Corollary discharge in precerebellar nuclei of sleeping infant rats
Figures
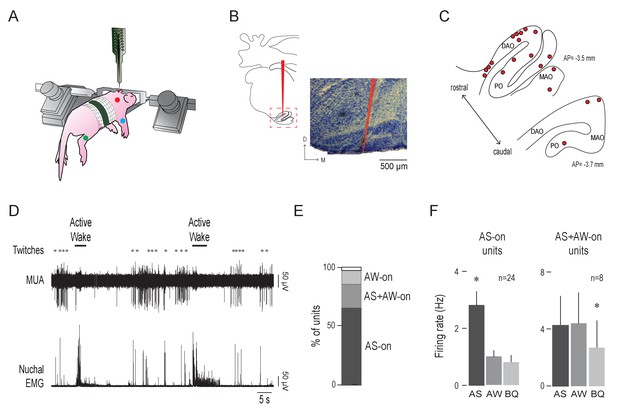
Olivary activity predominates during active sleep.
(A) Illustration of a head-fixed rat pup in a recording apparatus instrumented with nuchal (red), forelimb (blue), and hindlimb (green) EMG electrodes. (B) Left: Reconstruction of a representative electrode placement within the IO (red line). Red dashed box circumscribes the IO. Right: Representative coronal Nissl-stained brain section. Red line is the trace of a DiI-coated electrode placed within the IO. D: dorsal; M: medial (C) Electrode placements (red circles) within the IO in two coronal sections across all subjects. DAO: dorsal accessory olive; MAO: medial accessory olive; PO: principal olive; AP: antero-posterior distance in relation to lambda. (D) Representative recording of rectified nuchal EMG activity and multiunit activity (MUA) in the IO during spontaneous sleep-wake cycling. Asterisks denote twitches and horizontal bars denote periods of active wake movements as scored by the experimenter. (E) Stacked plot showing the percentage of IO units that were AS-on, AS+AW-on, and AW-on. (F) Mean (+SEM) firing rates of AS-on (left) and AS+AW-on (right) units across behavioral states. Each individual unit included in these means was significantly state dependent. * significant difference from the other two behavioral states, p < 0.008. AS: active sleep; AW: active wake; BQ: behavioral quiescence.
-
Figure 1—source data 1
Source data for panels E and F.
- https://doi.org/10.7554/eLife.38213.003
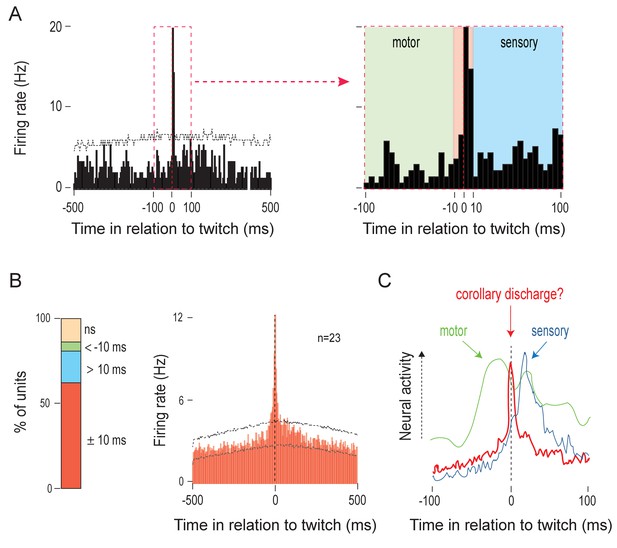
Twitches trigger sharp, short-latency olivary activity.
