Orbital frontal cortex updates state-induced value change for decision-making
Figures
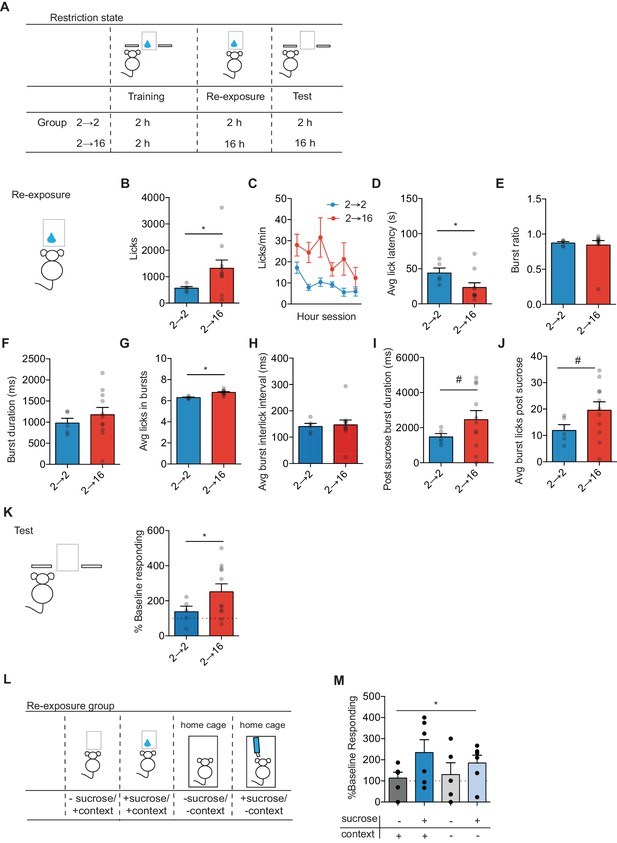
Positive incentive learning in mice.
(A) Schematic showing training, re-exposure and testing schedule for positive incentive learning. Group n’s: 2→ 2: n = 5; 2→ 16: n = 11. Data points and bar graphs represent the mean ± SEM. (B) Number of licks and (C) licking rate (10 min bins) during the re-exposure session. (D) The average latency to begin licking after a sucrose delivery (s). (E) The ratio of licks that occur in bursts, (F) average duration of bursts (ms), (G) average number of licks within a burst, and (H) average interlick interval within bursts (ms). (I) Average burst duration after a sucrose delivery and (J) average number of licks within a burst after a sucrose delivery. (K) Response rate during the 5 min non-rewarded test as a percent of acquisition response rate (last 2 days of training). (L) Schematic of training, re-exposure, and testing schedule for context positive incentive learning. Group n’s: context + sucrose -: n = 5; context + sucrose + : n = 11; context - sucrose -: n = 11; context – sucrose +: n = 11 (M). (J) Percent of baseline responding (last two training days) for mice not exposed to sucrose, not exposed to sucrose nor the context, exposed to sucrose in the context, and exposed to sucrose in the home cage. * indicates p=0.05, # indicates p=0.06.
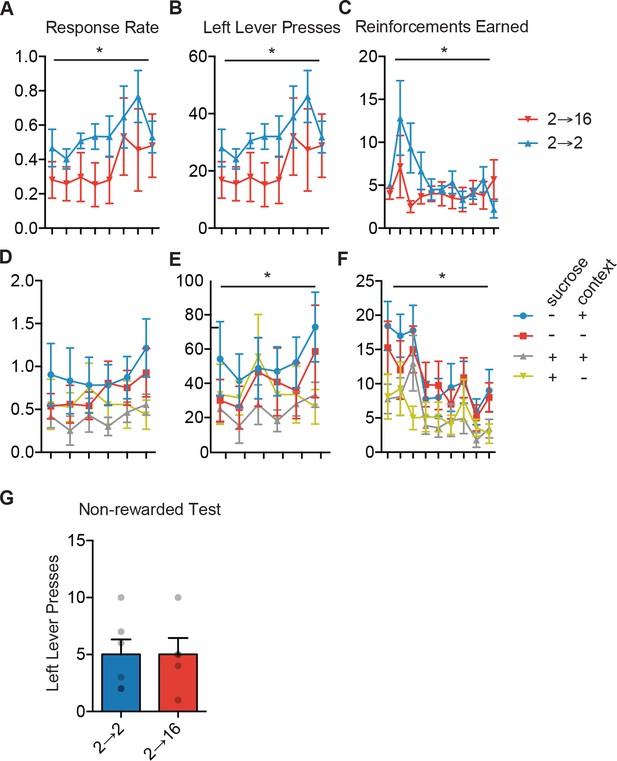
Acquisition of lever pressing for positive and context incentive learning.
