Human VMPFC encodes early signatures of confidence in perceptual decisions
Figures
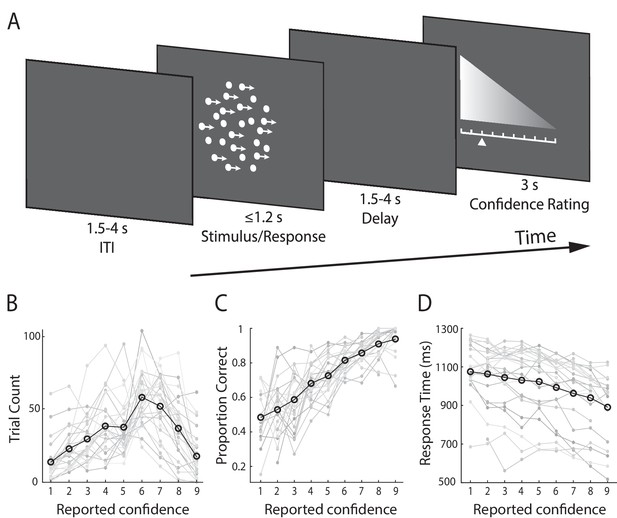
Experimental design and behavioural performance.
(A) Schematic representation of the behavioural paradigm. Subjects made speeded left vs. right motion discriminations of random dot kinematograms calibrated to each individual’s perceptual threshold. Stimulus difficulty (i.e., motion coherence) and was held constant across trials. Stimuli were presented for up to 1.2 s, or until a behavioural response was made. After each direction decision, subjects rated their confidence on a 9-point scale (3 s). The response mapping for high vs. low confidence ratings alternated randomly across trials to control for motor preparation effects, and was indicated by the horizontal position of the scale, with the tall end representing high confidence. All behavioural responses were made on a button box, using the right hand. (B) Mean confidence rating behaviour, showing the frequency with which subjects selected each point on the confidence scale. (C) Mean proportion of correct direction choices as a function of reported confidence. (D) Mean response time as a function of reported confidence. Faint grey lines in (B), (C), and (D) indicate individual subject data. For (C) and (D) we excluded any trial averages based on fewer than five trials.
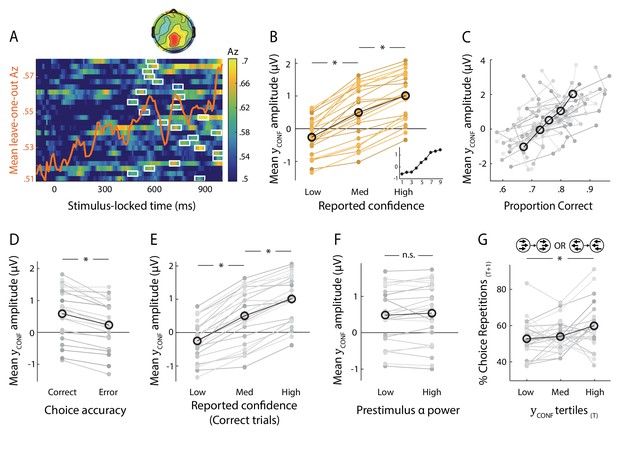
Neural representation of confidence in the EEG.