(A) Left: Perievent histogram (5-ms bins) showing sharp, short-latency activity of a representative IO unit in relation to nuchal muscle twitches. Upper confidence band (p < 0.01 for each band) is indicated by the horizontal dashed line (lower confidence band is at zero). The red dashed box demarcates the ±100-ms time window around twitches. Right: IO unit activity within the ±100-ms period around twitches. Three time windows are shown: <-10 ms (green), ±10 ms (red), and >10 ms (blue), respectively. (B) Left: Stacked plot showing the percentage of IO units that exhibited significant increases in firing within the three time windows around twitches. Right: Perievent histogram (5-ms bins) showing IO unit activity in relation to twitches for those units that were significantly active within the ±10 ms time window. Data are pooled across 23 units and triggered on 6602 twitches. Upper and lower confidence bands (p < 0.01 for each band) are indicated by horizontal dashed lines. ns: not significant. (C) Illustrative comparison of IO activity in relation to twitches (red line; from B) with the neural activity of a representative motor structure (green line; data for the RN from Del Rio-Bermudez et al., 2015) and sensory structure (blue line; data for the ECN from Tiriac and Blumberg, 2016).
-
Figure 2—source data 1
Source data for panels A-C.
- https://doi.org/10.7554/eLife.38213.007
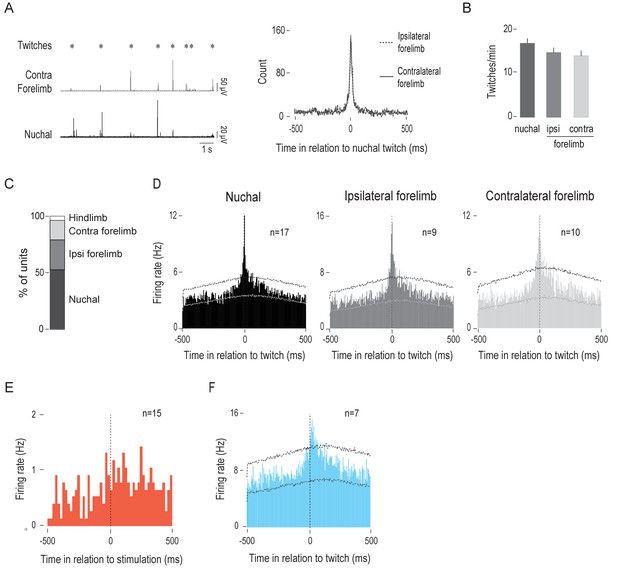
IO units respond predominantly to nuchal and forelimb twitches.
(A) Left: Representative recordings of nuchal and contralateral forelimb EMG activity to show the temporal relations among twitches in those muscles. Asterisks denote experimenter-scored twitches. Right: Cross-correlogram (5-ms bins) of nuchal muscle twitches with ipsilateral (dotted line) and contralateral (solid line) forelimb twitches. (B) Mean (+SEM) twitching per min for nuchal muscle and ipsilateral (ipsi) and contralateral (contra) forelimb muscles across 15 P8 rats. Ipsi: ipsilateral; contra: contralateral. (C) Stacked plot showing the percentage of IO units that exhibited significant activity within ±10 ms of twitch onset in relation to nuchal, ipsilateral forelimb, contralateral forelimb, and hindlimb twitches. (D) Perievent histograms (5-ms bins) showing IO unit activity in relation to nuchal, ipsilateral forelimb, and contralateral forelimb twitches. Data are pooled across 17, 9, and 10 units, respectively. Upper and lower confidence bands (p < 0.01 for each band) are indicated by horizontal dashed lines. (E) Perievent histogram (20-ms bins) showing IO unit activity in relation to peripheral limb stimulation for units that were most active within ±10 ms of twitch onset. Data are pooled across 15 units and triggered on 369 stimulations (F) Perievent histogram (5-ms bins) showing twitch-related IO unit activity for units that were most active >10 ms after twitch onset. Data are pooled across 7 units and triggered on 2059 twitches. Upper and lower confidence bands (p < 0.01 for each band) are indicated by horizontal dashed lines.
-
Figure 2—figure supplement 1—source data 1
Source data for panels A-F.