(A) Responses per minute during training days on the left lever for positive incentive learning group. Two-way repeated measured ANOVA: no interaction; no effect of group; main effect of training day (F (7, 70)=3.375, p=0.0037). (B) Left lever presses for each training day for incentive learning. Two-way repeated measures ANOVA: no interaction; no effect of group; main effect of training day: F (7,70)=3.37, p=0.0037. (C) Sucrose outcomes earned each training day during positive incentive learning. Two-way repeated measures ANOVA: no interaction; no effect of group; main effect of training day: F(10,100) = 2.58, p=0.008. (D) For context experiment, mice were trained at a 2 hr food restriction and tested at a 16 hr restriction. Mice were either re-exposed to sucrose in the operant context, in their home cage, or were not re-exposed to sucrose and had the same amount of time in the operant context or home cage. Responses per minute of mice trained at a 2 hr restriction. Two-way repeated measures ANOVA: No interaction or main effects (Fs < 1.52, ps >.19) (E) Left lever presses over each training day. Two-way repeated measures ANOVA: no interaction: F = 1.02, p=0.44; no main effect of Group: F = 0.90, p=0.46; main effect of training day: F(5,130) = 2.82, p=0.0187. (F) Sucrose outcomes earned over training days. Two-way repeated measures ANOVA: no Group x training day interaction, F = 0.90, p=0.61; no main effect of group, F = 2.38, p=0.09; main effect of training day: F(8,232) = 9.72, p<0.0001. (G) Left lever presses on non-rewarded test day for positive incentive learning. Unpaired t-test: F < 1.01, p>0.99.
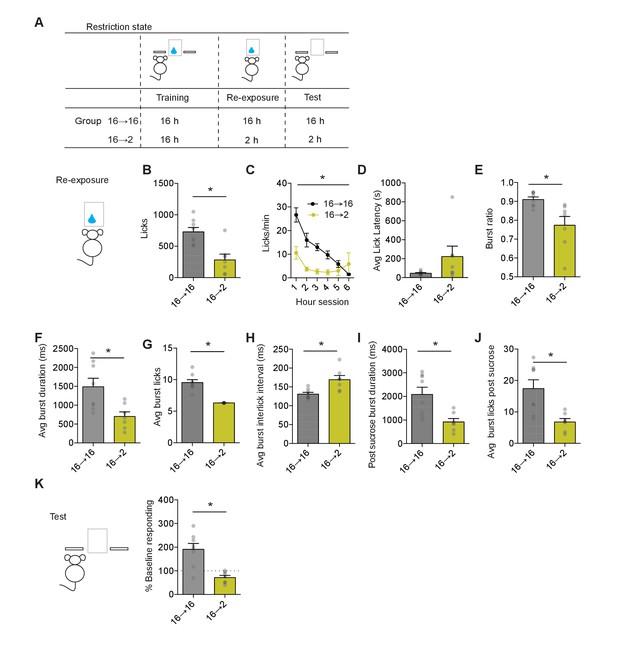
Negative incentive learning in mice.
(A) Schematic showing training, re-exposure, and testing schedule for negative incentive learning. Group n’s: 16→ 16: n = 8; 16→ 2: n = 7. Data points and bar graphs represent the mean ± SEM. (B) Number of licks and (C) licking rate (10 min bins) during the re-exposure session. (D) The average latency to begin licking after a sucrose delivery (s). (E) The ratio of licks that occur in bursts, (F) average duration of bursts (ms), (G) average number of licks within a burst, and (H) average interlick interval within bursts (ms). (I) Average burst duration after a sucrose delivery and (J) average number of licks within a burst after a sucrose delivery. (K) Response rate during the 5 min non-rewarded test as a percent of acquisition response rate (last 2 days of training). * indicates p<0.05.
