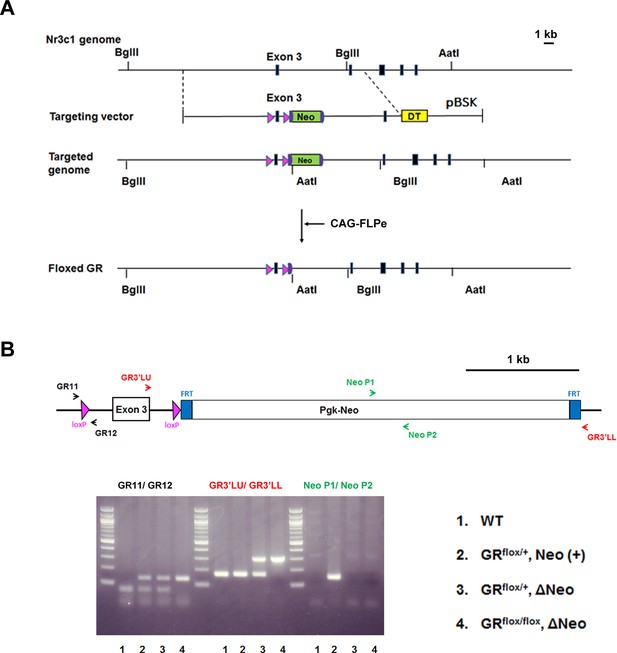
Generation of floxed GR mice.
(A) Strategy for targeting the GR (Nr3c1) gene locus. The coding regions of GR exons are indicated by closed boxes. The construct included two loxP sites and the pgk-neo genes flanked by two FRT sites. DT, diphtheria toxin gene; pBSK, pBluescriptII SK; GR, glucocorticoid receptor. The chimeric mice obtained were crossed with CAG-FLP mice to delete the pgk-neo cassette and establish a floxed GR mouse line. (B) Genotyping PCR of genomic DNA prepared from WT, GRflox/+, Neo (+), GR flox/+, ΔNeo, and GR flox/flox, ΔNeo mice. The locations of PCR primers used are indicated.
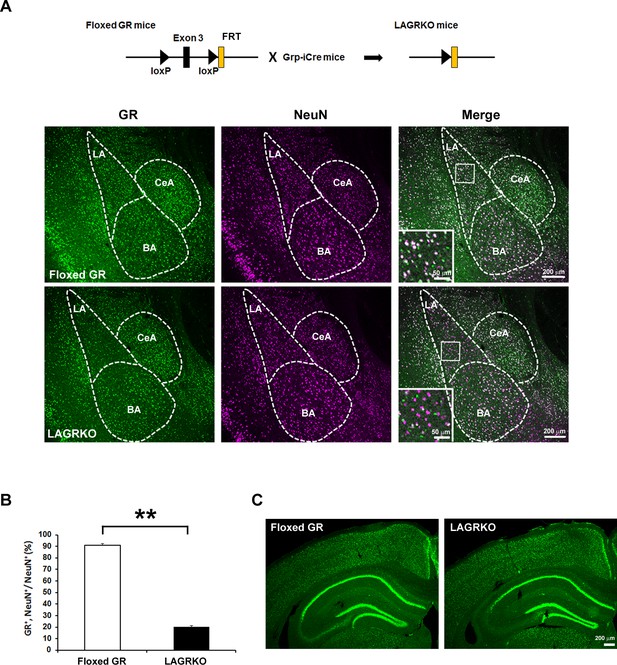
Generation and characterization of lateral amygdala (LA)-selective glucocorticoid receptor (GR) knockout (LAGRKO) mice.
(A) The LAGRKO mouse line (GRflox/flox, Grp-iCre+/-) was established by crossing floxed GR (GRflox/flox) and Grp-iCre mice. Double immunofluorescence staining of GR (green, left panels) and NeuN (magenta, middle panels) in coronal brain sections from floxed GR and LAGRKO mice. The overlap of green and magenta signals (white, right panels) indicates the expression of GR in LA neurons in floxed GR mice (upper), which was apparently reduced in LAGRKO mice (lower). Magnified images of the boxed areas are shown in the insets. LA, lateral nucleus of the amygdala; BA, basal nucleus of the amygdala; CeA, central nucleus of the amygdala. (B) Quantification of GR+ and NeuN+ cells in the LA of floxed GR and LAGRKO mice (n = 9 sections from three mice). Data are presented as mean ± S.E.M. **p<0.001. (C) Expression of GR in the cerebral cortex and hippocampus.
-
Figure 2—source data 1
Raw data for generating Figure 2B.