- https://doi.org/10.7554/eLife.38213.006
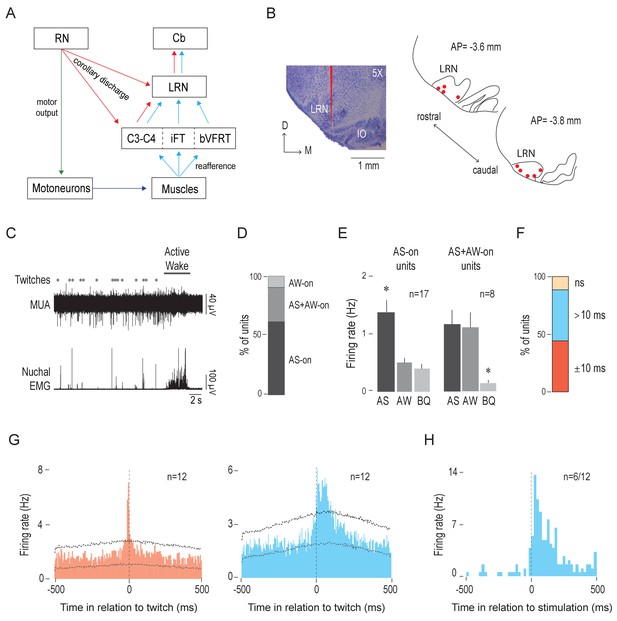
The LRN receives twitch-related corollary discharge and reafference signals.
(A) Diagram depicting afferent and efferent connections of the LRN. Pathways conveying motor commands (green), reafference (blue), and corollary discharge (red) are shown (see Alstermark and Ekerot, 2013). C: cervical segment; Cb: cerebellum; iFT: ipsilateral forelimb tract; bVFRT: bilateral ventral flexor reflex tract. (B) Left: Representative coronal Nissl-stained brain section to show the trace of a DiI-coated electrode placed within the LRN (red line). Right: Electrode placements (red circles) within the LRN in two coronal sections across all P8 subjects (n = 9). D: dorsal; M: medial; AP: antero-posterior distance in relation to lambda. (C) Representative recording of rectified nuchal EMG activity and multiunit activity (MUA) in the LRN during spontaneous sleep-wake cycling. Asterisks denote twitches and the horizontal bar denotes a period of active wake movements as scored by the experimenter. (D) Stacked plot showing the percentage of LRN units that were AS-on, AS+AW-on, and AW-on. (E) Mean (+SEM) firing rates of AS-on (left) and AS+AW-on (right) units across behavioral states. Each individual unit included in these means was significantly state dependent. * significant difference from the other two behavioral states, p < 0.02. (F) Stacked plot showing the percentage of LRN units that significantly increased their firing rates within two time windows in relation to twitch onset: ±10 ms (red) and >10 ms (blue). ns: not significant. (G) Perievent histograms (5-ms bins) showing LRN unit activity in relation to twitches. Left: Data pooled across 12 units (triggered on 3688 twitches) that significantly increased their activity in the ±10-ms time window (red). Right: Data pooled across the 12 units (triggered on 5264 twitches) that exhibited a significant peak in the >10-ms time window (blue). Upper and lower confidence bands (p < 0.01 for each band) are indicated by horizontal dashed lines. (H) Perievent histogram (20-ms bins) showing LRN unit activity in response to forelimb or hindlimb stimulation for those units (6/12) that significantly increased their activity in the >10-ms time window (blue histogram in G). Black vertical dashed line denotes stimulation onset as determined using EMG activity.
-
Figure 3—source data 1
Source data for panels D-H.