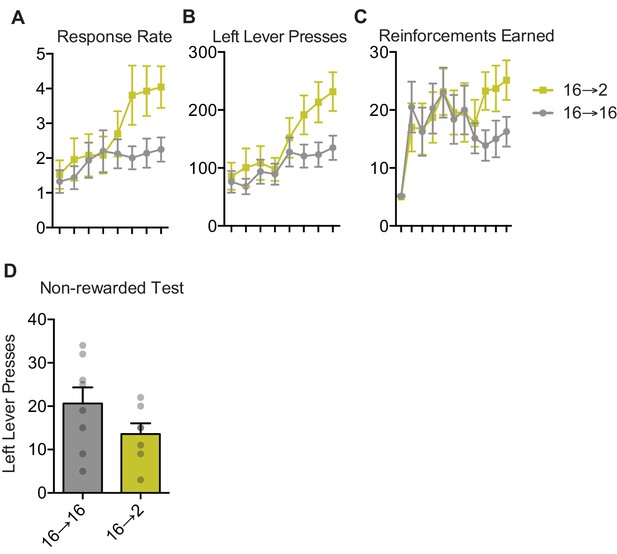
Acquisition of lever pressing for negative incentive learning.
(A) Responses per minute during training days on the left lever for negative incentive learning group. Two-way ANOVA: interaction (F (7,91)=3.97, p=0.0008); no effect of group; main effect of training day (F (7, 91)=9.64, p<0.0001). (B) Left lever presses for each training day for negative incentive learning. Negative incentive learning: interaction: F (7,91)=3.29, p=0.0036; no effect of group; main effect of training day: F (7,91)=16.14, p<0.0001. (C) Sucrose outcomes earned each training day for negative incentive learning. Negative incentive learning: no interaction; no effect of group; main effect of training day: F (10,130)=7.77, p<0.0001. (D) Left lever presses on non-rewarded test day for negative incentive learning. Unpaired t-test: F = 2.58, p=0.1489.
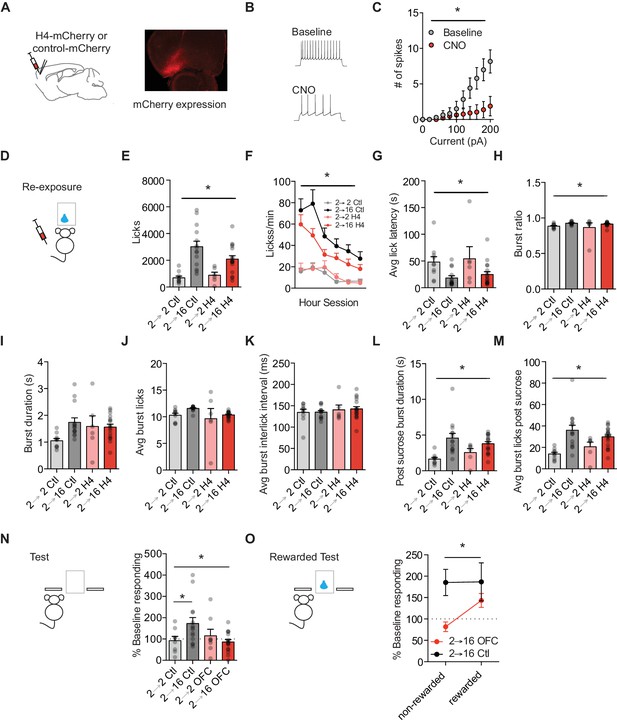
Orbitofrontal cortex attenuation prevents positive incentive learning.
(A) Schematic of injection site (left) and representative mCherry expression in OFC (right). (B) Representative traces and (C) summary data from ex vivo physiological whole cell recordings in HMD4i expressing OFC projection neurons during baseline and following CNO bath application to H4 slice. (cells n = 8). (D) Training and testing schematic showing when OFC manipulations were given, with CNO given only during the re-exposure session. Group n’s: 2→ 2 Ctl: n = 9; 2→ 16 Ctl: n = 14; 2→ 2 H4: n = 7; 2 → 16 H4: n = 17. (E) Number of licks and (F) licking rate (10 min bins) during the re-exposure session. (G) The average latency to begin licking after a sucrose delivery (s). (H) The ratio of licks that occur in bursts, (I) average duration of bursts (s), (J) average number of licks within a burst, and (K) average interlick interval within bursts (ms). (L) Average burst duration after a sucrose delivery (s) and (M) average number of licks within a burst after a sucrose delivery. (N) Response rate during the 5 min non-rewarded test as a percent of acquisition response rate (last 2 days of training). (O) Percent of baseline responding from non-rewarded to the rewarded test Data points represent individual subjects and bar graphs and error bars represent the mean ± SEM. * indicates p<0.05.