(A) Classifier performance (Az) during High- vs. Low-confidence discrimination for stimulus-locked data. Each row represents the Az as a function of time, for a single subject (warm colours indicate higher values). The overlapping line (orange) shows the mean classifier performance across subjects. Outlined in white are the pre-response time windows of peak confidence discrimination used subsequently to extract single-trial measures of confidence (i.e., discriminant component amplitudes). In selecting these, we considered only the discrimination period ending, on average, at least 100 ms (across-subject mean 271 ± 162 ms) prior to subjects’ mean response times, to minimise potential confounds with activity related to motor execution, due to a sudden increase in corticospinal excitability in this period (Chen et al., 1998). Inset shows average (normalised) topography associated with the discriminating component at subject-specific times of peak confidence discrimination. (B) Mean amplitude of the confidence discriminant component as a function of reported confidence, showing a parametric effect across the Low, Medium, and High bins. The mean component amplitudes for individual confidence ratings (weighted by each subjects’ trial count per rating) are also shown (inset). (C) Trial-by-trial confidence discriminant component amplitudes were positively correlated with accuracy. To visualise this relationship, single-trial component amplitudes were grouped into five bins. (D) Mean amplitude of the confidence discriminant component for correct vs. error responses, showing a significant effect of choice accuracy.(E) Mean amplitude of the confidence discriminant component as a function of reported confidence, for correct trials only (in order to control for accuracy). The same pattern as in (B) is observed. (F) Mean amplitudes of the confidence discriminant component did not differ significantly between trials associated with High vs. Low prestimulus oscillatory power in the alpha band (which we used as a proxy for subjects’ prestimulus attentional state). (G) Relationship between the strength of electrophysiological confidence signals on the current trial (i.e., confidence-discriminating component amplitudes) and the tendency to repeat a choice on the immediately subsequent trial, for trial pairs showing stimulus motion in the same direction (i.e., nominally identical stimuli). Faint orange (in B) and grey lines (in C–G) represent individual subject data.
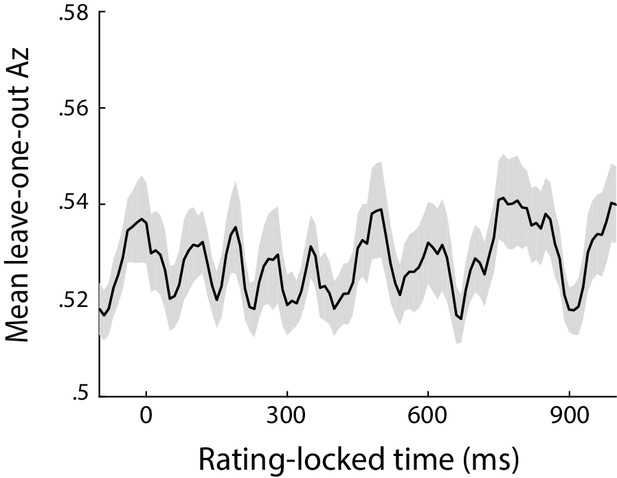
Classifier performance (Az) during High- vs Low-confidence discrimination, for data locked to the rating phase of the trial (defined as the onset of the rating prompt).
EEG signals during this time window are likely to be heavily contaminated by rating-related motor responses (due to field spread), thus precluding reliable characterisation of confidence representations. Accordingly, poor confidence discrimination can be observed during this stage of the trial.
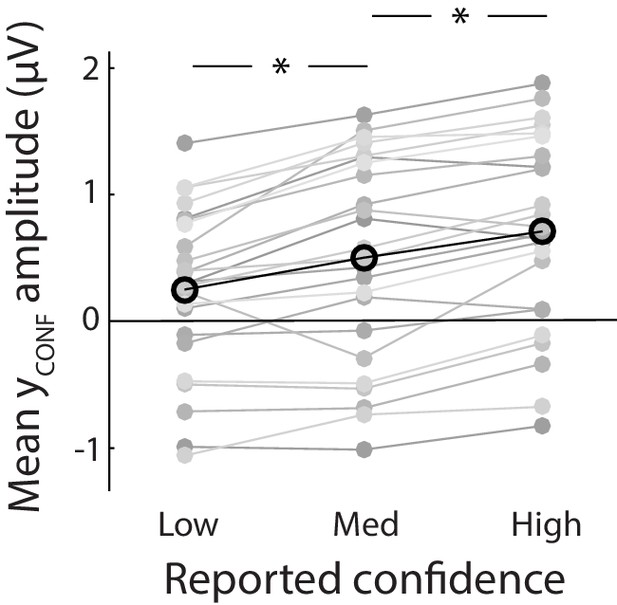
Mean amplitude of the confidence discriminant component showing parametric modulation by reported confidence.
Importantly, component estimates illustrated here were obtained fully out of sample from a leave-one-out cross-validation procedure.