- https://doi.org/10.7554/eLife.38213.009
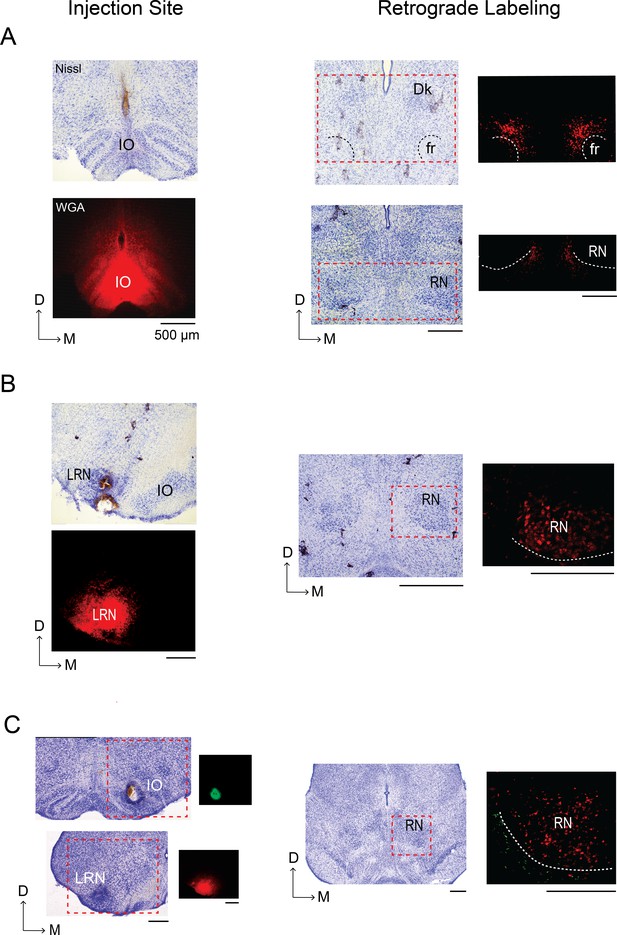
Retrograde labeling of the mesodiencephalic junction (MDJ) after infusion of WGA into the IO and LRN of P8 rats.
(A) Left column: Coronal section depicting WGA-555 diffusion at the injection site in the IO; an adjacent Nissl-stained section is shown above. Right column: Nissl-stained coronal sections within the MDJ; retrograde labeling in the regions within the red-dashed boxes is shown at right for adjacent sections. No labeling was seen in the nucleus of Darkschewitsch (Dk) or RN, consistent with published work in adult rats (Ruigrok et al., 2014). White dashed lines show the boundaries of the fasciculus retroflexus (fr) and RN. (B) Left column: Coronal section depicting WGA-555 diffusion at the injection site in the LRN; an adjacent Nissl-stained section is shown above. Right column: Nissl-stained coronal section at the level of the RN; retrograde labeling in the region within the red-dashed box is shown at right for an adjacent section. White dashed line shows the ventromedial boundary of the RN. (C) Left column: Nissl-stained coronal sections from a single P8 rat to show the sites of WGA injection in the IO (WGA-555, red) and contralateral LRN (WGA-488, green); red-dashed boxes denote regions for the adjacent fluorescent sections shown at right. Right column: Nissl-stained coronal section at the level of the RN; retrograde labeling in the region within the red-dashed box is shown at right for an adjacent section. White dashed line shows the ventromedial boundary of the RN. D: dorsal; M: medial. All scale bars are 500 µm.
