Integrin-based diffusion barrier separates membrane domains enabling the formation of microbiostatic frustrated phagosomes
Figures
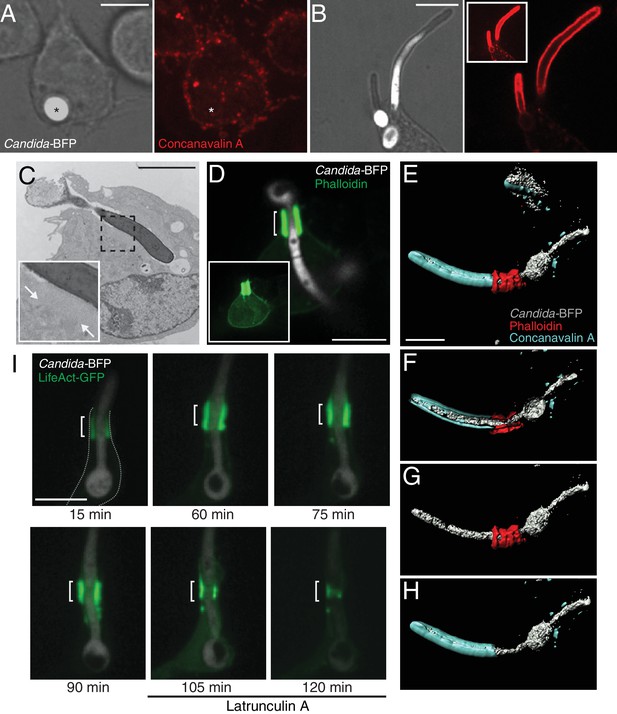
Partial phagocytosis of C. albicans hyphae is associated with formation of an actin cuff.
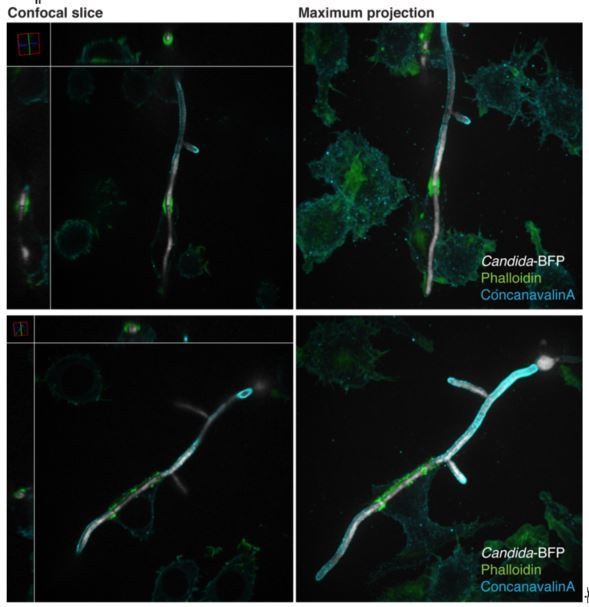
Phagocytosis of C. albicans yeast (A) or hypha (B) by RAW-Dectin1 cells. After incubation with Candida-BFP, RAW-Dectin1 cells were fixed and extracellular C. albicans stained using Alexa594-conjugated concanavalin A (red). The fluorescence of the BFP is shown in white here and elsewhere to reveal the location of the Candida-BFP. Inset in (B): overexposure of the concanavalin A signal to show less intense, staining of the macrophage membrane (as in A). Scale bars: 5 μm and 10 μm, respectively. (C) Transmission electron micrograph of a RAW-Dectin1 cell with a partially internalized C. albicans hypha. Area of organelle clearance corresponding to the cuff structure is indicated in inset by arrows. Scale bar: 5 μm. (D) F-actin enrichment at the neck of partial phagosome. RAW-Dectin1 cells were allowed to internalize C. albicans hyphae, fixed and stained with fluorescent phalloidin (green). Actin cuff indicated with a bracket. Inset: overexposure to show the less intense cellular actin. Scale bar: 10 μm. (E–H) 3D rendering of a C. albicans hypha partially internalized by a RAW-Dectin1 cell. After incubation with Candida-BFP (white), RAW-Dectin1 cells were fixed and extracellular portions of the hyphae stained using Alexa647-conjugated concanavalin A (blue). Actin was stained with fluorescent phalloidin (red). Scale bar: 5 μm. (F) 3D rendering sliced near the middle of the tubular phagosome, (G) same as E showing only the hypha (white) and actin (red), and (H) same as E showing only the hypha (white) and concanavalin A (blue). (I) Stability of the actin cuff assessed by live cell imaging. RAW-Dectin1 cells expressing LifeAct-GFP were allowed to internalize C. albicans hyphae and imaged at defined intervals. Where indicated (105 min) 1 µM latrunculin A was added and recording continued. Actin cuff location indicated by bracket. Scale bar: 10 μm. Images are representative of ≥30 fields from ≥3 separate experiments of each type. In this and subsequent figures the outline of the phagocyte (when not readily apparent) is indicated by a dotted grey line.
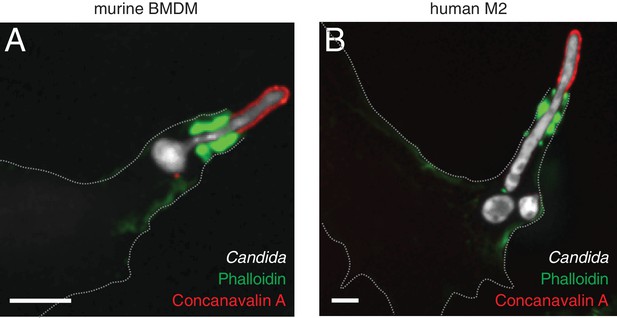
Actin cuffs are observed in both murine and human primary macrophages infected with C. albicans hyphae.
Phagocytosis of Candida-BFP hyphae by murine BMDM (A) or human monocyte-derived macrophages (B). Following phagocytosis, cells were fixed and extracellular C. albicans stained using Alexa594 conjugated concanavalin A (red). F-actin was stained using fluorescent phalloidin (green). Scale bars: 5 μm.
