ErbB4 deletion in noradrenergic neurons in the locus coeruleus induces mania-like behavior via elevated catecholamines
Figures
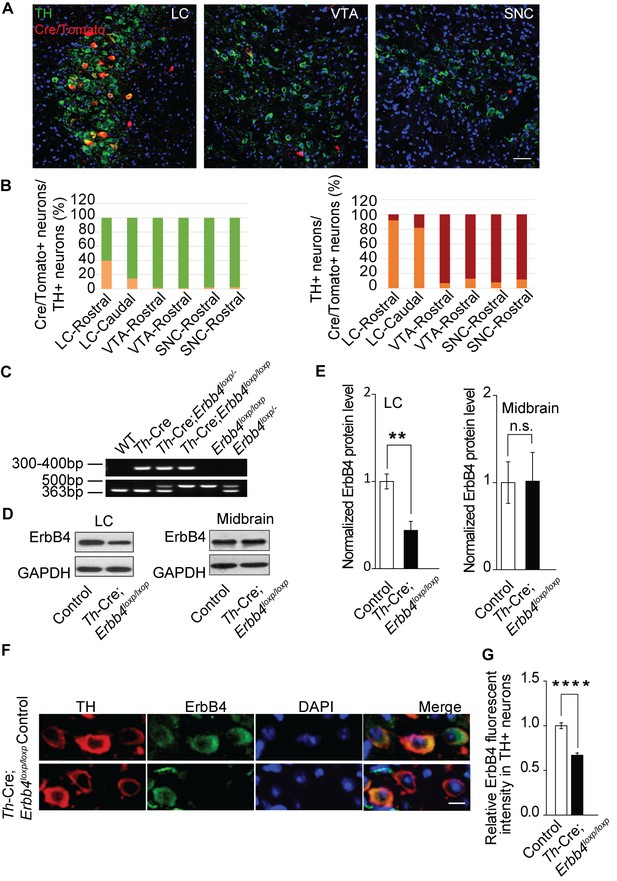
ErbB4 is primarily deleted in NE neurons of the LC in Th-Cre;Erbb4loxp/loxp mice.
(A) Representative micrographs of Cre/Tomato distribution (red) in the locus coeruleus (LC), ventral tegmental area (VTA), and substantia nigra pars compacta (SNC). Slices were obtained from Ai9;Th-Cre mice and stained with antibody to TH (green), a marker of NE and dopaminergic neurons. Scale bar, 50 µm. (B) Colocalization of TH and Cre/Tomato. Three mice were studied, with three slices chosen for each mouse. (C) Genotyping of Th-Cre;Erbb4loxp/loxp mice. The Erbb4 primers generated a 363-base pair (bp) product for the wild-type allele or a 500 bp product for the loxP-flanked allele. The Th-Cre primers generated a band between 300 and 400 bp. (D), (E) Quantification of the fold change in ErbB4 protein expression relative to control mice. Unpaired two-tailed Student’s t-test. Data are expressed as means ± s.e.m. **p<0.01. (F) Specific deletion of ErbB4 in NE neurons of the LC. Sections from Th-Cre mice and Th-Cre;Erbb4loxp/loxp mice were stained with ErbB4-specific antibody and TH-specific antibody. Sections were also stained with DAPI to indicate nuclei. Scale bar, 10 µm. (G) Quantification of ErbB4 fluorescent intensity in TH-positive (TH+) cells. n = 13 slices (control), random 16 – 30 TH+ cells were quantified from each slice. n = 11 slices (Th-Cre;Erbb4loxp/loxp), random 19 – 30 TH+ cells were quantified from each slice.
-
Figure 1—source data 1
Statistical reporting of Figure 1.
- https://doi.org/10.7554/eLife.39907.007
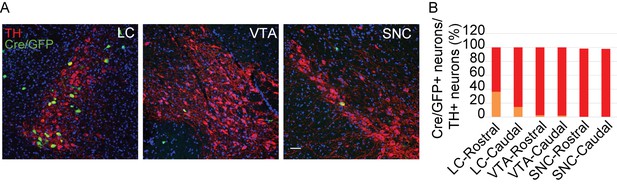
Cre/GFP was primarily expressed in NE neurons of the LC in Ai3;Th-Cre mice.
(A) Representative micrographs of Cre/GFP distribution (green) in Ai3;Th-Cre mice. Locus coeruleus (LC), ventral tegmental area (VTA), and substantia nigra pars compacta (SNC) slices were obtained from Ai3;Th-Cre mice and stained with antibody to TH (red), a marker of NE and dopaminergic neurons. Scale bar, 50 µm. (B) Cartogram of Cre expression in TH-positive neurons in the LC, VTA, and SNC. Three mice were studied, with three slices for each mouse.
