Spatial cell firing during virtual navigation of open arenas by head-restrained mice
Figures
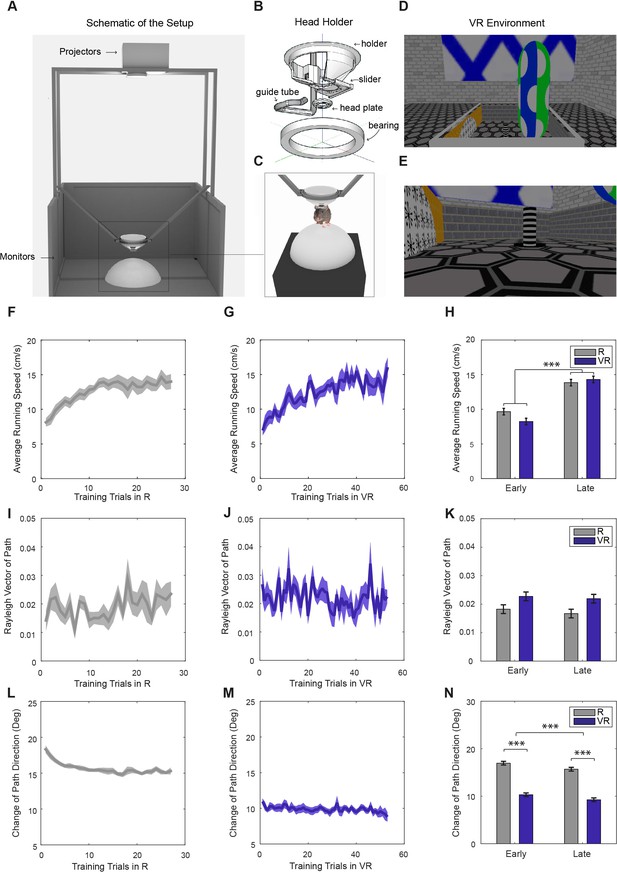
Virtual reality setup and behavior within it.
(A) Schematic of the VR setup (VR square). (B) A rotating head-holder. (C) A mouse attached to the head-holder. (D–E) Side views of the VR environment. (F–G) Average running speeds of all trained mice (n = 11) across training trials in real (‘R’; F) and virtual reality (‘VR’; G) environments in the main experiment. (H) Comparisons of the average running speeds between the first five trials and the last five trials in both VR and R environments, showing a significant increase in both (n = 11, p<0.001, F(1,10)=40.11). (I–J) Average Rayleigh vector lengths of running direction across training trials in R (I) and VR (J). (K) Comparisons of the average Rayleigh vector lengths of running direction between the first five trials and the last five trials in both VR and R. Directionality was marginally higher in VR than in R (n = 11, p=0.053, F(1,10)=4.82) and did not change significantly with experience. (L–M) Average changes of running direction (absolute difference in direction between position samples) across training trials in R (L) and VR (M). (N) Comparisons of the changes of running direction between the first five and last five trials in both R and VR. Animals took straighter paths in VR than R (n = 11, p<0.001, F(1,10)=300.93), and paths became straighter with experience (n = 11, p<0.001, F(1,10)=26.82). Positions were sampled at 2.5 Hz with 400 ms boxcar smoothing in (I–N). All error bars show s.e.m.
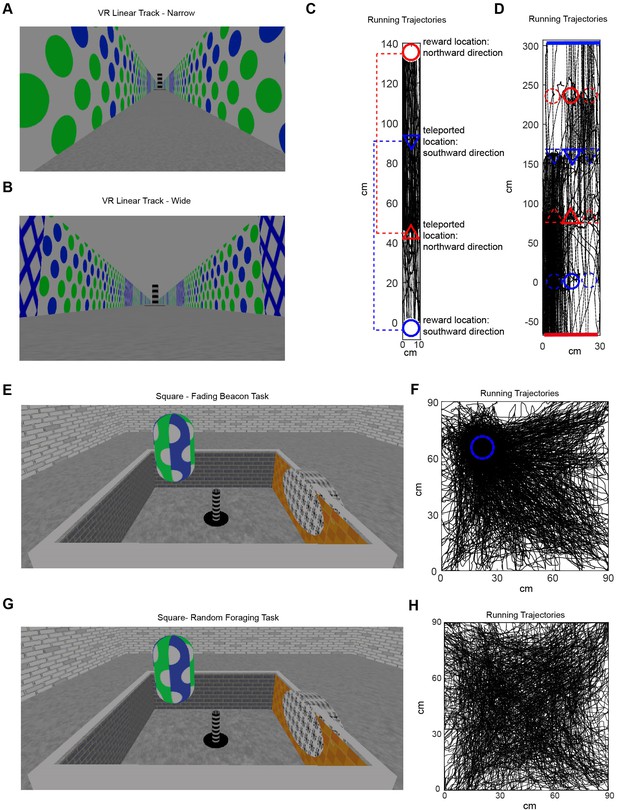
Example paths in the three training stages and the recording stage.