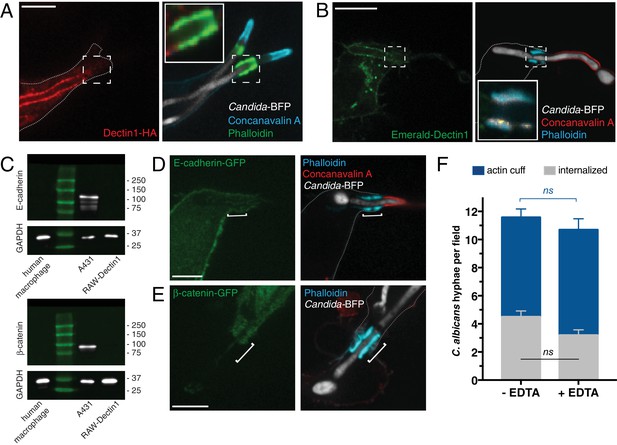
Assessing the contribution of Dectin1 and cadherin/catenin to the formation of the actin cuff.
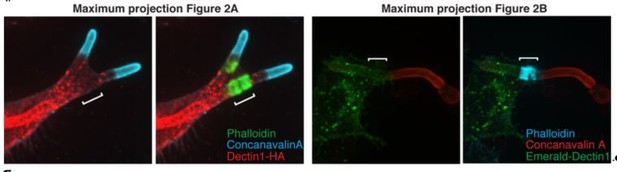
After incubation with Candida-BFP hyphae, RAW-Dectin1 cells were fixed and monolayers stained and visualized as follows. (A) The distribution of Dectin1-HA was detected by immunostaining (red). Actin was stained using fluorescent phalloidin (green); concanavalin A (blue). Inset: actin cuff shows little colocalization (yellow) with Dectin1-HA. (B) Visualization of Emerald-Dectin1 (green). Actin was stained using fluorescent phalloidin (blue); concanavalin A (red). Inset: poor colocalization of actin cuff with Emerald-Dectin1, in yellow. (C) The expression of E-cadherin (top panel) and β-catenin (bottom panel) was assessed by immunoblotting in human macrophages, A431 and RAW-Dectin1 cells; GAPDH was used as loading control. Visualization of: (D) E-cadherin-GFP or (E) β-catenin-GFP transiently transfected into RAW-Dectin1 cells. For both (D) and (E), after phagocytosis and fixation, extracellular C. albicans was stained using Alexa594-conjugated concanavalin A (red), and actin stained using fluorescent phalloidin (blue). Scale bars: 5 μm. (F) RAW-Dectin1 cells were allowed to internalize C. albicans-hyphae in the presence or absence of 4 mM EDTA. Following phagocytosis, extracellular C. albicans was stained using concanavalin A, and actin stained with phalloidin. The number of C. albicans hyphae that were fully internalized or partially internalized with actin cuffs per 37.5x field was counted by confocal microscopy, and the average number per field calculated. Average number of C. albicans per field was 12.7 ± 1.0. For each condition, three independent experiments were quantified, with ≥15 fields counted per replicate. p value was calculated using the unpaired, 2-tailed students t-test. Data are means ±SEM.
-
Figure 2—source data 1
Numerical data corresponding to Figure 2F.
- https://doi.org/10.7554/eLife.34798.008
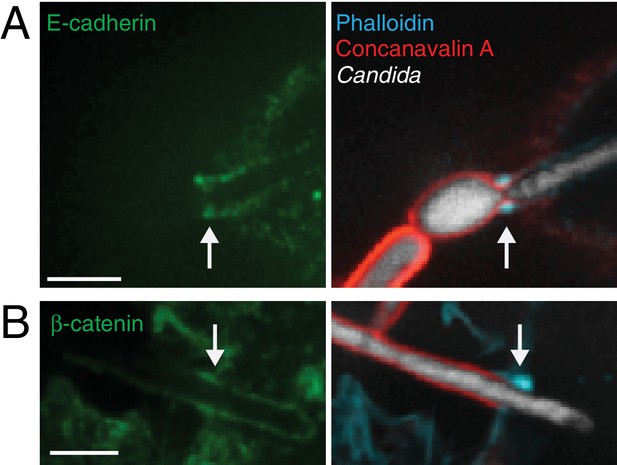
Cadherins accumulate at the actin cuff of epidermal cells.
Following incubation with Candida-BFP hyphae, A431 cells were fixed and extracellular C. albicans stained using Alexa594-conjugated concanavalin A (red). After permeabilization the cells were immunostained for endogenous (A) E-cadherin or (B) β-catenin (green). Actin was stained using fluorescent phalloidin (blue). For all panels, actin cuffs indicated by arrows. Scale bars: 5 μm.
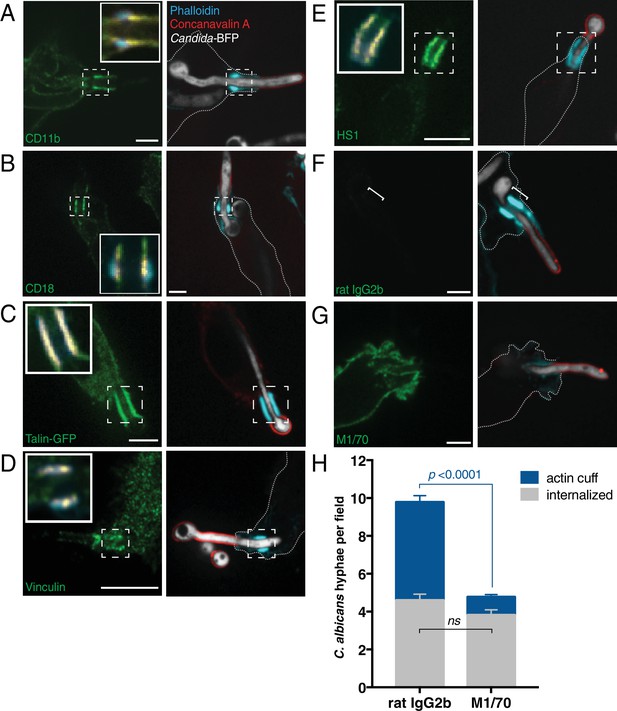
Engagement of integrin αMβ2 (CD11b/CD18) is necessary for formation of the actin cuff.