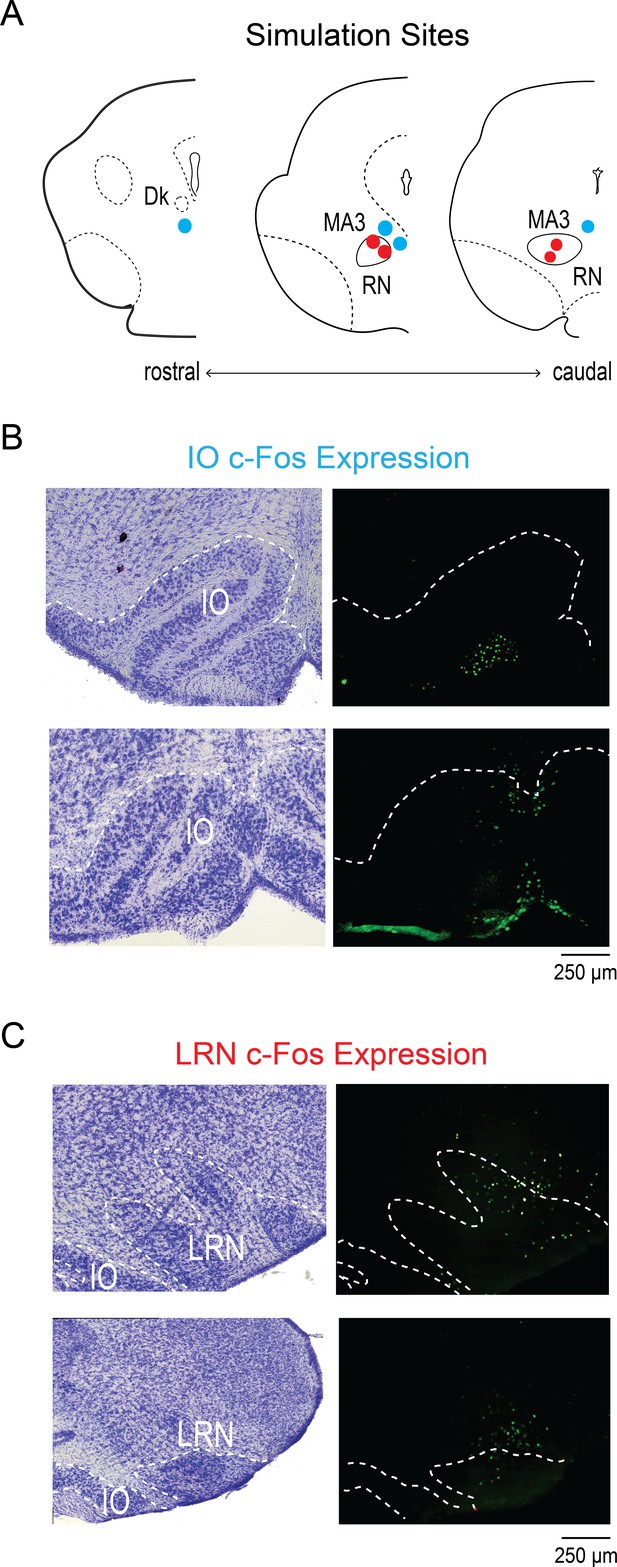
MDJ stimulation increases c-Fos expression in the IO and LRN.
(A) Coronal reconstructions of the stimulation sites within the red nucleus (RN; red dots) and in the MDJ region outside the RN (blue dots) in P8 rats. Dk: nucleus Darkschewitsch; MA3: accessory oculomotor nucleus. (B) For two different pups, Nissl-stained sections at the level of the IO (left) and adjacent sections showing c-Fos expression in response to stimulation within the MDJ but outside the RN (blue dots in A). The boundary of the IO is indicated by white dotted lines. (C) For the same pup, Nissl-stained sections at the level of the LRN (left) and adjacent sections showing c-Fos expression in response to RN stimulation (red dots in A). The boundary of the LRN is indicated by white dotted lines.
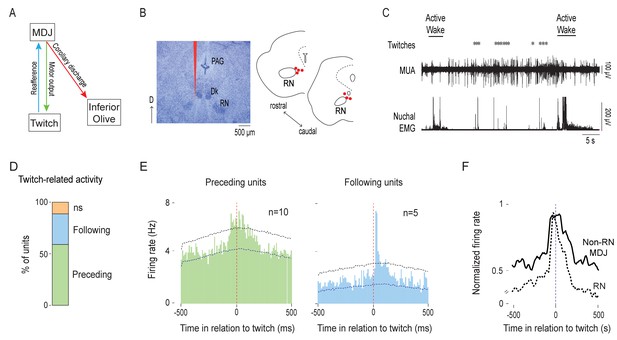
MDJ structures adjacent to the red nucleus exhibit twitch-preceding and twitch-following activity.