(A) A view of the VR narrow linear track at training stage 1. (B) A view of the VR wide linear track at training stage 2. (C) An example running trajectory in the narrow linear track. Circles indicate reward locations, triangles indicate start points where animals get teleported after getting rewards. The start points were associated with the reward positions with matching colors (dotted lines). For example, a mouse gets teleported back to the position indicated by the blue triangle from the reward position indicated by the blue cycle. (D) An example running trajectory in the wide linear track. Circles indicate reward locations, bars indicate end points of the track, triangles indicate start points where animals get teleported after getting rewards or reaching the ends of the track. (E) A view of the virtual square at training stage 3 – the ‘fading beacon’ task. (F) An example trajectory when a mouse performing a ‘fading beacon’ task in the VR square. The dotted blue circle indicates the fixed location of every fourth reward. (G) A view of the virtual square during the recording stage – random foraging. (H) An example trajectory from a mouse foraging for randomly-positioned rewards in the VR square.
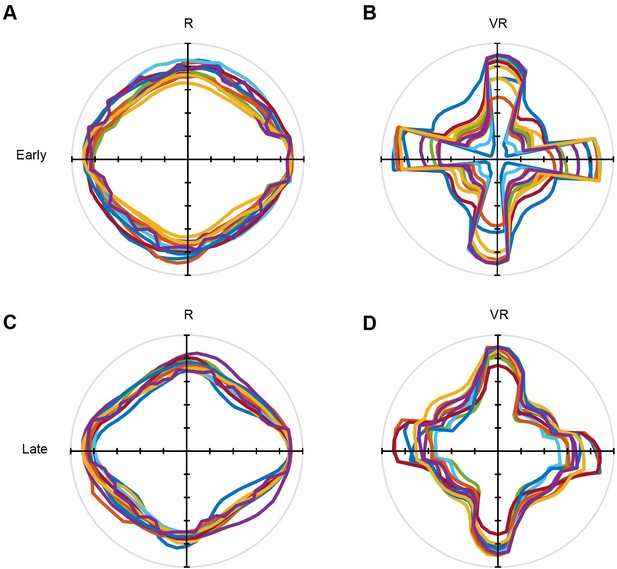
Directional polar plots of running directions in the VR square environment (n = 11 mice).
(A–B) Average polar plot of running directions over the first five training trials in R (left column) and VR (right column). (C–D) Average polar plot of running directions over last five training trials in R (left column) and VR (right column). Each color represents one animal from the main experiment.
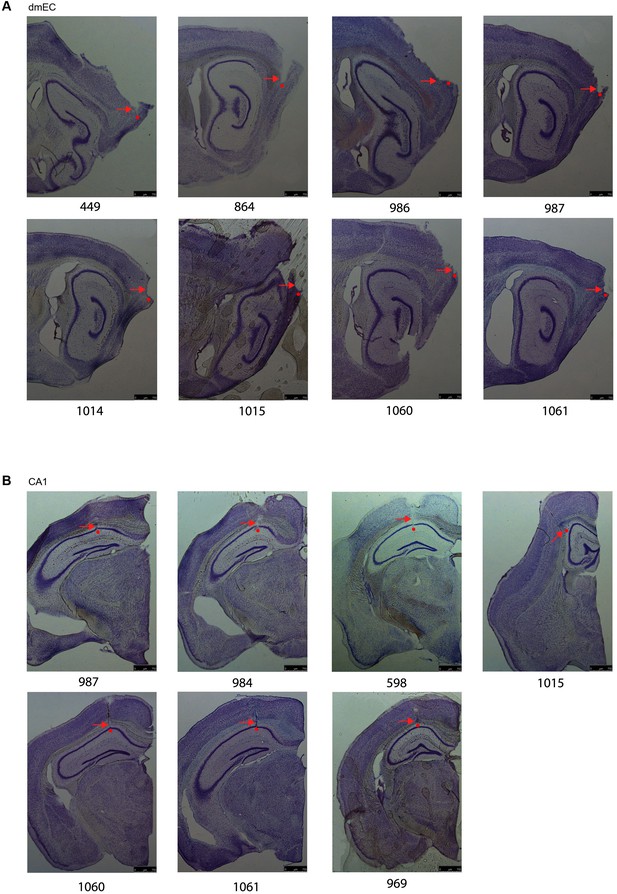
Nissl-stained brain sections from the 11 mice in the main experiment.
(A) Eight sections from mice with tetrodes aimed at dorsomedial Entorhinal Cortex and (B) seven sections from mice with tetrodes aimed at dorsal CA1. The red arrows indicated the electrode tracks and the red dots marked the end points of the tracks. We note some technical difficulties with processing (e.g. A 1015, B 969).