- https://doi.org/10.7554/eLife.34135.006
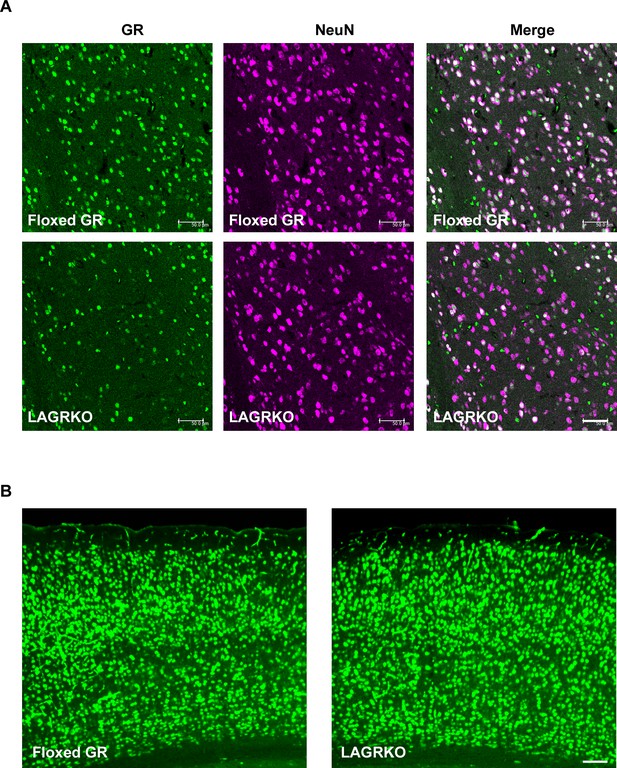
Expression of GR in the LA and cerebral cortex of floxed GR and LAGRKO mice.
(A) Double immunofluorescence staining of GR (green, left panels) and NeuN (magenta, middle panels) in the LA. The overlap of green and magenta signals (white, right panels) indicates the expression of GR in neurons. Scale bar = 50 μm. (B) Expression of GR in the cerebral cortex. Scale bar = 100 μm.
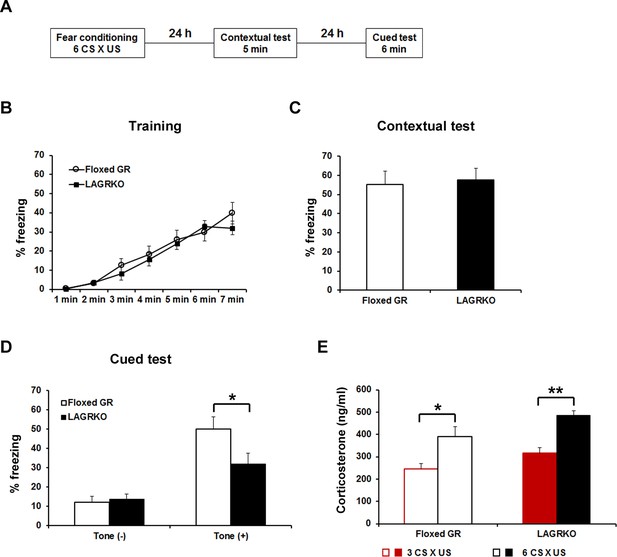
Genetic disruption of LAGR impairs auditory-cued fear memory following strengthened conditioning.
(A) Experimental protocol for fear conditioning. Mice were trained with a protocol using six CS × US pairings. Contextual and auditory-cued fear memory were tested 24 and 48 hr after training, respectively. Freezing levels during the training (B), contextual test (C), and cued test (D). During the cued test, LAGRKO mice (n = 10) exhibited significantly lower freezing levels in the presence of the tone than did floxed GR mice (n = 10). There was no significant difference in freezing levels between the two genotypes during the training and contextual test. (E) Plasma corticosterone levels 90 min after training were significantly higher in mice conditioned with six CS × US pairings (n = 7) than in mice conditioned with 3 CS × US pairings (n = 6) in both genotypes. Data are presented as mean ± S. E. M. *p<0.05, **p<0.001.
-
Figure 3—source data 1
Raw data for generating Figure 3B–E.
- https://doi.org/10.7554/eLife.34135.010
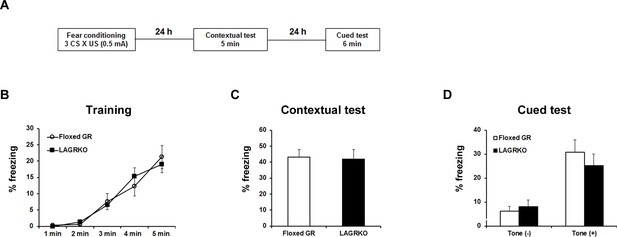
LAGRKO mice did not exhibit fear memory deficits with the moderate fear conditioning protocol.
