Dopamine maintains network synchrony via direct modulation of gap junctions in the crustacean cardiac ganglion
Figures
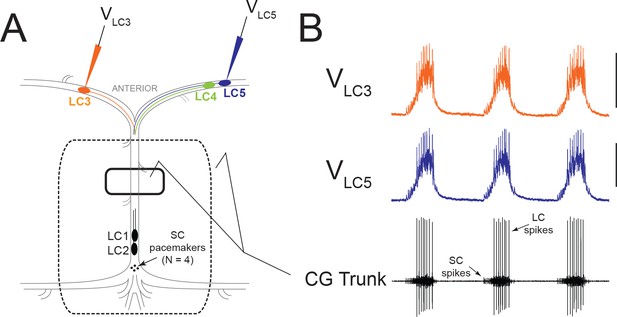
Experimental Setup and Typical Activity in the CG.
(A) Intracellular electrodes recorded simultaneously from LC3 and either LC4 or LC5. Extracellular recordings were taken from a petroleum jelly well on the CG trunk (solid line). For experiments which applied modulators or TEA exclusively to anterior LCs, the extracellular recording was taken from a single large well allowing anterior LCs to be exposed to the perfusate while protecting the remainder of the ganglion (dashed line). (B) Representative control activity showing the rhythmic synchronized bursting of LCs, with small cell (SC) pacemaker and Large Cell (LC) motor neuron spikes labeled on the extracellular trace (Scale bars = 10 mV, recording length = 14 s).
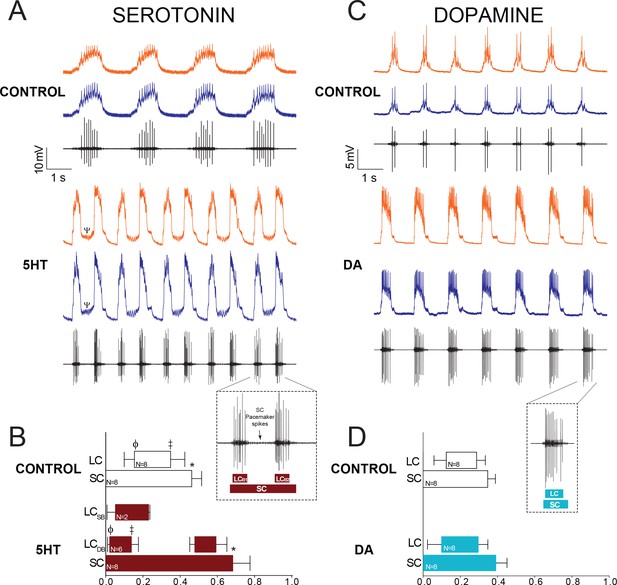
Effects of 10−6M 5HT and 10−5M DA on output of the CG network.
(A) Effects of 10−6M 5HT on the cardiac network. For each condition (Control and 5HT) the top two traces are intracellular recordings from two different LCs in the same network (LC3 and LC4) and the bottom trace is a simultaneous extracellular recording from the CG trunk. These recordings are taken from the same preparation before and after exposure to 5HT. In this case, the preparation exhibited ‘double-bursting’ output in 5HT, whereby two LC bursts are generated from continuous input from SC pacemaker cells. Note how the LC membrane potential does not return to baseline between two bursts of one full pacemaker cycle (designated by a Ψ in the 5HT intracellular recording). (B) Phase relationships of SCs and LCs in 5HT. Phase relationships for double-bursting (LCDB, N = 6) and single-bursting (LCSB, N = 2) LCs are shown separately for the 5HT condition. Bars represent mean ±SD. Inset shows how extracellular traces were used to quantify the phase parameters for the SC pacemakers and double-bursts (LCB1, LCB2) in 5HT preparations. Significant differences (p<0.01, paired t-tests) relative to control are denoted with the presence of distinct symbols for SC off (*), LC on (ϕ), and LC off (‡). These were analyzed only for the double-bursting preparations, where statistical power was sufficient. Although double-bursting is a distinct form of output unique to the 5HT condition, we compared the LC on and LC off for the first LC burst to demonstrate a significant phase advance of the initial LC bursting in this condition. (C) Effects of 10−5M DA on the cardiac network. Recordings as in panel A. (D) Phase relationships of SCs and LCs in DA (N = 8). DA does not initiate a distinct double-bursting output in LCs, and there were no significant changes in phase relationships in DA. Inset shows how extracellular traces were used to quantify the phase parameters for the SC pacemakers and single LC bursts in DA preparations.