After incubation with Candida-BFP hyphae, RAW-Dectin1 cells were fixed and extracellular C. albicans stained using Alexa594-conjugated concanavalin A (red). For panels (A–G) F-actin was stained using fluorescent phalloidin (blue), and actin cuff location indicated with a dashed box or bracket. (A) Anti-CD11b immunostaining (green). Inset: Colocalization of actin cuff with CD11b, in yellow. Scale bar: 5 μm. (B) Anti-CD18 immunostaining (green). Inset: Colocalization of actin cuff with CD18, in yellow. Scale bar: 5 μm. (C) Visualization of transfected Talin-GFP. Inset: Colocalization of actin cuff with talin, in yellow. Scale bar: 10 μm. (D) Immunostaining of endogenous vinculin (green). Inset: Colocalization of actin cuff with vinculin, overlaid in yellow. Scale bar: 10 μm. (E) Immunostaining of endogenous HS1 (green). Scale bar: 10 μm. (F–H) Internalization of Candida-BFP hyphae was allowed to proceed in the presence of the CD11b blocking antibody M1/70 or an isotype-matched (rat IgG2b) control antibody. Following phagocytosis, extracellular C. albicans was stained using Alexa594-conjugated concanavalin A (red), and actin stained using fluorescent phalloidin (blue). Immunostaining (green) for rat IgG2b isotype control (F, left panel) or M1/70 (G, left panel). Scale bars: 5 μm. Images shown are representative of at least 3 experiments of each kind. (H) The number of C. albicans hyphae that were fully internalized or partially internalized with actin cuffs per 37.5x field was counted by confocal microscopy. Average number of C. albicans per field was 11.7 ± 0.5. For each condition, four independent experiments were quantified, with ≥15 fields counted per replicate. p value was calculated using the unpaired, 2-tailed students t-test. Data are means ±SEM.
-
Figure 3—source data 1
Numerical data corresponding to Figure 3H.
- https://doi.org/10.7554/eLife.34798.011
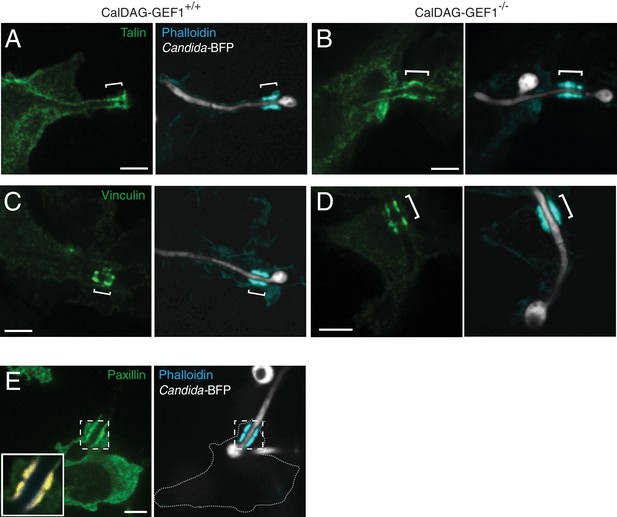
Novel activation of CR3 during actin cuff formation.
(A–D) Role of CalDAG-GEF1 in actin cuff formation. After incubation with Candida-BFP hyphae, CalDAG-GEF1+/+ or −/− BMDM, cells were fixed, permeabilized and F-actin stained using fluorescent phalloidin (blue); actin cuff location is indicated with a bracket. (A) and (B) Talin immunostaining (green). (C) and (D) Vinculin immunostaining (green). (E) Paxillin is enriched in the actin cuff. Following phagocytosis of C. albicans hyphae, RAW-Dectin1 cells were fixed and immunostained for paxillin (green). Inset: Colocalization of actin cuff with paxillin, in yellow. Scale bars: 5 μm throughout.
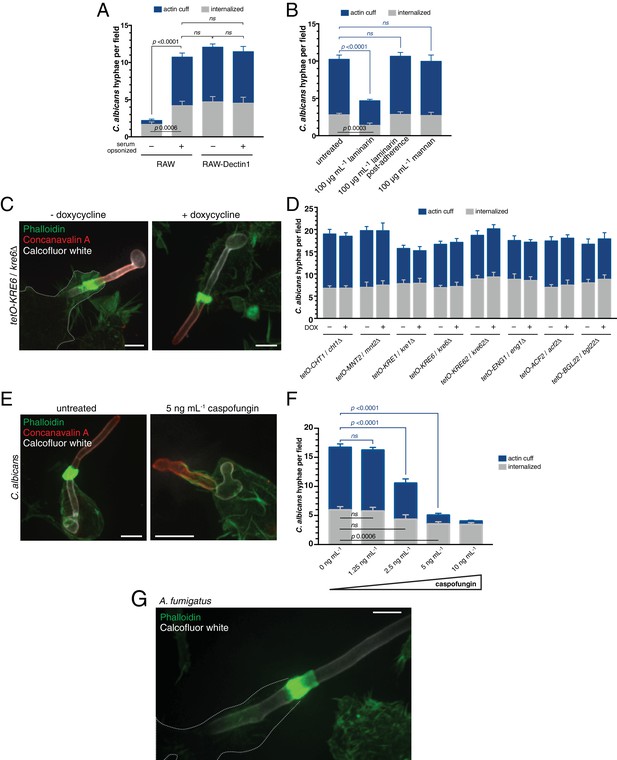
Assessing the contribution of C. albicans cell wall components to actin cuff formation.