(A) Experimental design for fear conditioning. Mice were trained with three CS × US pairing protocol. Contextual and auditory fear memories were tested 24 and 48 hr after training, respectively. Freezing levels during the training (B), contextual test (C), and cued test (D). Data are presented as mean ± S. E. M. Floxed GR, n = 10; LAGRKO, n = 16. Data are presented as mean ± S. E. M.
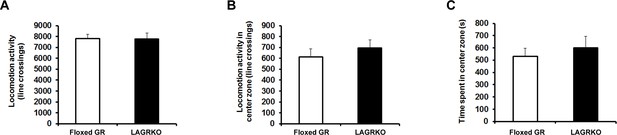
LAGRKO mice exhibited no changes in locomotor activity and anxiety level as determined using the open field test.
Distance of travel throughout the apparatus (A) and in the center zone of the open field (B) and time spent in the center of the open field (C) did not differ between the two genotypes. Floxed GR, n = 15; LAGRKO, n = 10. Data are presented as mean ± S. E. M.
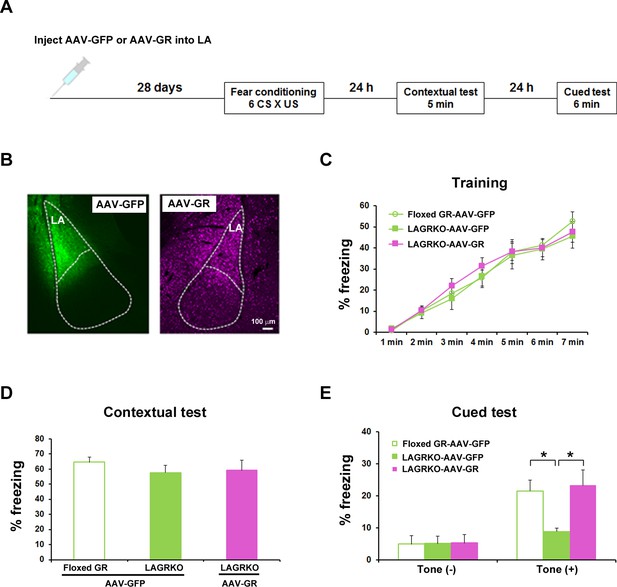
Rescue of the auditory-cued fear memory deficit in LAGRKO mice by restoring GR expression levels in the LA.
(A) The experimental protocol for adeno-associated virus (AAV) vector injection and fear conditioning. (B) Representative images of the expression of green fluorescent protein (GFP, green) and GR (magenta) in the LA of LAGRKO mice injected with AAV-GFP or AAV-GR. The expression levels of GFP and GR were assessed after the behavioral test was completed. Freezing levels during the training (C), contextual test (D), and cued test (E). During the cued test, LAGRKO mice (n = 9) injected with AAV-GR and floxed GR injected with AAV-GFP exhibited significantly higher levels of freezing in the presence of the tone than did AAV-GFP-injected LAGRKO mice (n = 9). There were no significant differences between the three groups during the training and contextual tests. Data are presented as mean ± S. E. M. *p<0.05.
-
Figure 4—source data 1
Raw data for generating Figure 4B–E.
- https://doi.org/10.7554/eLife.34135.012
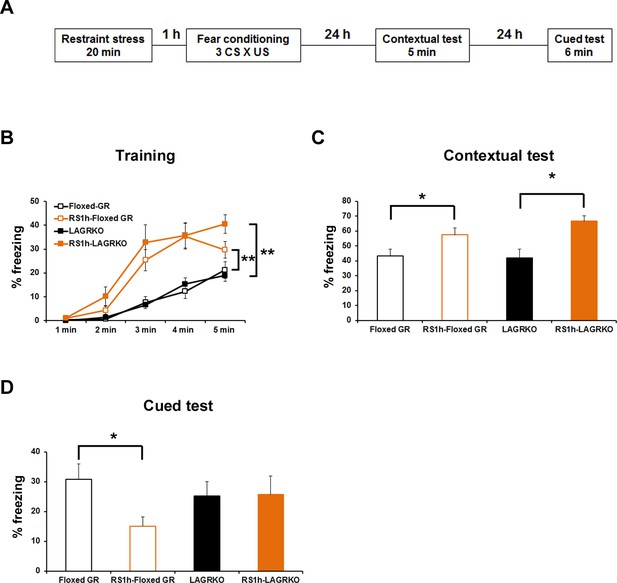
Effect of acute prior stress on auditory-cued fear conditioning.