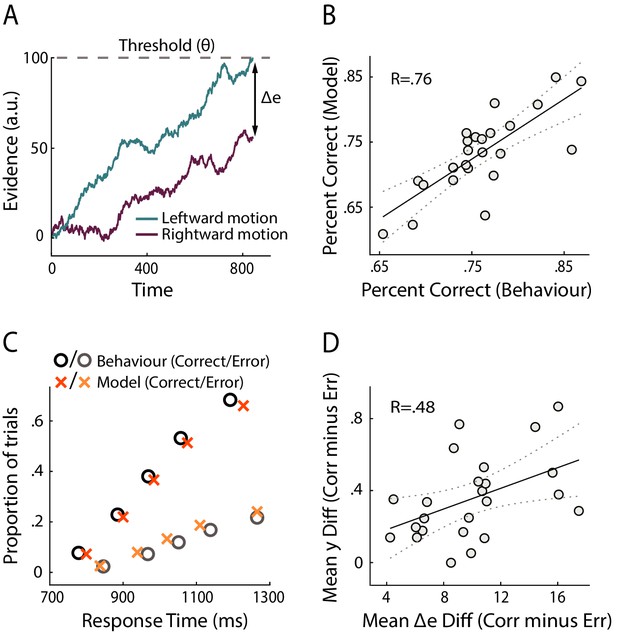
Modelling results.
(A) Schematic representation of the decision model for one trial. Evidence in favour of the two choice alternatives (here, leftward and rightward motion) accumulates gradually over time. A decision is made when one of the accumulators reaches a decision threshold (θ). The model quantifies confidence as the absolute difference in the accumulated evidence for the two options, at the time of decision (Δe). (B) Correlation between behavioural vs. model-predicted choice accuracy. Each point represents trial-averaged data for one subject. (C) Behavioural (circles) and model-predicted (crosses) response time distribution. On the x axis from left to right, data points represent the RT below which 10%, 30%, 50%, 70% and 90% of the data, respectively, are situated. The y axis shows the associated proportion of data for correct (upper symbols) and incorrect (bottom symbols) responses. (D) Across-subject correlation between the model-predicted and neurally observed relationship of confidence with choice accuracy (quantified as the difference in confidence estimates between correct and error trials). Each dot represents data for one subject.
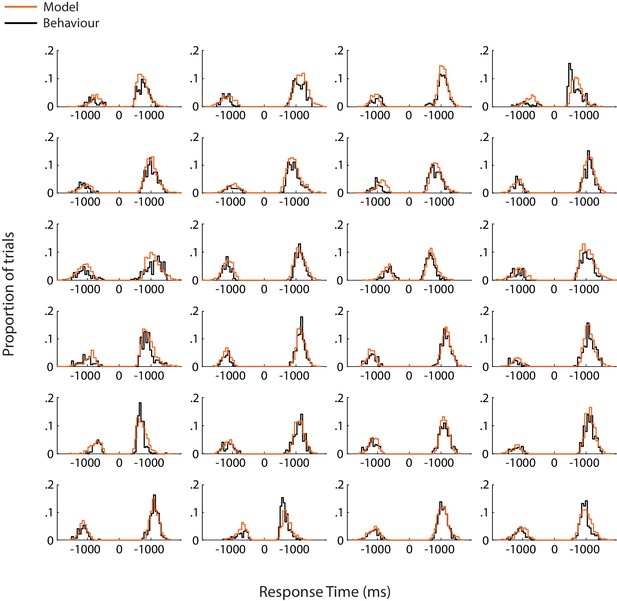
Model fits for individual subjects.
Each panel shows response time distributions of model-predicted (orange) and actual (black) data, for correct and error responses. Response times for error trials have been mirrored onto the negative axis in order to combine them with those for correct trials in a single distribution.