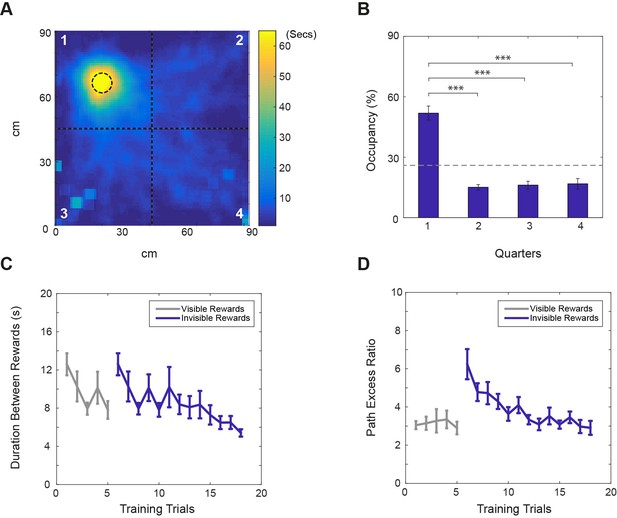
Performance on the ‘fading beacon’ task.
(A) An example heat map showing the distribution of locations between the third and the fourth rewards during a 40 min trial (mouse#987, trial#24). The dotted circle in the first quadrant shows the location of the faded reward. (B) Average time spent (as % of total time) in each quadrant of the VR square (numbered in A) showed a clear bias (n = 11, p<0.001, F(3,30)=39.03), with time spent in the first quadrant was significantly higher than in the others (*** denotes significance at p<0.001, ** at p<0.01). (C) Average durations between the third and the fourth rewards across training trials. (D) Average path excess ratios between the third and the fourth rewards across training trials (means ± s.e.m). Note that in each set of four rewards, the first, second and third rewards appeared at random locations in the virtual square, marked by visual beacons, the fourth reward was located at a fixed location. Grey lines show trials when the fixed-location rewards were marked by visual beacons. Blue lines show trials when the fixed rewards were not marked. See Supplementary video.
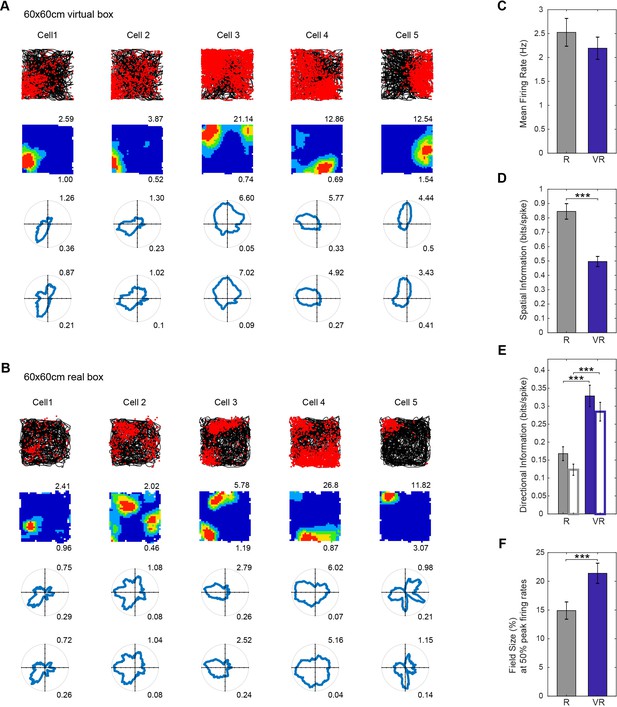
Place cell firing in real and virtual environments.
(A–B) The same five place cells recorded in a 60 × 60 cm virtual square (A) and in a 60 × 60 cm real square (B, one cell per column). Top row: 40 min running trajectory (black line) with red dots showing the locations of spikes; 2nd row, firing rate maps, maximum firing rate (Hz) shown at top right, spatial information (bits/spike) bottom right; third and fourth row: polar plots of directional firing rates (third row: standard binning; fourth row: after fitting a joint ‘pxd’ model to account for in homogeneous sampling), maximum firing rate top right, directional information bottom right. (C–F) Comparison between R (grey bars) and VR (blue bars): (C) Mean firing rates, higher in R than VR but not significantly so (n = 154, t(153)=1.67, p=0.10); (D) Spatial information, significantly higher in R than in VR (n = 154, t(153)=8.90, p<0.001); (E) Directional information rates using standard (solid bars) and pxd binning (open bars), greater in VR than in R (standard n = 154, t(153)=6.45, p<0.001; pxd, n = 154, t(153)=7.61, p<0.001). (F) Field sizes (bins with firing above 50% of peak firing rate, as a proportion to the size of the test environment), were larger in VR than in R (n = 154, t(153)=4.38, p<0.001).