(A) The experimental design for prior restraint stress (RS) exposure and fear conditioning. Floxed GR and LAGRKO mice were exposed to a 20 min RS and fear-conditioned 1 hr later. Contextual and auditory-cued fear memories were tested 24 and 48 hr after training, respectively. (B) Mice exposed to RS 1 hr before conditioning (brown, RS1h-Floxed GR, n = 11; RS1h-LAGRKO, n = 10) exhibited significantly higher freezing levels than did nonstressed mice (black, Floxed GR, n = 10; LAGRKO, n = 16) during the training session in both genotypes. (C) Mice exposed to RS exhibited significantly higher freezing levels than did nonstressed mice during the contextual fear memory test. (D) During the cued test, floxed GR mice exposed to RS 1 hr before training exhibited significantly lower freezing levels than did the nonstressed floxed GR mice. Previous RS exposure had no effect on auditory-cued fear memory in LAGRKO mice. Data are presented as mean ± S. E. M. *p<0.05; **p<0.001.
-
Figure 5—source data 1
Raw data for generating Figure 5B–D.
- https://doi.org/10.7554/eLife.34135.015
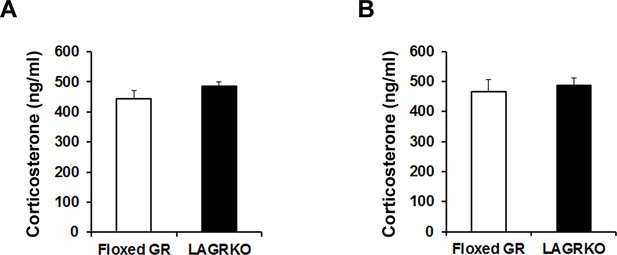
Restraint stress- and auditory fear conditioning-induced release of corticosterone was comparable between floxed GR and LAGRKO mice.
(A) Plasma corticosterone levels 1 hr after RS exposure (n = 5). (B) Plasma corticosterone levels 90 min after RS exposure followed by AFC (n = 5). Data are presented as mean ± S. E. M.
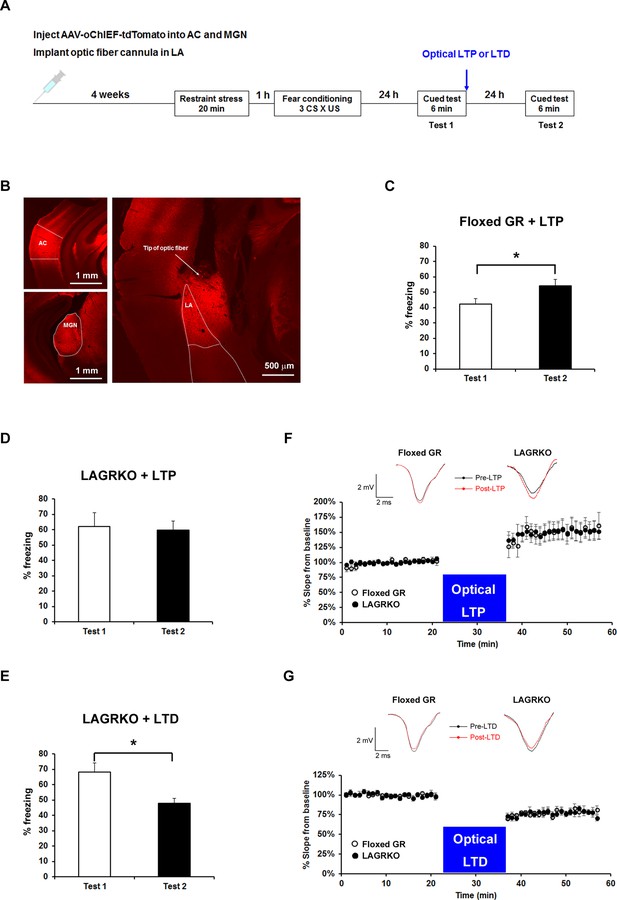
Optogenetic manipulation of LTP or LTD at auditory inputs to the LA alters the effect of acute stress on auditory-cued fear memory.