(A) Diagram showing anatomical connections of the MDJ regions that lie adjacent to the red nucleus. Proposed pathways conveying motor commands (green line), reafference (blue line), and corollary discharge (red line) are shown. (B) Left: Representative Nissl-stained coronal brain section. Red line is the trace of a DiI-coated electrode placed within the MDJ but outside the RN. Right: Reconstruction of electrode placements (red circles) in the MDJ in two coronal sections across all pups (n = 7). D: dorsal; PAG: periaqueductal gray; Dk: nucleus of Darkschewitsch. (C) Representative recording of rectified nuchal EMG activity and multiunit activity (MUA) in the MDJ during spontaneous sleep-wake cycling. Asterisks denote twitches and horizontal bars denote periods of active wake movements as scored by the experimenter. (D) Stacked plot showing the percentage of twitch-preceding (motor; green) and twitch-following (sensory; blue) units in the MDJ. ns: not significant. (E) Left: Perievent histogram (10-ms bins) showing activity of twitch-preceding MDJ units in relation to twitches. Data are pooled across 10 units and triggered on 2877 twitches. Right: Perievent histogram (10-ms bins) showing activity of twitch-following MDJ units in relation to twitches. Data are pooled across 5 units and triggered on 1382 twitches. Upper and lower confidence bands (p < 0.05 for each band) are indicated by horizontal dashed lines. (F) Perievent histograms (10-ms bins) comparing normalized firing rate in relation to twitch onset for twitch-preceding units in the red nucleus (RN; dashed black line; data from Del Rio-Bermudez et al., 2015) with that of non-RN MDJ units adjacent to the red nucleus (solid black line; redrawn from E, left).
-
Figure 5—source data 1
Source data for panels D-F.
- https://doi.org/10.7554/eLife.38213.015
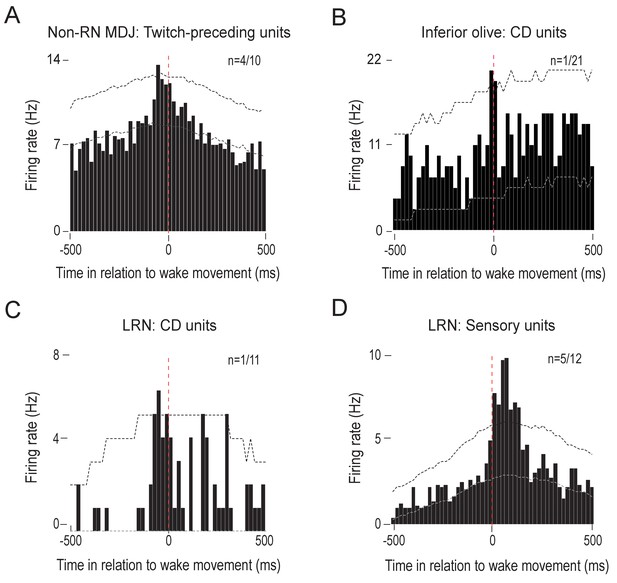
Neural activity in relation to wake-movement onset.
(A) Perievent histogram showing non-RN MDJ unit activity in relation to wake-movement onset.
Data are pooled across the 4 units (out of a total of 10 twitch-preceding units) that showed significant increases in activity 10–100 ms before wake movement onset (triggered on 331 wake movements). In this and the other panels, upper and lower confidence bands (p < 0.05 for each band) are indicated by horizontal dashed lines. (B) Perievent histogram showing IO unit activity in relation to wake-movement onset. The data are from the 1 unit (out of a total of 21 twitch-related CD units) that showed a significant increase in activity within ±10 ms of wake-movement onset (triggered on 33 wake movements). (C) Perievent histogram showing LRN unit activity in relation to wake-movement onset. Data are from the 1 unit (out of a total of 11 twitch-related CD units) that showed a significant increase in activity within ±10 ms of wake-movement onset (triggered on 45 wake movements). (D) Perievent histogram showing LRN unit activity in relation to wake-movement onset. Data are pooled across the 5 units (out of a total of 12 sensory units) that showed significant increases in activity >10 ms after twitch onset (triggered on 364 wake movements).