(A) RAW or RAW-Dectin1 cells were incubated with Candida-BFP hyphae that had been either untreated or serum-opsonized. Following phagocytosis, extracellular C. albicans was stained using concanavalin A, and actin stained with phalloidin. The number of C. albicans hyphae that were fully internalized or partially internalized with actin cuffs per 37.5x field was counted by confocal microscopy. Average number of C. albicans per field was 15.7 ± 1.3. For each condition, three independent experiments were quantified, with ≥4 fields counted per replicate. p value was calculated using the unpaired, 2-tailed students t-test. Data are means ±SEM. (B) RAW-Dectin1 cells were allowed to internalize Candida-BFP hyphae in the presence or absence of laminarin or mannan. For laminarin, RAW-Dectin1 cells were also allowed to adhere C. albicans 15 min prior to the addition of laminarin, as indicated. Other details as in A. Average number of C. albicans per field was 12.9 ± 0.7. (C–D) Evaluation of C. albicans GRACE strain cell wall mutants for actin cuff formation. GRACE strains were induced to form hyphae in the absence or presence of doxycycline (DOX) to repress target gene expression, and incubated with RAW-Dectin1 cells. Following phagocytosis, monolayers were fixed and C. albicans stained with 10 μg mL−1 calcofluor white (white), extracellular C. albicans stained using concanavalin A (red), and actin stained with phalloidin (green). Image in C is representative of ≥30 fields from ≥3 separate experiments of each type. Scale bar: 5 μm. (D) The number of C. albicans hyphae that were fully internalized or partially internalized with actin cuffs per 37.5x field was counted by confocal microscopy, and the average number per field calculated. Average number of C. albicans per field was 20.6 ± 0.6. For each condition, three independent experiments were quantified, with ≥4 fields counted per replicate. p value was calculated using the unpaired, 2-tailed students t-test. Data are means ±SEM. (E) Role of C. albicans β-(1,3)-glucan in actin cuff formation. The GRACE wild-type strain was incubated and induced to form hyphae in the presence or absence of 5 ng mL−1 caspofungin and incubated with RAW-Dectin1 cells for phagocytosis. Following phagocytosis, cells were fixed and C. albicans stained with 10 μg mL−1 calcofluor white (white), extracellular C. albicans stained using fluorescent concanavalin A (red), and actin stained with fluorescent phalloidin (green). Image is representative of ≥30 fields from ≥3 separate experiments. Scale bar: 5 μm. (F) The effect of β-(1,3)-glucan synthase inhibition on actin cuff formation. Hyphae were prepared as in (E), with varying concentrations of caspofungin, as indicated. Phagocytosis, fixation and staining as in (E). Other details as in (A). Average number of C. albicans per field was 19.7 ± 0.8. (G) Actin cuffs are observed during phagocytosis of A. fumigatus hyphae. After incubation with hyphae, monolayers were fixed and A. fumigatus stained with 10 μg mL−1 calcofluor white (white). Actin stained with phalloidin (green). Image representative of ≥30 fields from ≥2 separate experiments. Scale bar: 5 μm.
-
Figure 4—source data 1
Numerical data corresponding to Figure 4A.
- https://doi.org/10.7554/eLife.34798.014
-
Figure 4—source data 2
Numerical data corresponding to Figure 4B.
- https://doi.org/10.7554/eLife.34798.015
-
Figure 4—source data 3
Numerical data corresponding to Figure 4D.
- https://doi.org/10.7554/eLife.34798.016
-
Figure 4—source data 4
Numerical data corresponding to Figure 4F.
- https://doi.org/10.7554/eLife.34798.017
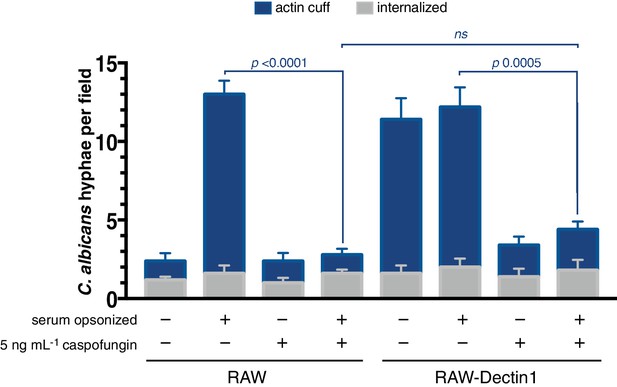
C. albicans β-(1,3)-glucan is required for actin cuff formation.
Candida-BFP was induced to form hyphae in the presence or absence of 5 ng mL−1 caspofungin as in Figure 4E, and additionally prepared with and without serum opsonization as in Figure 4A. RAW or RAW-Dectin1 cells were incubated with prepared C. albicans hyphae. Following phagocytosis, extracellular C. albicans was stained using concanavalin A, and actin stained with phalloidin. The number of C. albicans hyphae that were fully internalized or partially internalized with actin cuffs per 37.5x field was counted by confocal microscopy. Average number of C. albicans per field was 16.6 ± 1.5. For each condition, three independent experiments were quantified, with ≥5 fields counted per replicate. p value was calculated using the unpaired, 2-tailed students t-test. Data are means ±SEM.
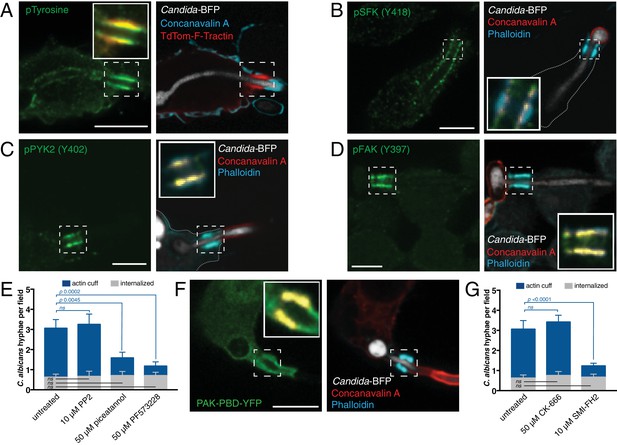
Signaling associated with actin polymerization at the phagocytic cup formed around C. albicans hyphae.
After incubation with Candida-BFP hyphae, RAW-Dectin1 cells were fixed and extracellular C. albicans stained using fluorescent concanavalin A. (A) Phosphotyrosine (pTyrosine) was detected by immunostaining (green). F-actin was visualized using TdTom-F-Tractin (red); concanavalin A (blue). Inset: Colocalization of actin cuff with pTyrosine, in yellow. Image is representative of ≥30 fields from ≥3 separate experiments. (B) Phospho-SFK (Y418) was detected by immunostaining (green); concanavalin A (red). Inset: Colocalization of actin cuff with pSFK, in yellow. (C) Phospho-PYK2 (Y402) was detected by immunostaining (green); concanavalin A (red). Inset: Colocalization of actin cuff with pPYK2, in yellow. (D) Phospho-FAK (Y397) was detected by immunostaining (green); concanavalin A (red). Inset: Colocalization of actin cuff with pFAK, in yellow. Images in B, C and D are representative of ≥30 fields from ≥2 separate experiments of each type. (E) Effect of tyrosine kinase inhibitors on actin cuff formation. RAW-Dectin1 cells were allowed to adhere Candida-BFP hyphae for 15 min and then incubated 45 min in the presence of vehicle, PP2, piceatannol or PF573228. Following phagocytosis, extracellular C. albicans was stained using concanavalin A, and actin stained with phalloidin. The number of C. albicans hyphae that were fully internalized or partially internalized with actin cuffs per 94.5x field was counted by confocal microscopy. Average number of C. albicans per field was 3.4 ± 0.6. For each condition, three independent experiments were quantified, with ≥4 fields counted per replicate. p value was calculated using unpaired, 2-tailed students t-test. Data are means ±SEM. (F) Active Rac/Cdc42 were visualized using PAK(PBD)-YFP as a probe (green). Actin was stained using fluorescent phalloidin (blue); concanavalin A (red). Inset: Colocalization of actin cuff with PAK(PBD), in yellow. Image is representative of ≥30 fields from ≥3 separate experiments. Scale bars: 10 μm. (G) Effect of actin assembly inhibitors on actin cuff formation. RAW-Dectin1 cells were allowed to adhere Candida-BFP hyphae for 15 min, then incubated 45 min in the presence of vehicle, CK-666 or SMI-FH2. Following phagocytosis, extracellular C. albicans was stained using concanavalin A, and actin stained with phalloidin. The number of C. albicans hyphae that were fully internalized or partially internalized with actin cuffs per 94.5x field was counted by confocal microscopy. Average number of C. albicans per field as in (E). For each condition, three independent experiments were quantified, with ≥5 fields counted per replicate. p value was calculated using the unpaired, 2-tailed students t-test. Data are means ±SEM.