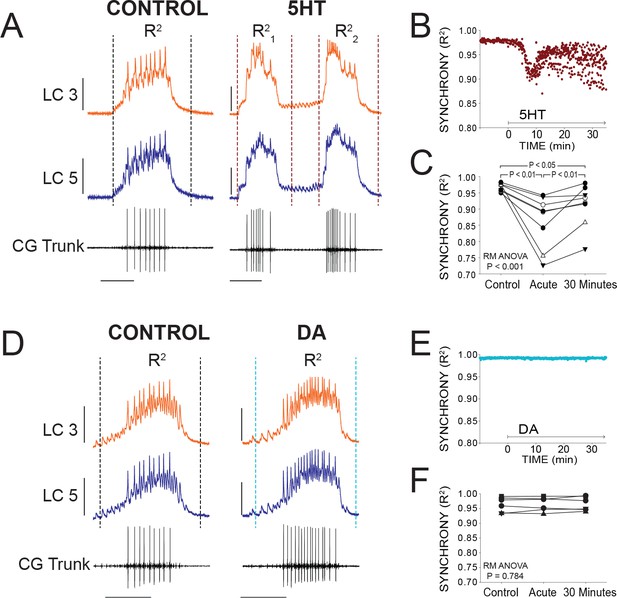
Effects of 5HT and DA on Synchrony of LC Voltage Waveforms.
(A) Representative traces show that LCs with virtually identical control activity produce different burst waveforms after application of 5HT. Double-bursting occurred 6 of 8 preparations in 5HT. Dashed lines designate points between which were used for calculation of R2 values (see Materials and methods). In 5HT double-bursting preparations, each LC burst waveform was treated as distinct for measurements of R2 (i.e. R21, R22). Scale bars = 10 mV and 1 s. (B) Waveform synchrony (R2) was calculated for every burst across a full experiment, and a scatterplot shows the synchrony of bursts for 10 min of control activity followed by 30 min of continuous perfusion of 5HT in one example preparation. An acute loss of synchrony accompanies the onset of modulation. (C) R2 was averaged for 10 consecutive bursts at each of 3 time points: control (5 min prior to perfusion), Acute (at the point R2 reached a minimum), and after 30 min of modulation. A one-way Repeated Measures ANOVA indicated that there was a significant effect of 5HT across groups (p<0.001, N = 8). Post-hoc testing revealed a significant decrease in R2 from control to acute 5HT (p<0.01, Tukey test), and a significant increase between acute and 30 min (p<0.01, Tukey test). Synchrony was not restored to control levels after 30 min (p<0.05, Tukey test). N = 8 preparations. (D) Representative traces show that excitability and network output are affected by DA, but LCs remain synchronized. Dashed lines designate points between which were used for calculation of R2 values. Scale bars = 10 mV and 1 s. (E) R2 was calculated for every burst across a full experiment. Scatterplot shows 10 min of control activity followed by 30 min in DA for a representative preparation. R2 values are largely unaffected by changes in activity caused by DA. (F) R2 was averaged for 10 consecutive bursts at each of 3 time points: control (5 min prior to perfusion), Acute (9.1 min), and after 30 min of DA. There were no significant effects of DA on synchrony indicated as a result of one-way Repeated Measures ANOVA (p=0.784, N = 8).
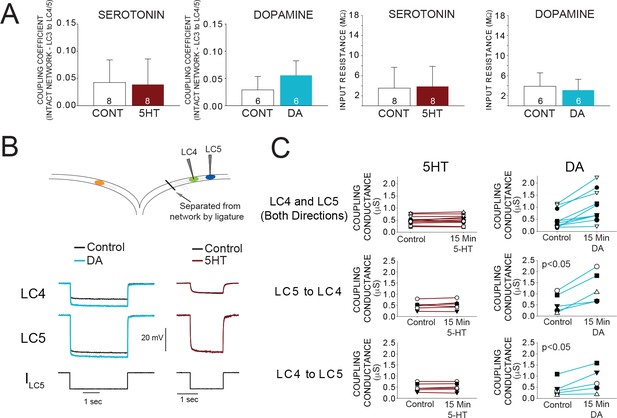
Effects of 5HT and DA on Electrical Coupling.
(A) Coupling Coefficients measured in the intact network for LC3-LC4 or LC3-LC5 were not significantly changed by 5HT. Mean coupling coefficient increased by 41% in DA (p<0.01, paired t-test). There were no effects of 5HT or DA on cell input resistance. Sample sizes noted in each bar. (B) A reduced preparation where LC4 and LC5 somata were physically isolated from the network by thread ligature was used to test the direct effect of 5HT and DA on coupling conductance. Representative traces of current injections in these isolated pairs of cells that were used to calculate coupling conductance are shown before and after DA exposure (left) and 5HT exposure (right). Traces in black show voltage responses of LC4 (top) and LC5 (middle) to a −8 nA hyperpolarizing current injection into LC5 (bottom). Overlaid traces show the voltage response to the same current injection after DA (left, blue) and 5HT (right, maroon). Control traces in the 5HT recordings are difficult to see due to near complete overlap when 5HT and Control recordings were superimposed. (C) Coupling conductance between LC4 and LC5 was unchanged by 5HT, and increased by DA. Top row in C shows coupling conductance in both directions (i.e. LC4 to LC5, and LC5 to LC4) for N = 5 preparations before and after modulation. These data are then separated by directionality. Coupling Conductance was significantly increased in DA in both directions (p<0.05 for each, mean increase 149%, N = 5).