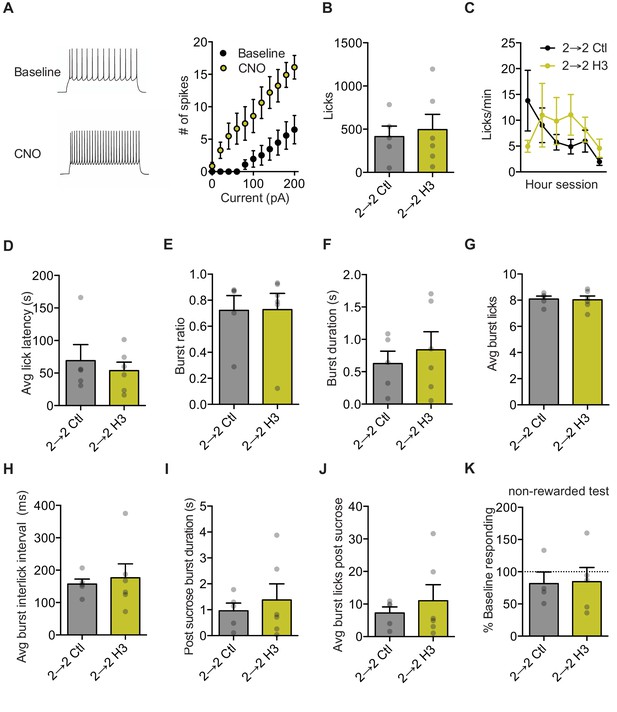
Orbitofrontal cortex excitation does not generate an increased motivational state.
(A) Spikes and trace from H3 slice data (cells n = 7). Two-way repeated measures ANOVA: interaction of Current x Treatment: F(10,60) = 2.94; Main effect of Treatment: F(1,6) = 23.09, p=0.003; main effect of current: F(10,60) = 29.63, p<0.0001. (B) Total licks during RT session. (Group n’s: (2→ 2 Ctl: 5), (2→ 2 H3: 6)) (C) Licking rate in 10 min bins over the 60 min re-exposure session. (D) Licks occurring in bursts over total licks. (E) Average burst duration. (F) Average number of licks within a burst (G) Average latency to begin licking after sucrose deliveries. (H). Average interlick interval within bursts. (I) Average burst duration after sucrose deliveries. (J) Average number of licks within a burst after an outcome delivery. (K) Percent of baseline responding during non-rewarded test. Data points and bar graphs represent the mean ±SEM. * indicates p<0.05. All t-tests for licking data and non-rewarded test day: p>0.05.
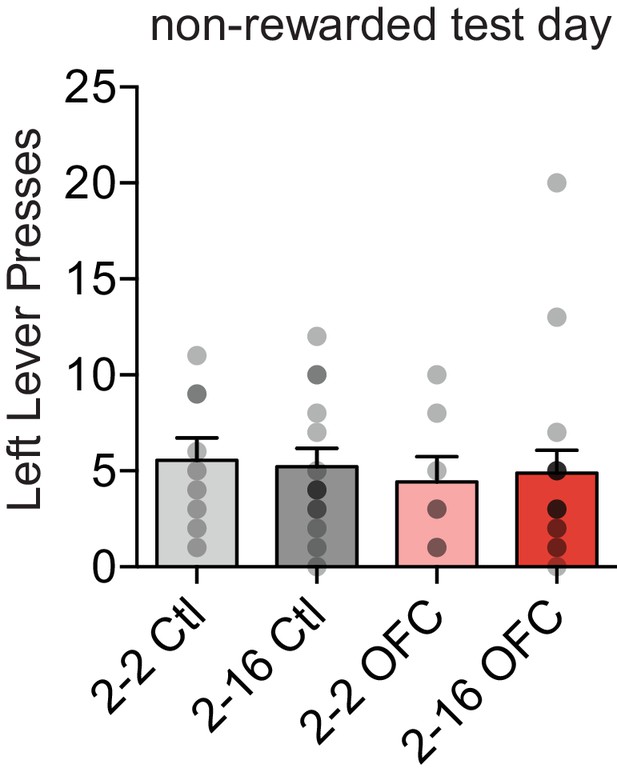
Left lever presses in OFC positive incentive learning during test day.