-
Figure 5—source data 1
Numerical data corresponding to Figure 5E.
- https://doi.org/10.7554/eLife.34798.021
-
Figure 5—source data 2
Numerical data corresponding to Figure 5G.
- https://doi.org/10.7554/eLife.34798.022
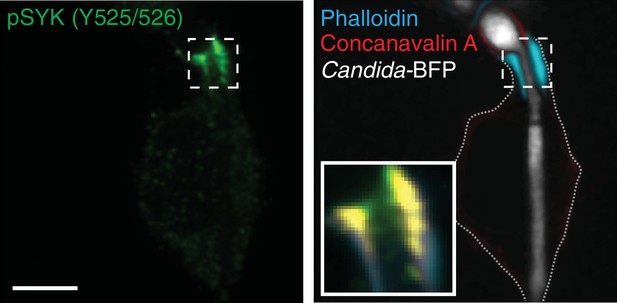
Localization of pSYK to the actin cuff.
After incubation with Candida-BFP hyphae, RAW-Dectin1 cells were fixed and extracellular C. albicans stained with concanavalin A. Phospho-SYK (Y525/526) was detected by immunostaining (green); concanavalin A (red). Inset: Colocalization of actin cuff with pSYK, in yellow. Scale bar: 5 μm. Image is representative of ≥30 fields from ≥2 separate experiments of each type.
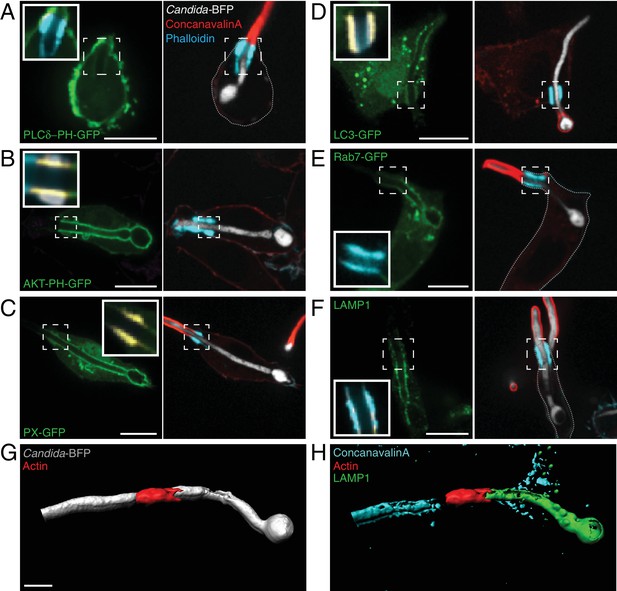
Distribution of phosphoinositides and endo-lysosomal markers.
After incubation with Candida-BFP hyphae, RAW-Dectin1 cells were fixed and extracellular C. albicans stained using Alexa594-conjugated concanavalin A (red). Actin was stained using fluorescent phalloidin (blue), and the location of the actin cuff is indicated by the dashed square. Visualization of: (A) PtdIns(4,5)P2 using PLCδ-PH-GFP; (B) PtdIns(3,4,5)P3/PtdIns(3,4)P2 using AKT-PH-GFP, inset: colocalization of actin cuff with AKT-PH, in yellow; (C) PtdIns(3)P using PX-GFP, inset: colocalization of actin cuff with PX, in yellow; (D) LC3-GFP, inset: colocalization of actin cuff with LC3, in yellow; (E) Rab7-GFP, inset: colocalization of actin cuff with Rab7, in yellow; (F) immunostained LAMP1 (green), inset: colocalization of actin cuff with LAMP1, in yellow. Scale bars: 10 μm. Images are representative of ≥30 fields from ≥3 separate experiments of each type. (G–H) 3D rendering of a RAW-Dectin1 cell with a partially internalized C. albicans hypha. After incubation with Candida-BFP, RAW-Dectin1 cells were fixed and extracellular portions of the hyphae were stained using concanavalin A (blue). (G) C. albicans (white) visualized with actin immunostaining (red). (H) Same 3D rendering as in (G), visualizing LAMP1 immunostaining (green), actin (red) and concanavalin A (blue). Scale bar: 5 μm.
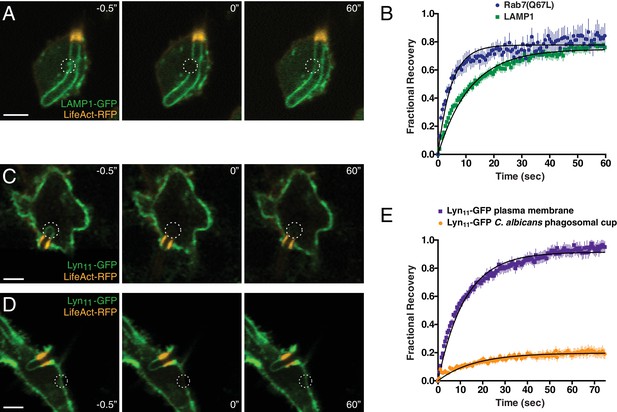
Formation of the actin cuff is associated with the establishment of a diffusional barrier.
