Serine is the major residue for ADP-ribosylation upon DNA damage
Figures
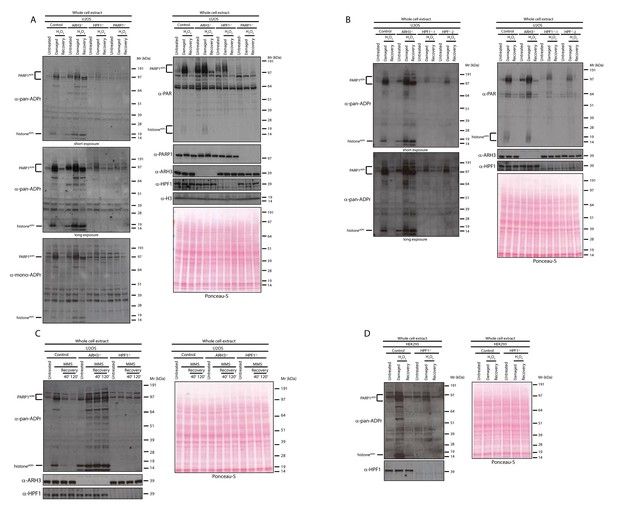
HPF1-dependent Ser-ADPr is the major form of ADPr upon genotoxic stress.
(A) Control, ARH3 KO (ARH3−/−), HPF1 KO (HPF1−/−), and PARP1 KO (PARP1−/−) U2OS cells were treated with 2 mM H2O2. After treatment/recovery, cells were lysed and proteins were separated by SDS-PAGE, analysed by western blot and probed for pan-ADPr, mono-ADPr, PAR, PARP1, ARH3, H3, and HPF1 antibodies. Additionally, Ponceau-S staining was used as loading control. (B) Control, ARH3 KO (ARH3−/−) and two independent clones of HPF1 KO (HPF1−/−−1 and HPF1−/−−2) U2OS cells were treated with 2 mM H2O2. After treatment/recovery, cells were lysed and proteins were separated by SDS-PAGE, analysed by western blot and probed for pan-ADPr, PAR, ARH3, and HPF1 antibodies. Ponceau-S staining was used as loading control. (C) Control, ARH3 KO (ARH3−/−), and HPF1 KO (HPF1−/−) U2OS cells were treated with 2 mM MMS. After the induction of DNA damage, the cells were left to recover from genotoxic stress for the indicated time points. After treatment/recovery, cells were lysed and proteins were separated by SDS-PAGE, analysed by western blot and probed for pan-ADPr, ARH3, and HPF1 antibodies. Ponceau-S staining was used as loading control. (D) Control and HPF1 KO (HPF1−/−) HEK293 cells were treated with 2 mM H2O2. After treatment/recovery, cells were lysed and proteins were separated by SDS-PAGE, analysed by western blot and probed for pan-ADPr, ARH3, and HPF1 antibodies. Ponceau-S staining was used as loading control.
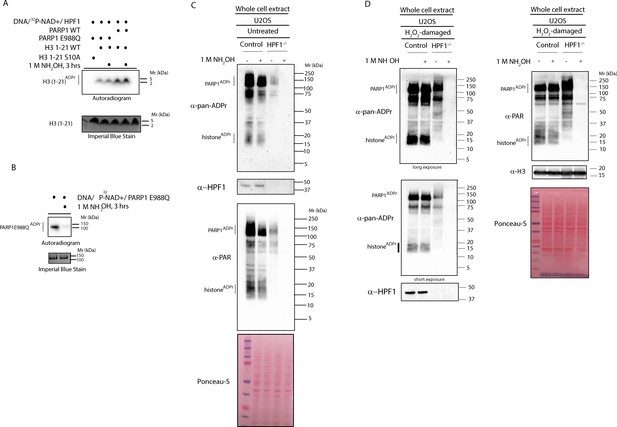
HPF1-dependent ADPr is resistant to hydroxylamine.
(A) Autoradiogram shows serine ADPr of two synthetic peptides (wild type (WT) or Ser10Ala (S10A) mutant) corresponding to amino acids 1–21 of human H3 by wild type PARP1 or PARP1 E988Q in the presence of HPF1, with or without treatment with 1M NH2OH (hydroxylamine). Imperial Blu staining was used to show equal loading of samples. (B) Autoradiogram shows auto-ADPr of PARP1 E988Q (at glutamate residues) and the effect of the treatment with 1M NH2OH. Imperial Blue staining was used to show equal loading of samples. (C) Whole cell extracts were prepared from pre-damaged U2OS wild type or HPF1 KO (HPF1−/−) cells. Extracts were either left untreated or treated with 1M hydroxylamine (NH2OH) for 3 hr prior to separation on SDS-PAGE gel and immunoblotting with pan-ADPr, PAR or HPF1 antibodies. Ponceau-S staining was used as loading control. (D) Whole cell extracts were prepared from U2OS wild type or HPF1 KO (HPF1−/−) cells following treatment with 2 mM H2O2 for 10’. Extracts were either left untreated or treated with 1M hydroxylamine (NH2OH) for 3 hr prior to separation on SDS-PAGE gel and immunoblotting with pan-ADPr, PAR, H3 or HPF1 antibodies. Ponceau-S staining was used as loading control.