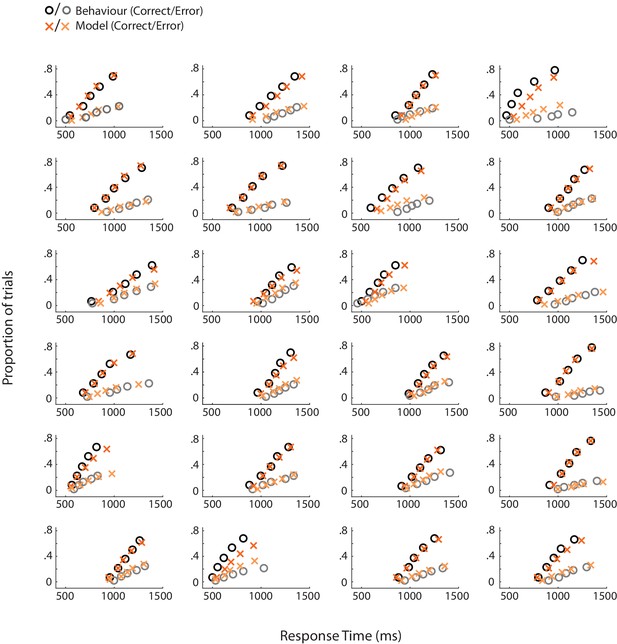
Behavioural (circles) and model-predicted (crosses) response time distribution for individual subjects.
On the x axis from left to right, data points represent the RT below which 10%, 30%, 50%, 70% and 90% of the data, respectively, are situated. The y axis shows the associated proportion of data for correct (upper symbols) and incorrect (bottom symbols) responses.
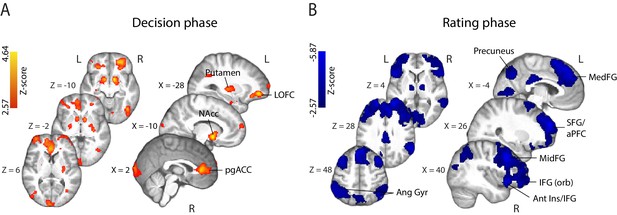
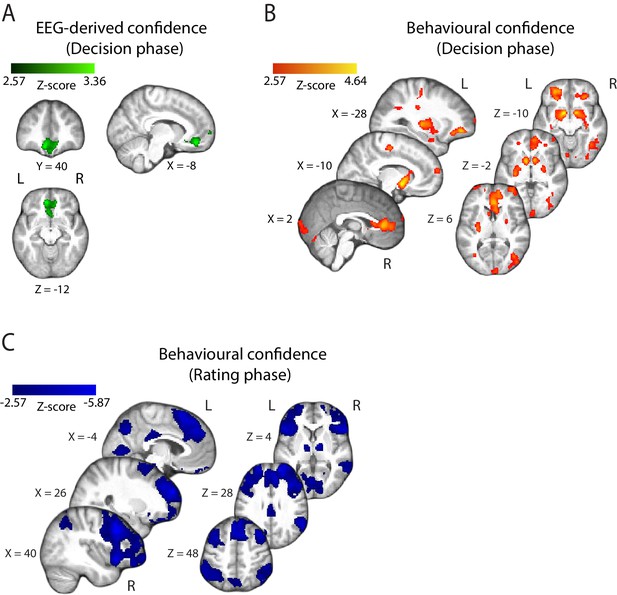
Parametric modulation of the BOLD signal by reported confidence.
(A) Clusters showing positive correlation with confidence during the decision phase of the trial. (B) Clusters showing negative correlation with confidence at the onset of the rating cue (i.e., rating phase). All results are reported at |Z| ≥ 2.57, and cluster-corrected using a resampling procedure (minimum cluster size 162 voxels; see Materials and methods). Ang Gyr, angular gyrus; Ant Ins, anterior insula; IFG (orb), inferior frontal gyrus (orbital region); LOFC, lateral orbitofrontal cortex; MedFG, medial frontal gyrus; MidFG, middle frontal gyrus; NAcc, nucleus accumbens; pgACC, pregenual anterior cingulate cortex; RLPFC, rostrolateral prefrontal cortex; SFG, superior frontal gyrus. The complete lists of activations are shown in Supplementary Tables 1 and 2 (Supplementary file 1).