Two-way ANOVA (Food Restriction x OFC Treatment): no interactions, no main effects (Fs < 0.33, ps > 0.56).
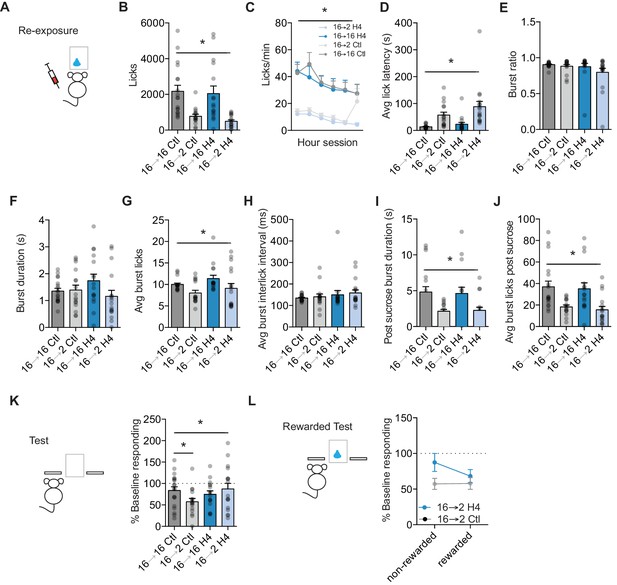
Orbital frontal cortex attenuation prevents negative incentive learning.
(A) Training, re-exposure, and testing schematic showing that OFC attenuation occurred only during the re-exposure session. Group n’s: 16→ 16 Ctl: n = 18; 16→ 2 Ctl: n = 16; 16→ 16 H4: n = 16; 16→ 2 H4: n = 17.(B) Number of licks and (C) licking rate (10 min bins) during the re-exposure session. (D) The average latency to begin licking after a sucrose delivery (s). (E) The ratio of licks that occur in bursts, (F) average duration of bursts (ms), (G) average number of licks within a burst, and (H) average interlick interval within bursts (ms). (I) Average burst duration after a sucrose delivery (s) and (J) average number of licks within a burst after a sucrose delivery. (K) Response rate during the 5 min non-rewarded test as a percent of acquisition average response rate (last 2 days of training). (L) Percent of baseline responding from non-rewarded to the rewarded test. * indicates p<0.05.
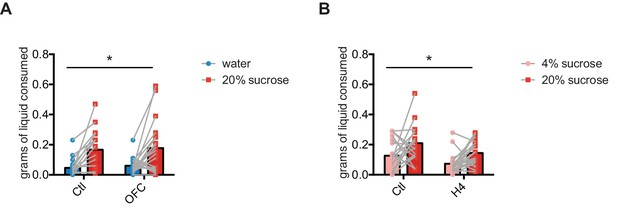
Orbitofrontal cortex inhibition does not change sucrose preference.
(A) Control mice and OFC-attenuated mice 20% sucrose solution and water consumption during a two-bottle choice test. Two-way ANOVA, main effect of liquid: F (1, 36)=24.47, p<0.0001; no effect of OFC treatment; no interaction. (B) Control mice and OFC-attenuated mice 20% and 4% sucrose solution consumption during a two-bottle choice test. Two-way ANOVA, main effect of sucrose concentration: F (1, 36)=11.11, p=0.002; main effect of OFC treatment: F (1, 36)=8.42, p=0.006; and no interaction.