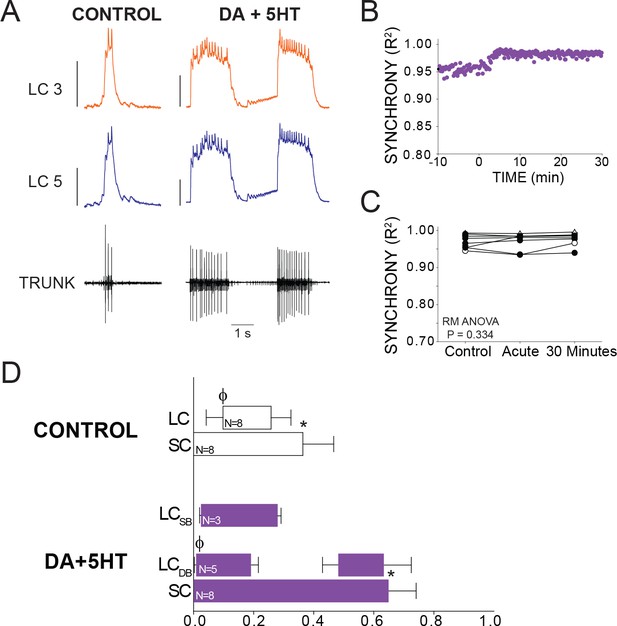
Effects of co-application of DA and 5HT on bursting output and synchrony.
(A) Representative traces show LCs maintain synchronized voltage waveforms during co-application of DA and 5HT. 5 out of 8 preparations transitioned to double-bursting, and network output shows increased number of spikes per burst, spike frequency in each burst, burst duration and LC duty cycle. Scale bars = 10 mV and 1 s. (B) R2 was calculated for every burst across a full experiment. Scatterplot shows this for 10 min of control activity followed by 30 min of perfusion with both modulators. (C) R2 was averaged for 10 consecutive bursts at each of 3 time points: control (5 min prior to perfusion), Acute, and after 30 min of modulation. There were no significant effects on synchrony detected across groups via one-way Repeated Measures ANOVA (p=0.334, N = 8). (D) Phase relationships of SCs and LCs in DA + 5 HT. Phase relationships for double-bursting (LCDB, N = 5) and single-bursting (LCSB, N = 3) LCs are shown separately. Bars represent mean ±SD. Significant differences (p<0.01, paired t-tests) relative to control are denoted with the presence of distinct symbols for SC off (*) and LC on (ϕ). These were analyzed only for the double-bursting preparations, where statistical power was sufficient. Although double-bursting is a distinct form of output unique to the 5HT condition, we compared the LC on and LC off for the first LC burst to demonstrate a significant phase advance of the initial LC bursting in this condition.
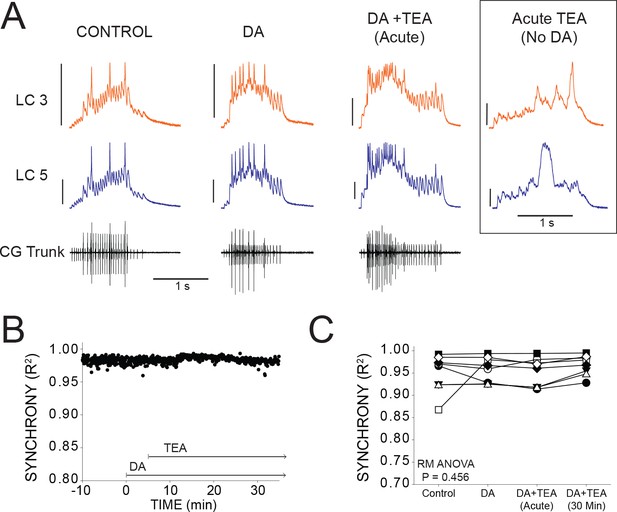
DA prevents desynchronization when co-applied with TEA.
(A) Representative traces for a single preparation in control, DA alone, and DA + TEA. Traces in the box at the far right illustrate acute desynchronization in TEA in the absence of DA (separate preparation). Scale bars = 10 mV and 1 s. (B) R2 was calculated for every burst across a full experiment. Scatterplot shows throughout 10 min of control activity followed by 5 min of DA exposure, followed by 30 min in DA and TEA for a single preparation. (C) R2 was averaged for 10 consecutive bursts at each of 4 time points: control (5 min prior to perfusion), after 5 min in DA, at the mean time point for desynchronization in TEA observed previously (Lane et al., 2016), and after 30 min exposure to the DA+TEA solution. No significant differences were detected across groups via one-way Repeated Measures ANOVA (p=0.456, N = 8).
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Chemical compound, drug | 5HT | Sigma | H7752 | 10^−6M |
| Chemical compound, drug | DA | Acros Organics | 122000100 | 10^−5M |
| Chemical compound, drug | TEA | Acros Organics | 150901000 | 10^−5M |
| Software, algorithm | Phaseburst | http://stg.rutgers.edu/Resources.html | Spike2 analysis script, provided by Dr. Dirk Bucher, New Jersey Institute of Technology |
Additional files
-
Transparent reporting form
- https://doi.org/10.7554/eLife.39368.008