RAW-Dectin1 cells were transfected with the indicated constructs, exposed to Candida-BFP, and used for FRAP determinations. F-actin was visualized with LifeAct-RFP (orange). (A) A region of interest (denoted by dotted circle) of LAMP1-GFP in the frustrated phagosome was selected (left panel, −0.5"), photobleached (middle panel, 0"), and allowed to recover for 60 s (right panel, 60"). Scale bar: 5 μm. Images in A, C and D are representative of ≥30 fields from ≥3 separate experiments of each type. (B) Quantitation of fractional recovery of fluorescence after photobleaching LAMP1 (green) or Rab7(Q67L) (blue). In both cases, data were normalized to fluorescence in unbleached regions of the C. albicans phagosomal cup. For either condition, four biological replicates, with a total of ≥30 cells, were quantified. (C–D) A region of interest in the frustrated phagosome (C) or in the plasma membrane (D) of cells expressing Lyn11-GFP was selected (left panel, −0.5"), photobleached (middle panel, 0"), and allowed to recover for 60 s (right panel, 60"). Scale bars: 5 μm. (E) Quantitation of fractional recovery of fluorescence of photobleached Lyn11-GFP in the plasma membrane (blue) or the frustrated C. albicans phagosomal cup (orange). In both cases, FRAP data was normalized to fluorescence in the plasma membrane. For either condition, three biological replicates, with a total of ≥35 cells, were quantified.
-
Figure 7—source data 1
Numerical data corresponding to Figure 7B.
- https://doi.org/10.7554/eLife.34798.027
-
Figure 7—source data 2
Numerical data corresponding to Figure 7E.
- https://doi.org/10.7554/eLife.34798.028
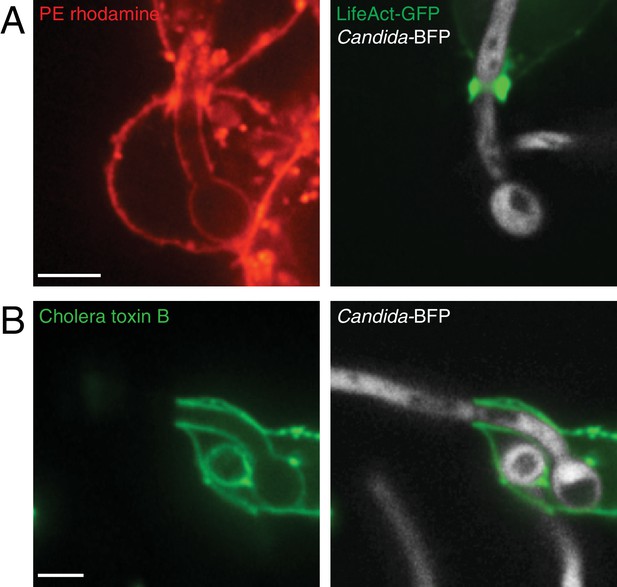
Diffusion of outer leaflet components is not restricted by the actin cuff.
Following phagocytosis of Candida-BFP hyphae, the plasma membrane of RAW-Dectin1 cells was labeled at 4°C with (A) PtdEth-rhodamine (red) or (B) Alexa488-conjugated cholera toxin B (green). After labeling, RAW-Dectin1 cells were incubated ≈5 min at 37°C and viewed live. Where indicated, actin cuff was visualized with transfected LifeAct-GFP. Scale bars: 5 μm.
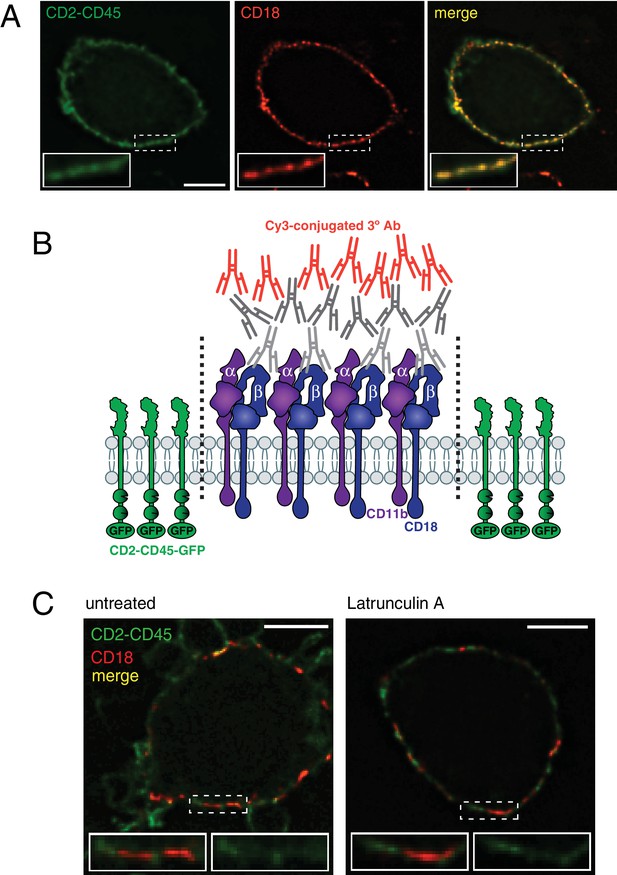
Clustering and patching of CR3 forms a diffusional barrier that excludes transmembrane proteins.
(A) Raw-Dectin1 cells transiently expressing CD2-CD45-GFP (left panel, green) and stained for external CD18 (middle panel, red). Right panel shows the colocalization of CD2-CD45 and CD18, in yellow. Panel insets: 2.1x magnification. Scale bar: 5 μm. (B) Diagram illustrating the method used to cluster CR3 in CD2-CD45-GFP-expressing Raw-Dectin1 cells, using M18/2 antibody to CD18, followed by secondary and tertiary antibodies. See Materials and methods for details. (C) Effect of CR3 patching and actin depolymerization. After clustering CR3 as in (B), cells were incubated 10 min in the absence (left) or presence (right) of 1 μM latrunculin A, and visualized for CD2-CD45-GFP (green) and extracellular CD18 (red). Colocalization of CD2-CD45 and CD18 channels shown in yellow. Panel insets: 2x magnification of both channels (left inset) and CD2-CD45 channel only (right inset). Scale bars: 5 μm.