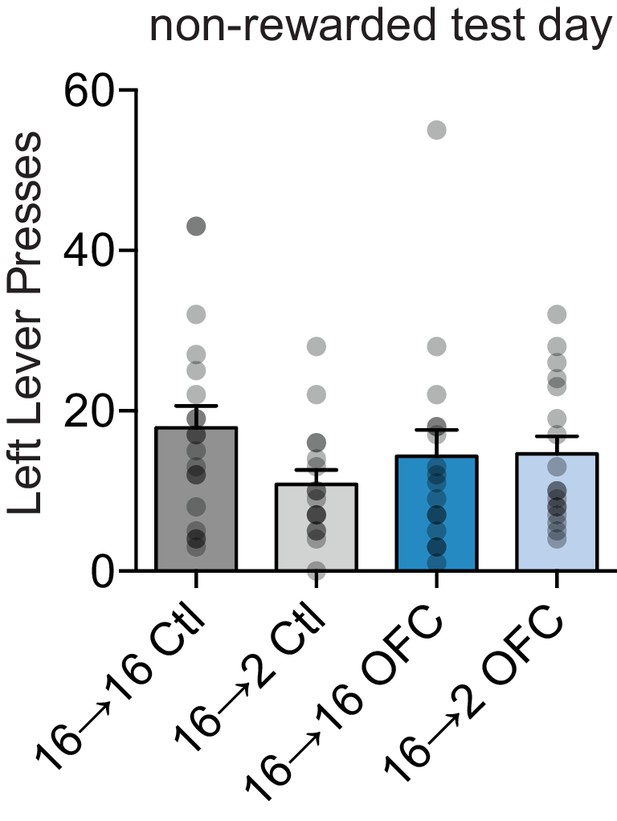
Left lever presses during OFC negative incentive learning test day.
Two-way ANOVA (Food Restriction x OFC Treatment): no interaction, no main effects (Fs < 2.1, ps > 0.15).
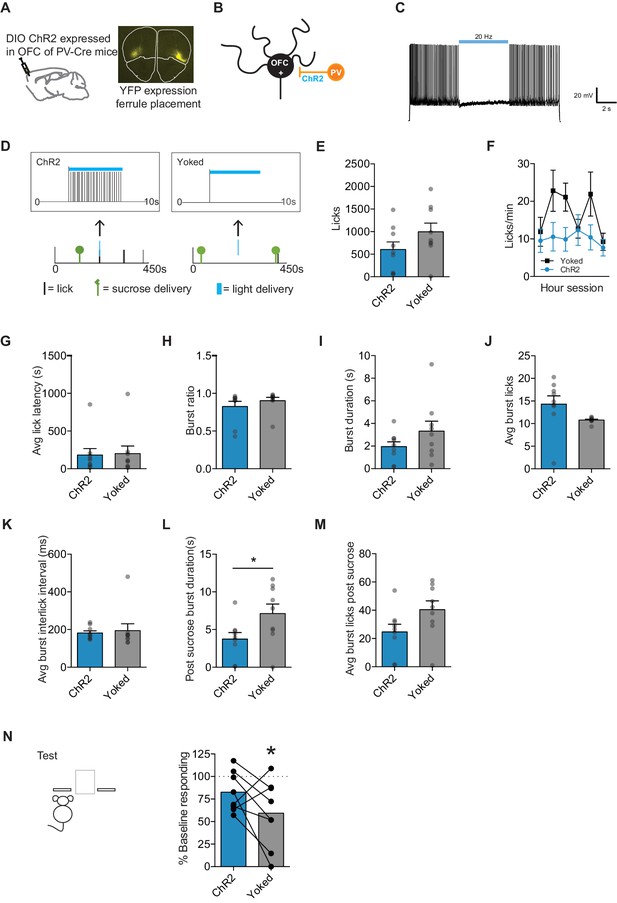
Optogenetic inhibition of OFC projection neurons during sucrose consumption prevents value updating.