-
Figure 5—figure supplement 1—source data 1
Source data for panels A-D.
- https://doi.org/10.7554/eLife.38213.014
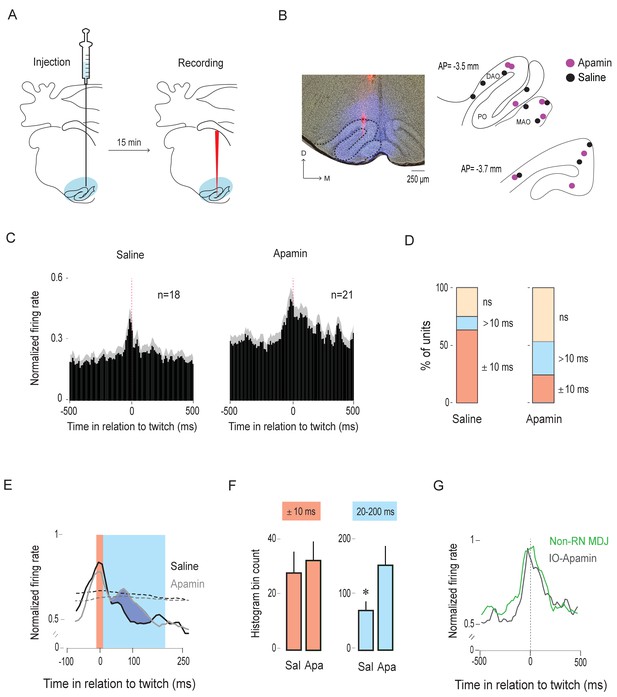
Apamin broadens the twitch-related peak in the IO.
(A) Diagram depicting experimental design. Apamin or saline, mixed with 4% Fluorogold, was microinjected into the IO (blue shading). Fifteen minutes after the injection, the microsyringe was withdrawn and a recording electrode, coated with DiI (red vertical line), was inserted into the IO. Unit activity was recorded for 30 min. (B) Left: Representative coronal section showing drug diffusion in the IO (blue) and placement of DiI-coated recording electrode (red). Right: Reconstruction of electrode placements within the IO in two coronal sections for all pups in the saline (black dots; n = 10 pups) and apamin (purple dots; n = 8 pups) groups. DAO: dorsal accessory olive; MAO: medial accessory olive; PO: principal olive; D: dorsal; M: medial; AP: antero-posterior distance in relation to lambda. (C) Perievent histograms (10-ms bins) showing mean (+SEM; gray shading) normalized firing rates across all units triggered on twitches in the saline (n = 18) and apamin (n = 21) groups. (D) Stacked plots showing the percentage of units with significant activity within ±10 ms of twitch onset (red) and >10 ms after twitch onset (blue) in the saline and apamin groups. ns: not significant. (E) Perievent histograms (10-ms bins) showing IO unit activity in relation to twitches in the saline (black line) and apamin (gray line) groups. Data for both groups are pooled across significant units only (red and blue stacks in D; n = 13 saline units and n = 11 apamin units) and smoothed (tau = 10 ms). Red shaded area denotes ±10-ms time window around twitches. Blue shaded area denotes 20–200-ms time window following twitches. Black and gray dashed lines denote upper confidence intervals (p < 0.05) for the event correlations in the saline and apamin groups, respectively. (F) Mean histogram bin counts (area under the curve, +SEM) for firing-rate data in two time windows: the ±10-ms window around twitches (red) and the 20–200-ms window following twitches (blue) for the units in the saline (Sal; n = 12) and apamin (Apa; n = 11) groups. *p = 0.03. (G) Perievent histograms comparing normalized firing rates in relation to twitch onset for twitch-preceding units in the non-RN MDJ region (green line, same as in Figure 5F) with that of IO units in the apamin group (black line).