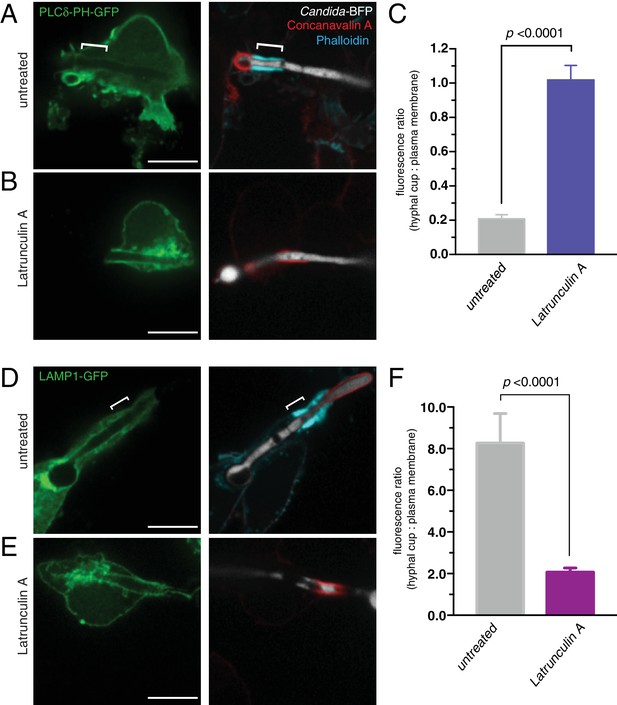
Actin depolymerization abolishes the diffusional barrier around C. albicans hyphae.
RAW-Dectin1 cells were transfected with the indicated constructs, exposed to Candida-BFP hyphae and incubated for 30 min in the presence (B and E) or absence (A and D) of latrunculin A. After treatment, cells were fixed and extracellular C. albicans stained using Alexa594-conjugated concanavalin A (red), and actin stained using fluorescent phalloidin (blue). (A and B) Cells transfected with PLCδ-PH-GFP. (C) Effect of latrunculin A on actin cuff-mediated segregation of PLCδ-PH-GFP to the plasma membrane, quantitated as the ratio of the fluorescence intensity of GFP in the phagocytic cup over the plasma membrane. (D and E) Cells transfected LAMP1-GFP. (F) Effect of latrunculin A on actin cuff-mediated segregation of LAMP1 to the frustrated phagocytic cup, quantitated as the ratio of the fluorescence intensity in the phagocytic cup over the plasma membrane. For A and D, location of the actin cuff is indicated with a bracket. Scale bars: 10 μm. Images are representative of ≥30 fields from ≥3 separate experiments of each type. For each condition in C and F, three independent experiments were quantified, with ≥10 fields counted per replicate. p value was calculated using unpaired, 2-tailed students t-test. Data are means ±SEM.
-
Figure 9—source data 1
Numerical data corresponding to Figure 9C.
- https://doi.org/10.7554/eLife.34798.031
-
Figure 9—source data 2
Numerical data corresponding to Figure 9F.
- https://doi.org/10.7554/eLife.34798.032
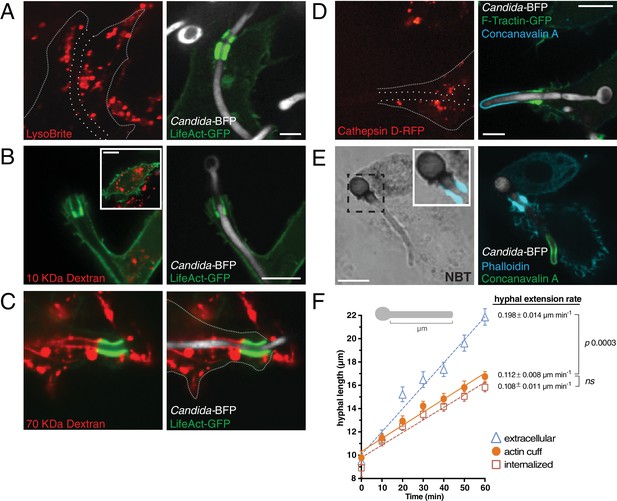
Analysis of the antimicrobial environment within the frustrated phagosome.
(A) After phagocytosis of Candida-BFP hyphae by RAW-Dectin1 cells, acidic compartments were labeled with LysoBrite (red). The open hyphal phagocytic cup is marked with a dotted outline. Actin was visualized using transfected LifeAct-GFP. Scale bar: 5 μm. (B and C) Prior to phagocytosis of C. albicans hyphae, the lysosomes of RAW-Dectin1 cells were loaded with (B) 10 kDa or (C) 70 kDa fluorescent dextran (red). After phagocytosis, retention of dextran in the frustrated hyphal phagocytic cup was assessed by live cell microscopy. Actin was visualized using transfected LifeAct-GFP. Scale bars: 10 μm. (D) Retention of lysosomal hydrolases was assessed using transfected cathepsin D-RFP as a marker (red). Following phagocytosis and fixation, extracellular C. albicans was stained using Alexa647-conjugated concanavalin A (blue). Actin was visualized using transfected F-Tractin-GFP. Frustrated hyphal phagocytic cup marked with a dotted outline. Scale bar: 5 μm. (E) Generation of superoxide within the frustrated hyphal cup was detected using NBT. Following phagocytosis and fixation, extracellular C. albicans was stained using Alexa594 conjugated concanavalin A (red). Actin was stained using fluorescent phalloidin (blue). Inset shows merged image of formazan precipitate and the actin cuff. Scale bar: 5 μm. Images are representative of ≥30 fields from ≥3 separate experiments of each type. (F) Effect of the frustrated phagosome on C. albicans hyphal extension rate. After incubation with Candida-BFP hyphae for 10 min, RAW-Dectin1 cells transiently expressing F-Tractin-GFP were fixed at 10 min intervals, and extracellular C. albicans stained using fluorescent concanavalin A and visualized by confocal microscopy. The length of C. albicans hyphae (see (F), top) was measured for hyphae identified as extracellular (Δ), fully internalized (□) or partially internalized with actin cuffs (•), and the average hyphal length at each time-point and hyphal extension rate calculated. Average number of C. albicans per time-point was 55.0 ± 3.1. For each condition, four independent experiments were quantified, with ≥10 fields (37.5x) counted per replicate. p values calculated using the unpaired, 2-tailed students t-test. Data are means ±SEM.
-
Figure 10—source data 1
Numerical data corresponding to Figure 10F.