(A) Schematic of injection site and ferrule implant (top), with DIO ChR2-eYFP detected at OFC injection site (bottom). (B) Schematic of setup of OFC excitatory neuron (OFC +) inhibition by activation of Parvalbumin (PV) interneurons (C) Confirmation of ChR2 function using ex vivo whole-cell recording. (D) Closed-loop behavioral control over light delivery. Example where in the ChR2 group the first lick after a sucrose delivery resulted in a 5 s light delivery (5 ms pulse, 20 Hz) (left), while Yoked group received light delivery at the same time independent of licking behavior (right). Group n’s: ChR2: n = 8, Yoked: n = 8. (E) Number of licks and (F) licking rate (10 min bins) during the re-exposure session. (G) The average latency to begin licking after a sucrose delivery (s). (H) The ratio of licks that occur in bursts, (I) average duration of bursts (ms), (J) average number of licks within a burst, and (K) average interlick interval within bursts (ms). (L) Average burst duration after a sucrose delivery (s) and (M) average number of licks within a burst after a sucrose delivery. (N) Response rate during the 5 min non-rewarded test as a percent of acquisition response rate (last 2 days of training). Data points and bar graphs represent the mean ± SEM. * indicates p<0.05.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Strain, strain background (Mus musculus) | Emx1-Cre | The Jackson Laboratory | RRID:IMSR_JAX:005628 | maintained on a C57BL6/J background |
| Strain, strain background (Mus musculus) | C57Bl/6J | The Jackson Laboratory | RRID:IMSR_JAX:000664 | |
| Strain, strain background (Mus musculus) | PV-Cre | The Jackson Laboratory | RRID:IMSR_JAX:017320 | maintained on a C57BL6/J background |
| Strain, strain background (Adeno-associated virus) | AAV5-hSyn-DIO- hM4D(Gi)-mCherry | UNC Viral Vector Core; Addgene | ||
| Strain, strain background (Adeno-associated virus) | AAV5-CamKIIa- GFP-Cre | UNC Viral Vector Core | ||
| Strain, strain background (Adeno-associated virus) | AAV5-hSyn-DIO- hM3D(Gq)-mCherry | UNC Viral Vector Core | ||
| Strain, strain background (Adeno-associated virus) | AAV5-hSyn-DIO -mCherry | UNC Viral Vector Core | ||
| Strain, strain background (Adeno-associated virus) | AAV Ef1a-DIO- hChR2(H134R)-eYFP | UNC Viral Vector Core | ||
| Software, algorithm | MATLAB | this paper | RRID:SCR_001622 | https://github.com/gremellab/lickingstructure; copy archived at https://github.com/elifesciences-publications/lickingstructure |
| Software, algorithm | Arduino | this paper | https://github.com/gremellab/arduinoLEDcontrol; copy archived at https://github.com/elifesciences-publications/arduinoLEDcontrol | |
| Software, algorithm | GraphPad Prism 6 | GraphPad | RRID:SCR_002798 | |
| Software, algorithm | Adobe Illustrator CS6 | Adobe | RRID:SCR_010279 | |
| Software, algorithm | AxographX | Axograph, Sydney, Australia | RRID:SCR_014284 | |
| Software, algorithm | JASP | JASP Team (2018). JASP (Version 0.8.6) | RRID:SCR_015823 | |
| Chemical compound, drug | clozapine-n-oxide | NIMH Chemical Synthesis and Drug Supply Program | C-929 | |
| Chemical compound, drug | NaCl | Thermo Fisher Scientific | S271 | |
| Chemical compound, drug | NaHCO3 | Thermo Fisher Scientific | S233 | |
| Chemical compound, drug | Dextrose | Thermo Fisher Scientific | D16 | |
| Chemical compound, drug | KCl | MilliporeSigma | P9541 | |
| Chemical compound, drug | NaH2PO4 | MilliporeSigma | S3139 | |
| Chemical compound, drug | Sucrose | MilliporeSigma | S8501 | |
| Chemical compound, drug | KMeSO4 | MilliporeSigma | 83000 | |
| Chemical compound, drug | HEPES | MilliporeSigma | H4034 | |
| Chemical compound, drug | EGTA | MilliporeSigma | 3777 | |
| Chemical compound, drug | MG-ATP | MilliporeSigma | A9187 | |
| Chemical compound, drug | Tris-GTP | MilliporeSigma | G9002 | |
| Chemical compound, drug | MgSO4 | MilliporeSigma | M2643 | |
| Chemical compound, drug | CaCl2 | MilliporeSigma | 223506 | |
| Chemical compound, drug | Picrotoxin | MilliporeSigma | P1675 |
Additional files
-
Supplementary file 1
Table 1: Effects of Strain on Responses Table 2: Comparison of Saline vs. CNO-treated Controls.
- https://doi.org/10.7554/eLife.35988.010
-
Transparent reporting form
- https://doi.org/10.7554/eLife.35988.015