-
Figure 6—source data 1
Source data for panels C-G.
- https://doi.org/10.7554/eLife.38213.019
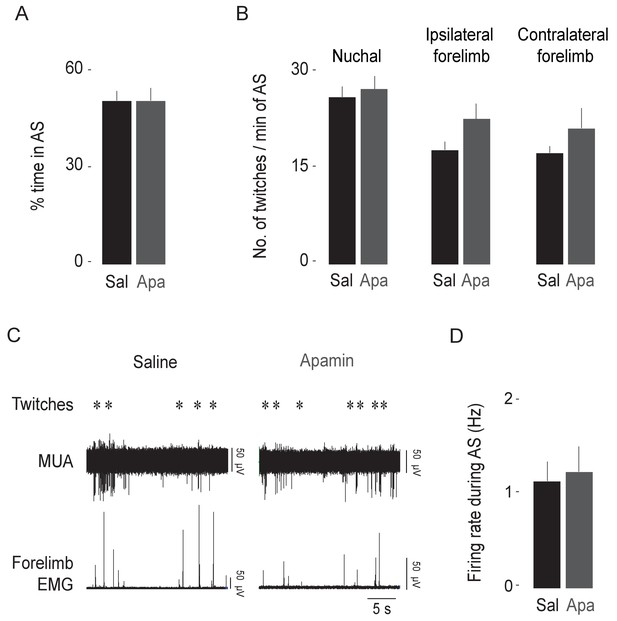
Apamin does not affect sleep-wake behavior.
(A) Mean (+SEM) percentages of time spent in active sleep in saline (Sal; n = 10 pups) and apamin (Apa; n = 8 pups) groups. (B) Mean (+SEM) rates of nuchal, contralateral forelimb, and ipsilateral forelimb twitching in saline (n = 10 pups) and apamin (n = 8 pups) groups. (C) Representative recording showing rectified forelimb EMG activity and multiunit activity (MUA) in the IO during spontaneous sleep-wake cycling in pups injected with saline (left) or apamin (right). Asterisks denote twitches as scored by the experimenter. (D) Mean (+SEM) unit firing rates during AS in saline (n = 18 units) and apamin groups (n = 22 units).
-
Figure 6—figure supplement 1—source data 1
Source data for panels A, B, and D.
- https://doi.org/10.7554/eLife.38213.018
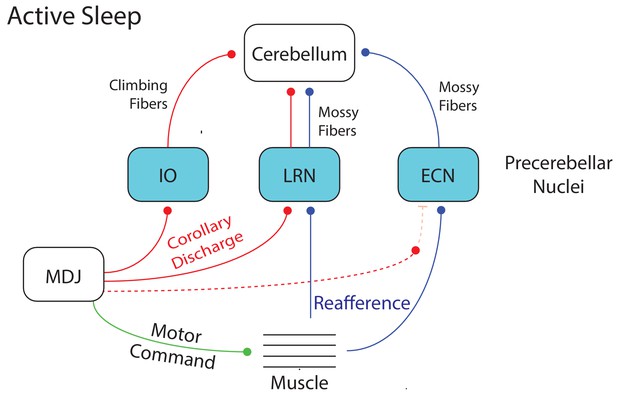
Summary diagram depicting the flow of twitch-related activity in the cerebellar system during active sleep in week-old rats.
A motor command from the MDJ to muscle (green line) produces a twitch. At the same time, twitch-related CD (red lines) is conveyed from the MDJ to the cerebellum via the IO and LRN. In addition, twitch-related reafference (blue lines) is conveyed to the cerebellum via the LRN. Also, as shown previously (Tiriac and Blumberg, 2016), reafference to the ECN is not gated during active sleep as it is during wake, thus allowing it to flow unimpeded to the cerebellum. Dotted lines denote hypothesized connections.
Additional files
-
Transparent reporting form
- https://doi.org/10.7554/eLife.38213.021