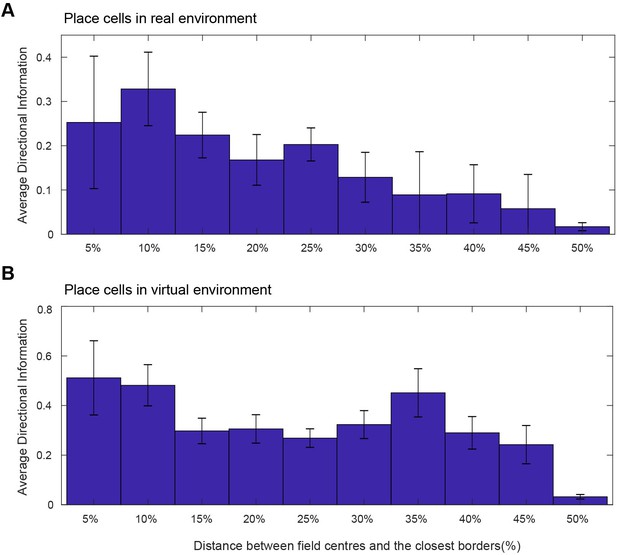
Directional information of place cell firing (bits/spike) as a function of the distance from the nearest wall (as % of the width of environment) in real (A) and virtual (B) environments (154 place cells from 11 animals).
https://doi.org/10.7554/eLife.34789.009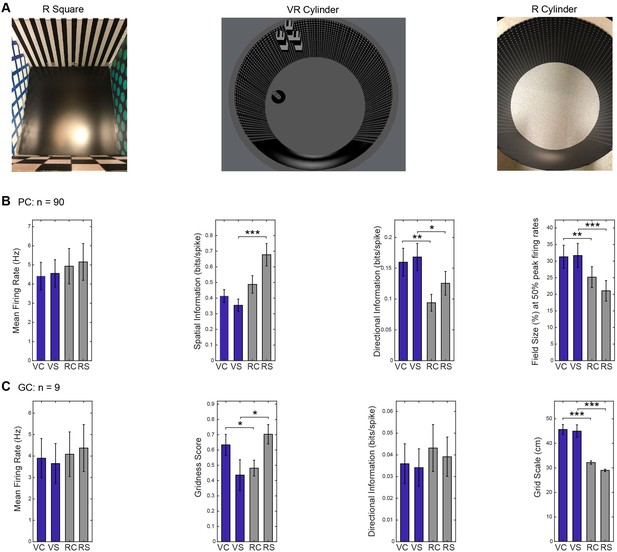
VR and R trials in square and cylindrical environments.
(A) Pictures taken for the environments used for recordings (see Figure 1 for the VR square). (B) Comparison of place cell properties in different environments (using two-way ANOVAs with factors VR vs R and cylinder vs square). Place cells had lower spatial information in VR, especially in the square environments (main effect of VR, n = 90, F(1,89)=25.20, p<0.001; interaction, F(1,89)=34.99, p<0.001; VR vs R square t(89)=-6.93, p<0.001; VR vs R cylinder t(89)=-1.73, p=0.09). Field size was larger in VR, and more so in the square environments (main effect, n = 90, F(1,89)=22.11, p<0.001; interaction, F(1,89)=4.24, p<0.05; VR vs R square: t(89)=5.07, p<0.001; VR vs R cylinder, t(89)=2.95, p<0.01). Directional information was higher in VR irrespective of environment shape (main effect VR vs R: n = 90, F(1,89)=12.16, p<0.001; interaction, n = 90, F(1,90)=1.10, p=0.30). (C) Comparison of grid cell properties in different environments. Grid cells had larger grid scale in VR irrespective of environment shape (main effect, n = 9, F(1,8)=63.74, p<0.001; interaction, n = 9, F(1,8)=3.47, p=0.10). Gridness score was lower in the VR square than the R square but higher in the VR cylinder than the R cylinder (n = 9, t(8)=2.33, p=0.05; interaction, n = 9, F(1,8)=13.35, p<0.01; VR vs R square: t(8)=-2.87, p<0.05; VR vs R cylinder: t(8)=2.33, p=0.05).
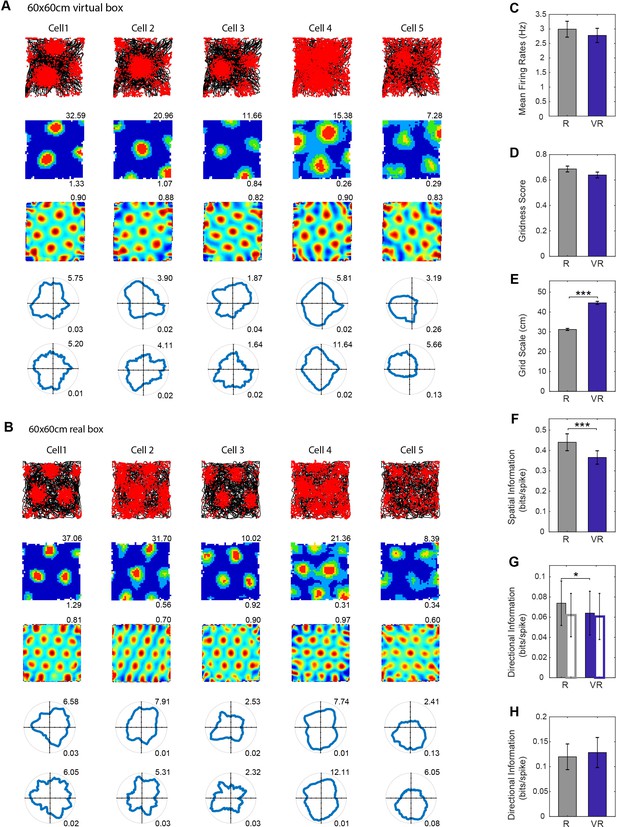
Grid cell firing in real and virtual environments.