- https://doi.org/10.7554/eLife.34798.036
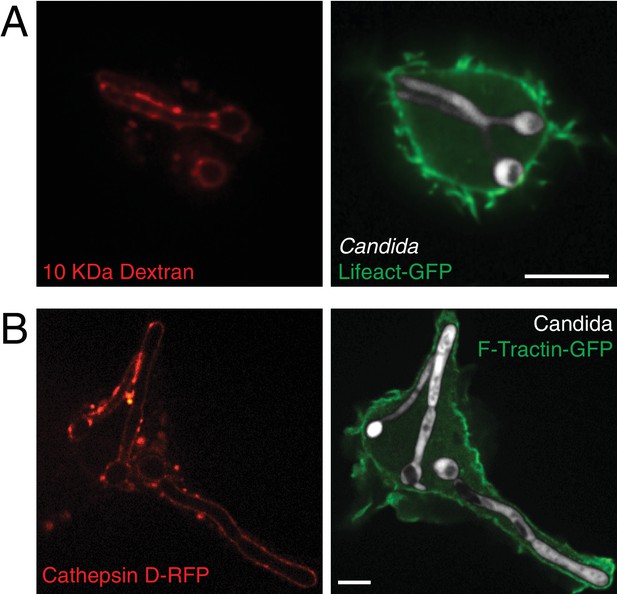
Fully internalized C. albicans hyphae contain typical lysosomal markers.
(A) Prior to phagocytosis of Candida-BFP hyphae, lysosomes of RAW-Dectin1 cells were loaded with 10 kDa fluorescent dextran. After phagocytosis, fully formed phagosomes were allowed to mature for 1 hr and the delivery of dextran (red) was assessed by live cell microscopy. Actin was visualized using transfected LifeAct-GFP. Scale bar: 10 μm. (B) Delivery of lysosomal hydrolases to the matured phagolysosome of fully internalized C. albicans hyphae was assessed as above, using transfected cathepsin D-RFP as a marker. Actin was visualized using transfected F-Tractin-GFP. Scale bar: 5 μm.
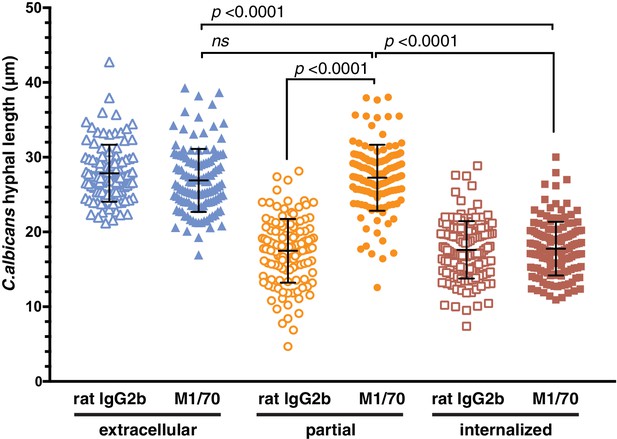
Engagement of integrin CR3 is necessary for the microbiostatic environment of the C. albicans frustrated phagosome.
Prior to phagocytosis, RAW-Dectin1 cells were incubated with CD11b blocking antibody M1/70 or an isotype-matched (rat IgG2b) control antibody as described in Materials and methods. Internalization of Candida-BFP hyphae was allowed to proceed for 60 min in the presence of M1/70 or rat IgG2b. Following phagocytosis, extracellular C. albicans was stained using Alexa594-conjugated concanavalin A, and actin stained using fluorescent phalloidin. The length of C. albicans hyphae was measured for hyphae identified as extracellular, internalized or with actin cuffs, and the average hyphal length per timepoint and hyphal extension rate calculated. For each condition, three independent experiments were quantified, with ≥10 37.5x fields counted per replicate. p values calculated using the unpaired, 2-tailed students t-test. Data are means ±SEM.
Videos
3D rendering of a RAW-Dectin1 cell with a partially internalized Candida-BFP hypha (white), showing the demarcation of concanavalinA (blue) by the actin cuff (red).
See Figure 1 for additional information.
3D rendering of a RAW-Dectin1 cell with a partially internalized Candida-BFP hypha (white), showing the demarcation of phagosomal LAMP1 (green) and concanavalin A (blue) by the actin cuff (red).
See Figure 6 for additional information.
Tables
C. albicans strains used in this study.
https://doi.org/10.7554/eLife.34798.018| Strain | Parent | Genotype | Gene function | Reference |
|---|---|---|---|---|
| Candida-BFP | SC5314 | Peno1-TagBFP-NATR | N/A | (Strijbis et al., 2013) |
| CaSS1 | CAI4 | ura3::imm434 / ura3::imm434 his3::hisG / his3::hisG leu2::tetR-GAL4AD-URA / LEU2 | N/A | (Roemer et al., 2003) |
| CHT1 | CaSS1 | tetO-CHT1 / cht1∆ | chitinase | (O'Meara et al., 2015) |
| CDA2 | CaSS1 | tetO-CDA2 / cda2∆ | chitin deacetylase | (O'Meara et al., 2015) |
| MNT2 | CaSS1 | tetO-MNT2 / mnt2∆ | α-(1,2)-mannosyl transferas | (O'Meara et al., 2015) |
| VRG4 | CaSS1 | tetO-VRG4 / vrg4∆ | GDP-mannose transporte | (O'Meara et al., 2015) |
| KRE1 | CaSS1 | tetO-KRE1 / kre1∆ | cell wall glycoprotein, β-(1,6)-glucan synthesis | (O'Meara et al., 2015) |
| KRE6 | CaSS1 | tetO-KRE6 / kre6∆ | β-(1,6)-glucan synthase subunit | (O'Meara et al., 2015) |
| KRE62 | CaSS1 | tetO-KRE62 / kre62∆ | β-(1,6)-glucan synthase subunit | (O'Meara et al., 2015) |
| KEG1 | CaSS1 | tetO-KEG1 / keg1∆ | integral membrane ER protein, β-(1,6)-glucan synthesis | (O'Meara et al., 2015) |
| ENG1 | CaSS1 | tetO-ENG1 / eng1∆ | endo-(1,3)-β-glucanase | (O'Meara et al., 2015) |
| ACF2 | CaSS1 | tetO-ACF2 / acf2∆ | endo-(1,3)-β-glucanase | (O'Meara et al., 2015) |
| BGL22 | CaSS1 | tetO-BGL22 / bgl22∆ | putative β-glucanase | (O'Meara et al., 2015) |
Additional files
-
Transparent reporting form
- https://doi.org/10.7554/eLife.34798.037