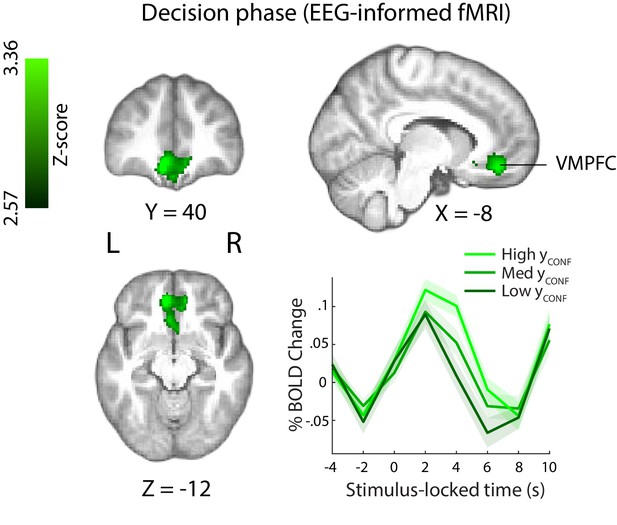
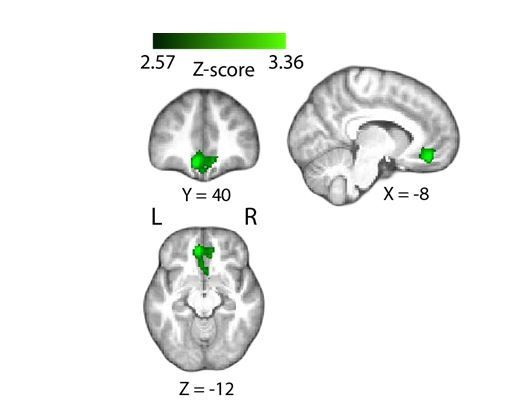
Positive parametric modulation of the BOLD signal by an EEG-derived single-trial confidence measure (see Materials and methods), during the decision phase of the trial.
Results are reported at |Z|≥2.57, and cluster-corrected using a resampling procedure (minimum cluster size 162 voxels). Bottom right: Time course of VMPFC BOLD response, showing parametric modulation by neural confidence (presented for illustration purposes only). Trials are separated by the strength of confidence-discriminating component amplitudes (CONF). VMPFC, ventromedial prefrontal cortex.
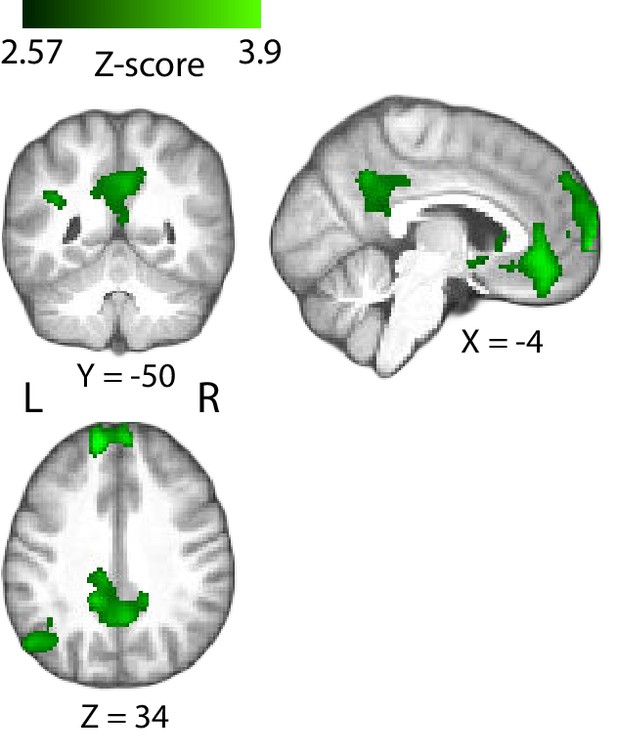
Positive correlation of the BOLD signal with the EEG-derived confidence measures in the posterior cingulate cortex (PCC), during the decision phase of the trial.
Note that for this GLM model, we excluded the regressor parametrically modulated by behavioural confidence at the time of decision (‘RatingsDEC’, see Materials and methods). Accordingly, the PCC clusters observed here (which did not show significant correlation with the EEG-derived measures of confidence in the original GLM analysis) likely reflect variability common to the behavioural and EEG-derived measures of confidence. Results are reported at |Z| ≥ 2.57, and cluster-corrected using a resampling procedure (minimum cluster size 162 voxels).