(A–B) The same five grid cells simultaneously recorded in a 60 × 60 cm virtual square (A) and in a 60 × 60 cm real square (B, one cell per column). Top row: 40 min running trajectory (black line) with red dots showing the locations of spikes; second row, firing rate maps, maximum firing rate (Hz) shown top right, spatial information (bits/spike) bottom right; third row: spatial autocorrelations, gridness scores top right; fourth and fifth rows: polar plots of directional firing rates (fourth row: standard binning; fifth row: ‘pxd’ binning to account for inhomogeneous sampling), maximum firing rate top right, directional information bottom right. (C–H) Comparison between R (grey bars) and VR (blue bars): (C) Mean firing rates, higher in R than VR but not significantly so (n = 61, t(60)=1.71, p=0.09); (D) Gridness scores, higher in R than VR but not significantly so (n = 61, t(60)=1.67, p=0.10); (E) Grid scales, larger in VR than in R (n = 61, t(60)=15.52, p<0.001); (F) Spatial information in bits/spike, higher in R than VR (n = 61, t(60)=4.12, p<0.001); (G) Directional information. Grid cell firing was slightly more directional in VR than in R (n = 61, t(60)=2.04, p<0.05), but the difference disappeared when calculated using pxd plots (open bars, n = 61, t(60)=0.32, p=0.75); (H) Directional information in individual grid firing fields, not significant difference between the R and VR trials based on pxd plots (n = 61, t(60)=0.53, p=0.60).
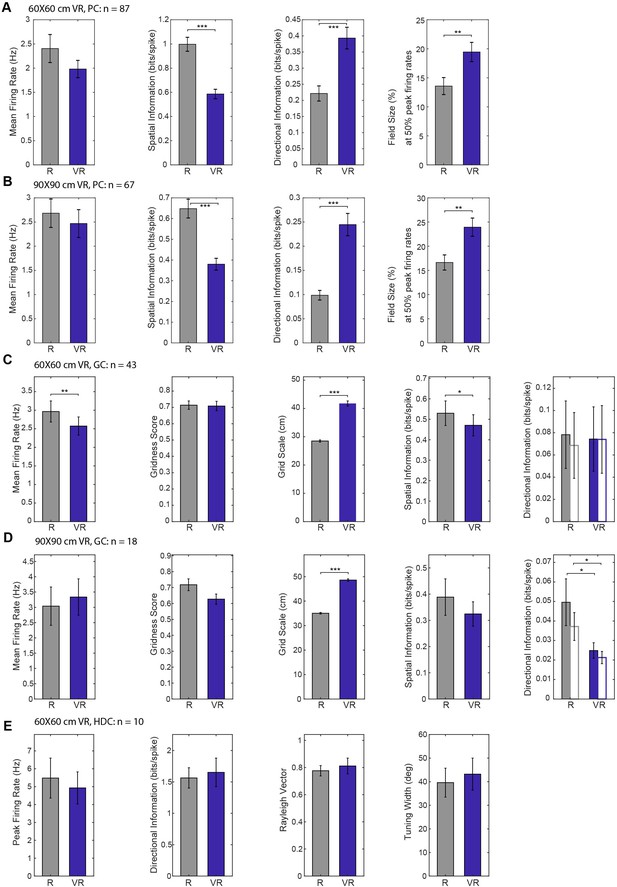
Breakdown of spatial firing properties in 60 × 60 cm or 90 × 90 cm VR environments.
(A) Place cell properties in 60 cm VR compared to 60 cm R square. Spatial information was lower (n = 87, t(86)=7.31, p<0.001), directional information higher (n = 87, t(86)=-4.71, p<0.001) and field size larger (n = 87, t(86)=-3.06, p<0.001). (B) Comparison of place cell properties in 90 cm VR compared to 60 cm R. Spatial information was lower (n = 67, t(66)=5.20, p<0.001), directional information higher (n = 67, t(66)=-4.52, p<0.001) and field size larger (n = 67, t(66)=-3.13, p<0.01). (C) Grid cell properties in 60 cm VR compared to 60 cm R square. Mean firing rates were lower (n = 43, t(42)=3.19, p<0.01), grid scale larger (n = 43, t(42)=-11.53, p<0.001) and spatial information lower (n = 43, t(42)=2.41, p<0.05). Directional information was similar with standard (solid bars) and pxd binning (open bars). (D) Grid cell properties in 90 cm VR compared to 60 cm R square. Grid scales were larger (n = 18, t(17)=-22.72, p<0.001) and directional information was lower for both standard (solid bars, n = 18, t(17)=2.62, p<0.05) and pxd binning (open bars, n = 18, t(17)=2.76, p<0.05). (E) Head direction cell properties in 60 cm VR and 60 cm R square. None of the measures showed significant difference between R and VR (n = 10).

Trial order and trial length effects on comparing firing properties across VR and R environments in additional data from four mice.