(A) Experimental schedule for AAV injection, RS exposure, auditory-cued fear conditioning, and optical LTP or LTD induction. (B) Representative fluorescence image of areas expressing AAV-oChIEF-tdTomato 4 weeks after virus injection in the medial geniculate nucleus (MGN) and auditory cortex (AC). LA, lateral nucleus of the amygdala. (C) Floxed GR mice (n = 8) that received immediate optical LTP after the first auditory-cued fear memory test (Test 1) exhibited significantly increased freezing levels in the second auditory-cued fear memory test (Test 2). (D) LAGRKO mice (n = 8) that received optical LTP stimulation exhibited the same freezing levels in Tests 1 and 2. (E) LAGRKO mice (n = 8) that received LTD stimulation immediately after Test 1 exhibited significantly lower freezing levels in Test 2 than in Test 1. Graphs show the freezing rate during the first 1 min of tone presentation at Tests 1 and 2. (F, G) A plot of the average of field EPSP slopes (normalized to the period before optical stimulation) before and after delivering an optical LTP (Floxed GR, n = 4, before LTP, 100 ± 0.8%, after LTP, 148.9 ± 3.2%; LAGRKO, n = 4, before LTP, 100 ± 0.6%, after LTP, 149.3 ± 2.8%) or LTD (Floxed GR, n = 4, before LTD, 100 ± 0.5%, after LTD, 77.4 ± 0.8%; LAGRKO, n = 4, before LTD, 100 ± 0.6%, after LTD, 76.7 ± 0.8%). Data are presented as mean ± S. E. M. *p<0.05.
-
Figure 6—source data 1
Raw data for generating Figure 6C–E.
- https://doi.org/10.7554/eLife.34135.018
-
Figure 6—source data 2
Raw data for generating Figure 6F,G.
- https://doi.org/10.7554/eLife.34135.019
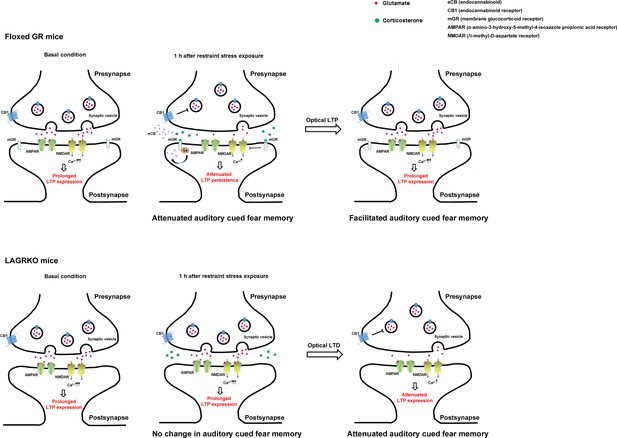
A putative model of LAGR-dependent mechanisms underlying alterations in synaptic plasticity during fear conditioning in basal condition or 1 hr after RS exposures.
In floxed GR mice, RS-induced release of corticosterone activates G protein-coupled postsynaptic membrane glucocorticoid receptors (mGRs), leading to the synthesis and release of endocannabinoid. The released endocannabinoid binds to the presynaptic CB1 endocannabinoid receptor to suppress the glutamate release. In addition, postsynaptic mGRs activation rapidly inhibit Ca2+ influx via the NMDA receptor. Therefore, when fear conditioning is conducted 1 hr after RS exposure, the reduced glutamate release and postsynaptic calcium entry may impair the persistence of LTP in the LA, thereby leading to suppression of auditory-cued fear memory, which can be rescued by delivery of an optical LTP protocol in the LA. In LAGRKO mice, which lack mGRs, RS exposure presumably fails to alter LTP persistence. Therefore, stressed-LAGRKO mice exhibit strong auditory-cued fear memory comparable to non-stressed LAGRKO mice, which can be suppressed by delivery of an optical LTD protocol in the LA.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers |
|---|---|---|---|
| Gene (Mus musculus) | Nr3c1 | Mouse Genome Informatics http://www.informatics.jax.org/marker/MGI:95824 | MGI:95824; NCBI Gene: 14815 |
| Gene (Mus musculus) | Grp | Mouse Genome Informatics http://www.informatics.jax.org/marker/MGI:95833 | MGI:95833; NCBI Gene: 225642 |
| Strain (C57BL/6-Tg(CAG-flpe) 36Ito/ItoRbrc) | CAG-FLPe mouse | RIKEN BioResource Center | RRID:IMSR_RBRC01834 |
| Strain (C57BL/6) | CAG-CAT-Z mouse | Proc Natl Acad Sci U S A 92: 160–164,1995 | |
| ES cell line | RENKA | Eur. J. Neurosci. 24: 2177–2190, 2006 | |
| Antibody | Rabbit anti-GR antibody | Santa Cruz Biotechnology Cat# sc-8992 | RRID:AB_2155784 |
| Antibody | Anti-NeuN antibody | Millipore Cat# MAB377 | RRID:AB_2298772 |
Additional files
-
Transparent reporting form
- https://doi.org/10.7554/eLife.34135.020