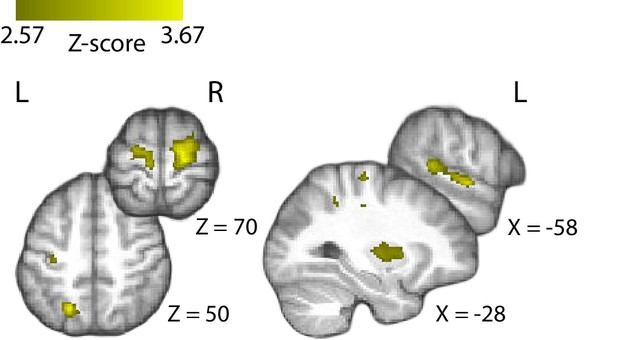
Positive parametric modulation of the BOLD signal by EEG-derived confidence at the confidence rating stage.
Results are reported at |Z| ≥ 2.57, and cluster-corrected using a resampling procedure (minimum cluster size 162 voxels).

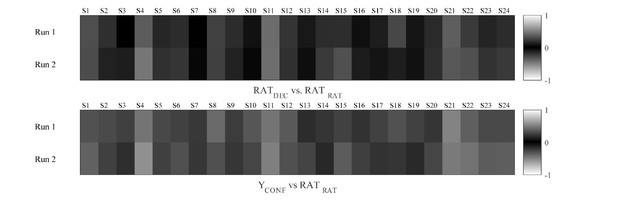
Correlations between HRF-convolved regressors locked to stimulus (i.e., decision phase) and confidence rating prompt (i.e., rating phase).
Colour scheme represents correlation coefficients in the same convention as in the FSL covariance matrices, ranging from 0 (shown in black) to 1/–1 (shown in white).
Parametric modulation of the BOLD signal by confidence, resulting from two GLM analyses whereby events pertaining to the decision and rating phases of the trial, respectively, were modelled separately.
Importantly, correlations with EEG-derived measures of confidence (A) and behavioural confidence reports (B and C) remained largely identical to those observed with our original GLM model which included predictors for both stages of the trial (see Figure 4 and Figure 5 for comparison). Results are reported at |Z| ≥ 2.57, and cluster-corrected using a resampling procedure (minimum cluster size 162 voxels).
Positive parametric modulation of the BOLD signal by EEG-derived measures of confidence resulting from a leave-one-trial-out cross validation procedure (shown in pink).
Our results remain unchanged relative to those observed with the original GLM analysis (shown in green). Results are reported at |Z| ≥ 2.57, and cluster-corrected using a resampling procedure (minimum cluster size 162 voxels).
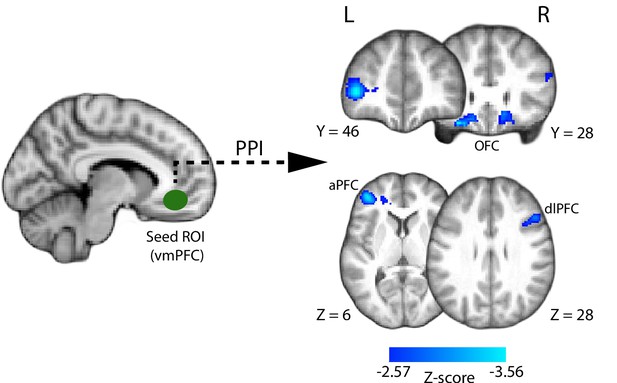
Psychophysiological interaction (PPI) analysis showing functional connectivity with the ventromedial prefrontal cortex (i.e., the seed region of interest; approximate location shown in green) during the perceptual decision phase of the trial.
Clusters in the anterior and dorsolateral prefrontal cortices, as well as the orbitofrontal cortex (shown in blue), show increased negative correlation with the VMPFC during the perceptual decision. All results are reported at |Z| ≥ 2.57, and cluster-corrected using a resampling procedure (minimum cluster size 162 voxels).
Additional files
-
Supplementary file 1
Supplementary Tables - Complete list of brain activations correlating with explicit confidence reports.
- https://doi.org/10.7554/eLife.38293.018
-
Transparent reporting form
- https://doi.org/10.7554/eLife.38293.019