Two-way ANOVAs with factors of environment (VR vs R) and trial order (VR first vs R first) were performed. (A) Place cell firing properties comparing 20 min R trials with 40 min VR trials. Irrespective of trial order, spatial information was lower in VR (main effect, n = 85, F(1,84)=72.61, p<0.001; interaction, F(1,84)=0.03, p=0.85), field sizes were larger (main effect, F(1,84)=38.5, p<0.001; interaction, F(1,84)=0.47, p=0.49). Directional information was higher for both standard (solid bars, main effect, F(1,84)=33.25, p<0.001) and pxd binning (open bars; main effect, F(1,84)=25.90, p<0.001), with a greater difference when VR trials preceded R trials for both standard (interaction, F(1,84)=6.44, p<0.05) and pxd binning (interaction, F(1,84)=17.95, p<0.001). (B) Place cell firing properties comparing 20 min R trials with the first 20 min of VR trials. Irrespective of trial order, spatial information was lower in VR (main effect, n = 85, F(1,84)=47.04, p<0.001; interaction, F(1,84)=0.19, p=0.66) and field sizes were larger (main effect, F(1,84)=20.74, p<0.001; interaction, F(1,84)=0.53, p=0.47). Directional information was higher in VR for both standard (main effect, F(1,84)=44.82, p<0.001) and pxd binning (main effect, F(1,84)=36.50, p<0.001) and more so when VR trials preceded R trials (standard binning: interaction, F(1,84)=3.92, p=0.051; pxd binning: interaction, F(1,84)=13.01, p<0.001). (C) Grid cell firing properties comparing 20 min R trials with 40 min VR trials. Irrespective of the order of trials, gridness scores were lower in VR (main effect, n = 20, F(1,19)=34.82, p<0.001; interaction, F(1,19)=1.14, p=0.30) and grid scale was larger (main effect, F(1,19)=74.41, p<0.001; interaction F(1,19)=1.97, p=0.18). (D) Grid cell firing properties comparing 20 min R trials with the first 20 min of VR trials. Irrespective of order, gridness scores were lower in VR (main effect, n = 20, F(1,19)=102.90, p<0.001; interaction, F(1,19)=0.80, p=0.38), grid scale was larger (main effect, F(1,19)=75.91, p<0.001; interaction, F(1,19)=0.14, p=0.72) and directional information was higher in both standard binning (main effect, F(1,19)=9.58, p<0.01; interaction, F(1,19)=0.01, p=0.91) and pxd binning (main effect, F(1,19)=8.18, p=0.01; interaction, F(1,19)=4.09, p=0.06).
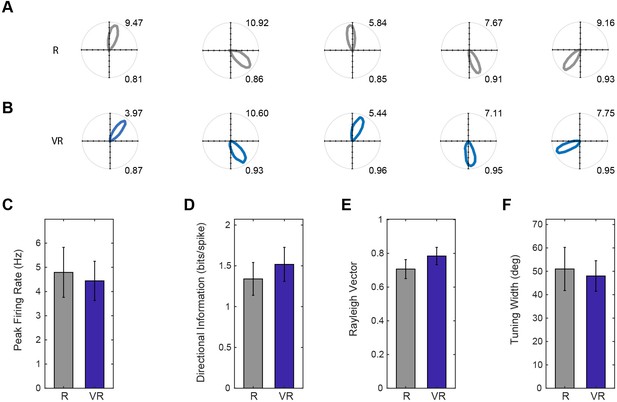
Head direction cell firing in real and virtual environments.
(A–B) Polar plots of the same five HD cells in dmEC simultaneously recorded in R (A) and VR (B, one cell per column). Maximum firing rates are shown top right, Rayleigh vector length bottom right. (C–F) Comparisons of basic properties of HD cells in dmEC between R and VR. There were no significant differences in peak firing rates (t(11)=0.65, p=0.53; (C); directional information (t(11)=1.38, p=0.19; D); Rayleigh vector length (t(11)=1.69, p=0.12; E); and tuning width (t(11)=0.48, p=0.64; F).
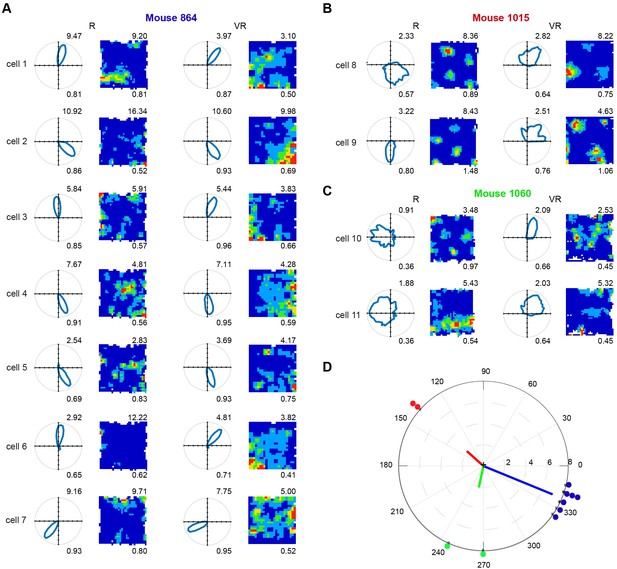
Eleven directional cells recorded in dmEC.