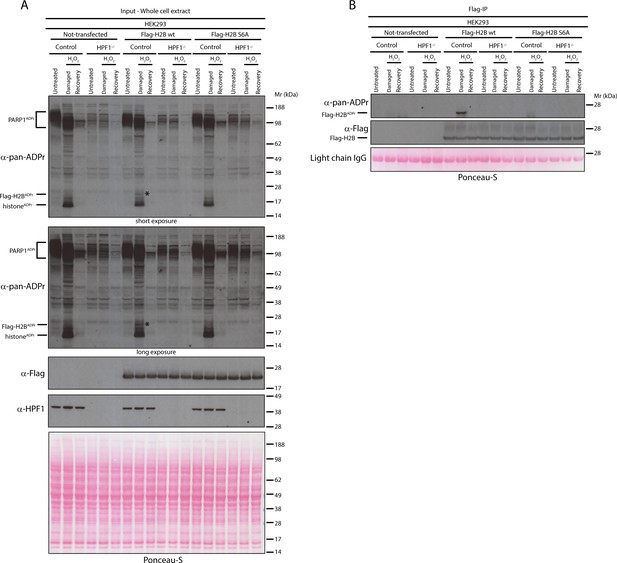
Serine six is the main ADPr site of histone H2B induced by DNA damage.
(A) Control and HPF1 KO (HPF1−/−) HEK293 cells were transfected or not with Flag-H2B wild type (wt) and Flag-H2B Ser6Ala mutant construct (S6A). 24 hr post-transfection, cells were treated with 2 mM H2O2. After treatment/recovery, cells were lysed and proteins were separated by SDS-PAGE, analysed by western blot and probed for pan-ADPr, Flag, and HPF1 antibodies. Ponceau-S staining was used as loading control. The black star marks the ADP-ribosylated Flag-tagged H2B protein in the whole cell wild type extract, which is absent in other extracts. (B) Flag-tagged H2B wild type (wt) and Ser6Ala mutant (S6A) were immunoprecipitated (IP) by using anti-Flag antibody from the lysates generated in Figure 3A. IPs were separated by SDS-PAGE, analysed by western blot and probed for pan-ADPr and Flag antibodies. Ponceau-S staining was used to stain light chains of Immunoglobulins (IgG) as loading control of the IP.
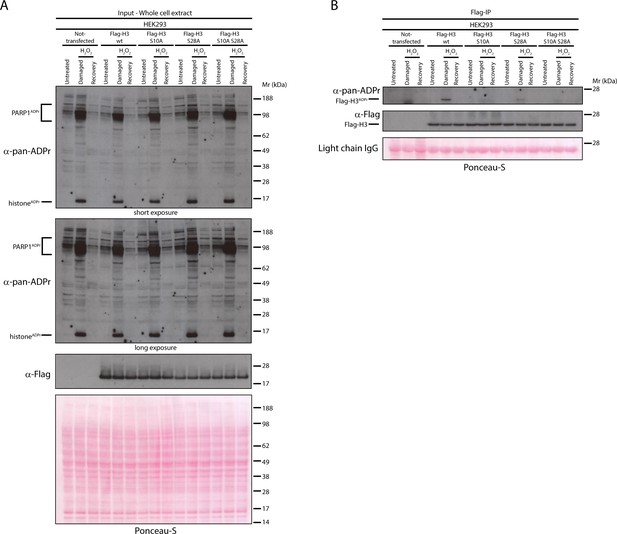
Serine 10 and serine 28 are the main ADPr sites of histone H3 induced by DNA damage.
(A) HEK293 cells were transfected or not with Flag-H3.1 (Flag-H3) wild type (wt), Flag-H3.1 Ser10Ala (S10A), Flag-H3.1 Ser28Ala (S28A), and Flag-H3.1 Ser10Ala Ser28Ala double mutant (S10A S28A) constructs. 24 hr post-transfection, cells were treated with 2 mM H2O2. After treatment/recovery, cells were lysed and proteins were separated by SDS-PAGE, analysed by western blot and probed for pan-ADPr and Flag antibodies. Ponceau-S staining was used as loading control. (B) Flag-tagged H3.1 (Flag-H3) wild type (wt), Ser10Ala (S10A), Ser28Ala (S28A), and Ser10Ala Ser28Ala double mutants (S10A S28A) were immunoprecipitated (IP) by using anti-Flag antibody from the lysates generated in Figure 4A. IPs were separated by SDS-PAGE, analysed by western blot and probed for pan-ADPr and Flag antibodies. Ponceau-S staining was used to stain light chains of immunoglobulins (IgG) as loading control of the IP.

Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| cell line (Homo sapiens) | U2OS | ATCC | HTB-96, RRID:CVCL_0042 | |
| cell line (Homo sapiens) | HEK293 | ATCC | CRL-3216, RRID:CVCL_0063 | |
| cell line (Homo sapiens) | U2OS ARH3 KO | Fontana et al., 2017 | ||
| cell line (Homo sapiens) | U2OS HPF1 KO | Gibbs-Seymour et al. (2016) | ||
| cell line (Homo sapiens) | U2OS PARP1 KO | Gibbs-Seymour et al. (2016) | ||
| cell line (Homo sapiens) | HEK293 HPF1 KO | Gibbs-Seymour et al. (2016) | ||
| antibody | anti-PAR (rabbit polyclonal) | Trevigen (Gaithersburg, MD, US) | 4336-BPC-100, RRID:AB_2721257 | WB 1:1000 |
| antibody | anti-pan-ADP-ribose (rabbit monoclonal) | Millipore (Billerica, MA, US) | MABE1016, RRID:AB_2665466 | WB 1:1500 |
| antibody | anti-mono-ADP-ribose (rabbit monoclonal) | Millipore (Billerica, MA, US) | MABE1076, RRID:AB_2665469 | WB 1:1000 |
| antibody | anti-PARP1 [E102] (rabbit monoclonal) | Abcam (Cambridge, UK) | ab32138, RRID:AB_777101 | WB 1:1000 |
| antibody | anti-histone H3, CT, pan (rabbit polyclonal) | Millipore (Billerica, MA, US) | 07–690, RRID:AB_417398 | WB 1:2000 |
| antibody | anti-ARH3/ADPRH (rabbit | Atlas Antibodies (Stockholm, Sweden) | HPA027104, RRID:AB_10601330 | WB 1:1000 |
| antibody | anti-HPF1 (rabbit polyclonal) | Gibbs-Seymour et al. (2016) | WB 1:1000 | |
| antibody | anti-Flag HRP-conjugated (mouse monoclonal) | Sigma-Aldrich (St. Louis, MO, US) | A8592, RRID:AB_439702 | WB 1:5000 |
| antibody | anti-Flag M2 agarose-conjugated (mouse monoclonal) | Sigma-Aldrich (St. Louis, MO, US) | A2220, RRID:AB_10063035 | IP |
| recombinant DNA reagent | pDONR221 (Gateway vector) | Thermo Fisher Scientific (Waltham, MA, US) | 12536017 | |
| recombinant DNA reagent | pDEST C3X (Gateway vector) | other | Laboratory of Fumiko Esashi | |
| recombinant DNA reagent | Flag-H2B wt (plasmid) | This paper | Progentiors: pDONR221-H2B; Gateway vector:pDEST C3X | |
| recombinant DNA reagent | Flag-H3.1 wt (plasmid) | This paper | Progentiors: pDONR221-H3.1; Gateway vector:pDEST C3X | |
| recombinant DNA reagent | Flag-H2B S6A (plasmid) | This paper | Made from Flag-H2B wt by site-directed mutagenesis | |
| recombinant DNA reagent | Flag-H3.1 S10A (plasmid) | This paper | Made from Flag-H3.1 wt by site-directed mutagenesis | |
| recombinant DNA reagent | Flag-H3.1 S28A (plasmid) | This paper | Made from Flag-H3.1 wt by site-directed mutagenesis | |
| recombinant DNA reagent | Flag-H3.1 S10A S28A (plasmid) | This paper | Made from Flag-H3.1 S10A by site-directed mutagenesis | |
| peptide, recombinant protein | Human PARP1 | Trevigen (Gaithersburg, MD, US) | 4668–02 K-01 | |
| peptide, recombinant protein | Human PARP1 E988Q | Fontana et al., 2017 | ||
| peptide, recombinant protein | Human HPF1 | Gibbs-Seymour et al. (2016) | ||
| peptide, recombinant protein | Human histone H3 fragment (1-21) wt | Bonfiglio et al., 2017a | ||
| peptide, recombinant protein | Human histone H3 fragment (1-21) S10A | Bonfiglio et al., 2017a | ||
| chemical compound, drug | Olaparib | Cayman Chemical (Ann Arbor, MI) | 10621 | |
| chemical compound, drug | ADP-HPD, dihydrate, ammonium salt | Calbiochem (La Jolla, CA) | 118415 | |
| chemical compound, drug | Hydrogen peroxide | Sigma-Aldrich (St. Louis, MO, US) | H1009 | |
| chemical compound, drug | Methyl methanesulfonate | Sigma-Aldrich (St. Louis, MO, US) | 129925 | |
| chemical compound, drug | Hydroxilamine | Sigma-Aldrich (St. Louis, MO, US) | 438227 |
Additional files
-
Transparent reporting form
- https://doi.org/10.7554/eLife.34334.006