(A) Polar plots (left column) and firing rate maps (right column) of seven cells found in mouse 1. (B) Two conjunctive grid cells found in mouse 2. (C) Two cells found in mouse 3. Numbers on the top right show maximum firing rates, and on the bottom show Rayleigh vector length (left columns) and spatial information (right columns). (D) The relative directional tuning difference of simultaneously recorded head-direction cells between VR and R: Mouse 1 (blue), 337.71 ± 8.28; Mouse 2 (red), 138.0 ± 0.00; Mouse 3 (green), 258.00 ± 16.97. The dots represent the relative directional tuning difference of individual cells between VR and R. The lines represent the mean tuning difference within the animals. Each dot represents one cell, and each color represents one animal.
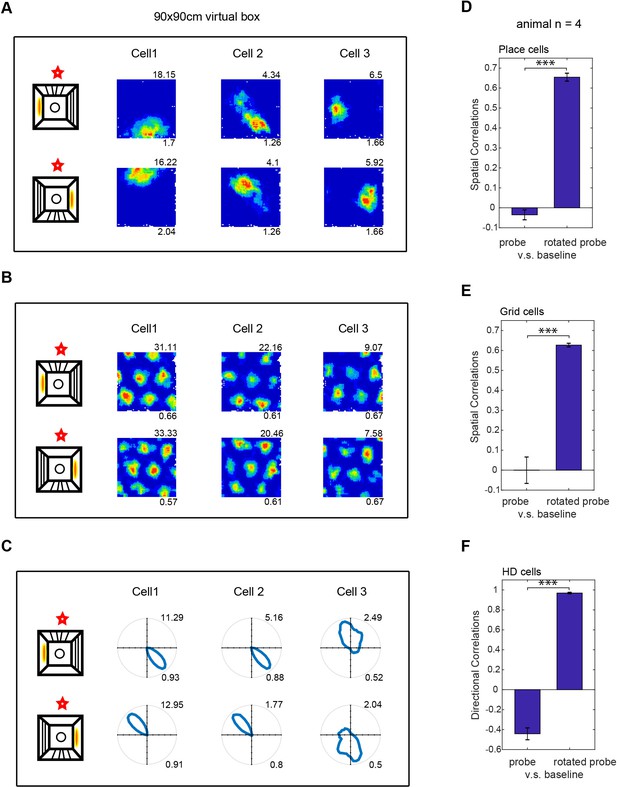
Effect of rotating the virtual environment on spatial firing patterns.
(A–C) Three simultaneously recorded CA1 place cells (A), dmEC grid cells (B) and dmEC head-direction cells (C). Upper rows show firing patterns in baseline trials, lower rows show the rotated probe trials. Schematic (far let) shows the manipulation: virtual cues and entry point were rotated 180o relative to the real environment (marked by a red star). Maximum firing rates are shown top right, spatial information (A), gridness (B) or Rayleigh vector length (C) bottom right. (D–F) Spatial correlations between probe and baseline trials were significantly higher when the probe trial rate map was rotated 180o than when it was not (spatial correlations for place cells, n = 123, t(122)=19.44, p<0.001; grid cells, n = 18, t(17)=9.41, p<0.001; HD cells, n = 17, t(16)=24.77, p<0.001).
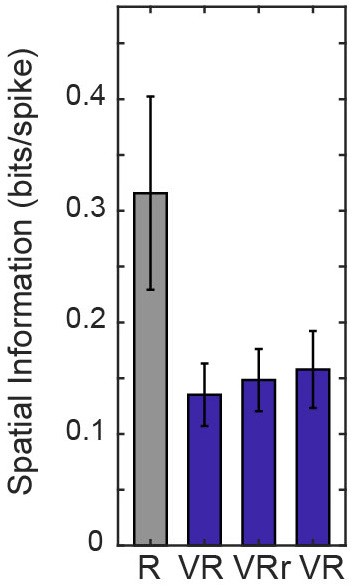
Spatial information of place cells that did not follow the 180 degree rotation of VR environment (n = 7, spatial information were 0.32 ± 0.23, 0.14 ± 0.07, 0.15 ± 0.07 and 0.16 ± 0.09 in R, VR control, VR rotated and VR control trials respectively).
https://doi.org/10.7554/eLife.34789.017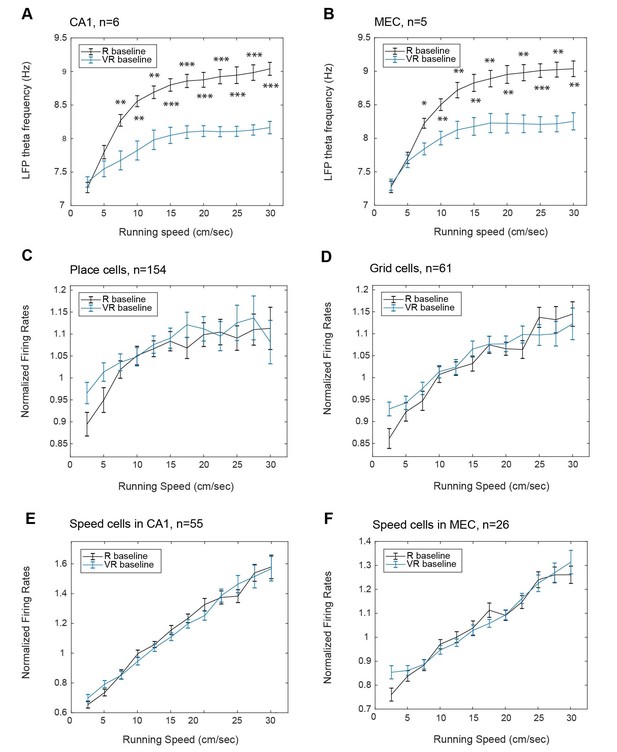
Effect of running speed on theta frequency and firing rates in real and virtual environments.
Relationship between running speed in VR (blue) and R (black) on instantaneous LFP theta frequency in CA1 (A, n = 6); instantaneous LFP theta frequency in dmEC (B, n = 5); firing rates of place cells in CA1 (C, n = 154); firing rates of grid cells in dmEC (D, n = 61); speed-modulated cells in CA1 (E, n = 55); firing rates of speed-modulated cells in dmEC (F, n = 26). Lines show the mean (±s.e.m) theta frequency in each running speed bin (2.5 cm/s to 30 cm/s).
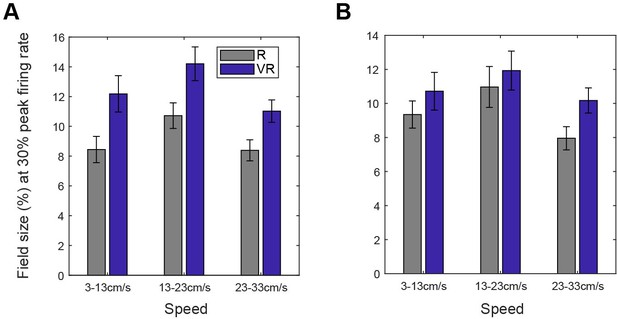
Spatial cell field size was modulated by running speed in a similar way in R and VR environments.
(A) Comparison of grid field size when sampled in different speed ranges. Field size (as a percentage of the area of the environment) were significantly different across the three speed ranges (n = 61, F(2, 120)=7.06, p<0.01) and between VR and R environments (n = 61, F(1, 60)=21.28, p<0.001) but there was no interaction between effects of speed and environment on size (n = 61, F(2, 120)=0.52, p=0.60). (B) Comparison of place cell field size when sampled in different speed ranges. Field sizes were different across speed ranges (n = 154, F(2, 306)=11.13, p<0.001), the effect of environment approached significance (F(1,153)=3.50, p=0.06) and there was no interaction between effects of environment and speed (F(2, 306)=1.98, p=0.14).
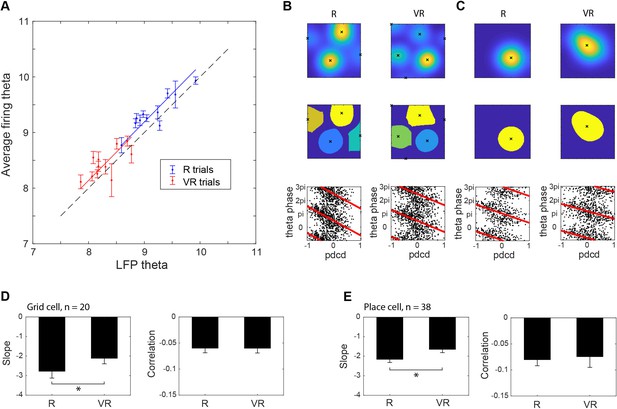
Theta phase precession.
(A) Theta frequency modulation of firing rate versus LFP theta frequency (175 theta modulated cells recorded from EC and CA1, including 20 grid cells and 38 place cells). (B) Example of a grid cell’s theta phase precession in real (left) and virtual environments (right), showing the detection of peaks in the smoothed firing rate map (above), division of data into separate firing fields (middle) and firing phase with respect to LFP theta plotted against distance through field along the current direction of motion (pdcd). (C) Example of a place cell’s theta phase precession in real (left) and virtual environments (right), shown as in B). (D) Comparison of grid cell theta phase precession in R and in VR. Precession slope is lower in absolute value in VR than in R (n = 20, t(19)=-2.55, p<0.05). Phase-pdcd correlation strengths were comparable in VR and in R (n = 20, t(19)=0.02, p=0.98). (E) Comparison of place cell theta phase precession in R and VR. Precession slope is lower in absolute value in VR than in R (n = 38, t(37)=-2.19, p<0.05). Phase-pdcd correlation strengths were not different in VR and in R (n = 38, t(37)=-0.24, p=0.82).
Videos
example of a mouse performing the ‘fading beacon’ task.
https://doi.org/10.7554/eLife.34789.007Additional files
-
Transparent reporting form
- https://doi.org/10.7554/eLife.34789.021