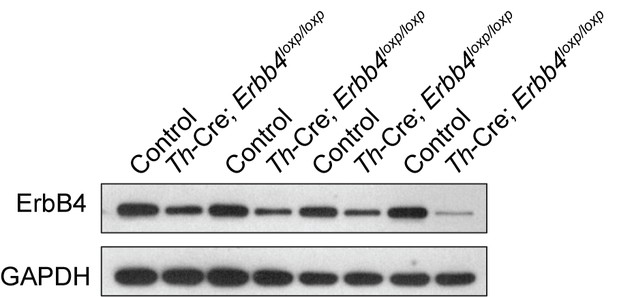
ErbB4 was primarily deleted in the LC of Th-Cre;Erbb4loxp/loxp mice.
Shown are representative Western blots of the ErbB4 protein (180 kDa) in Th-Cre;Erbb4loxp/loxp mice. Erbb4loxp/loxp mice and Th-Cre mice were used as controls. n = 4 (control); n = 4 (Th-Cre;Erbb4loxp/loxp).
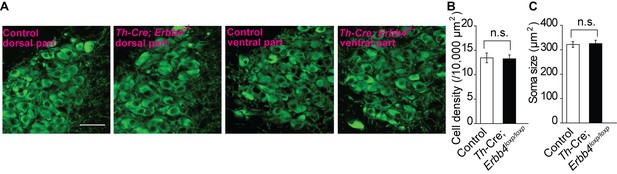
No obvious differences were detected in cell density or soma size of LC neurons between control and Th-Cre;Erbb4loxp/loxp mice.
(A) Representative micrographs of LC neurons in the dorsal and ventral parts of the LC in control and Th-Cre;Erbb4loxp/loxp mice. Coronal LC slices were obtained from control and Th-Cre;Erbb4loxp/loxp mice and were stained with antibody to TH (green), a marker of NE and dopaminergic neurons. Scale bar, 50 µm. (B), (C) Cell density and soma size of LC neurons did not differ significantly between control and Th-Cre;Erbb4loxp/loxp mice. Unpaired two-tailed Student’s t-test. Data are expressed as means ± s.e.m. n.s., not significant.
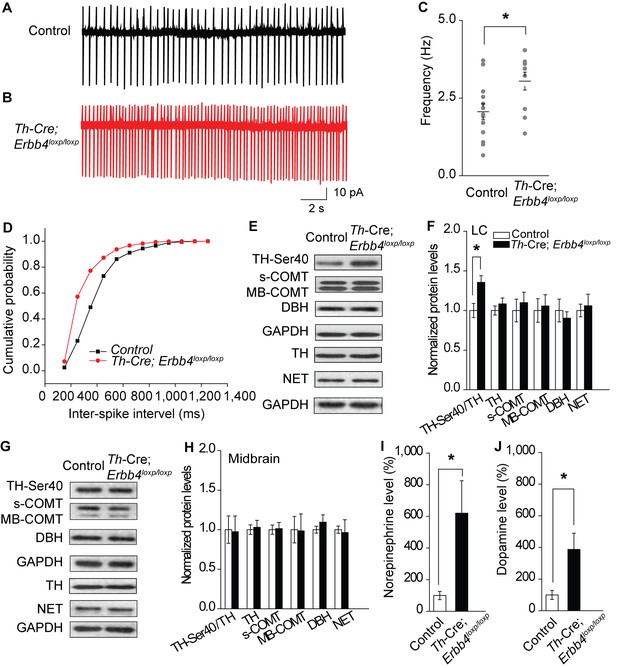
Increased spontaneous firing of LC-NE neurons, extracellular norepinephrine, and intracellular TH phosphorylation in Th-Cre;Erbb4loxp/loxp mice.
(A), (B) Representative firing of LC-NE neurons from control (black) and Th-Cre;Erbb4loxp/loxp mice (red). (C) Spontaneous firing frequency of LC-NE neurons was increased in Th-Cre;Erbb4loxp/loxp mice. n = 11 from three mice (control); n = 13 from three mice (Th-Cre;Erbb4loxp/loxp). (D) Interspike intervals were calculated over 2 min of firing from each neuron. Interspike intervals were decreased in Th-Cre;Erbb4loxp/loxp mice compared with control mice. n = 11 from three mice (control); n = 13 from three mice (Th-Cre;Erbb4loxp/loxp). Two-sample Kolmogorov-Smirnov test and data in (D) are presented in a cumulative frequency plot. ****p<0.0001. (E), (F) Protein levels of TH-Ser40 were increased in the LC of Th-Cre;Erbb4loxp/loxp mice. NET, norepinephrine reuptake transporter; DBH, dopamine beta-hydroxylase; S-COMT, soluble catechol-o-methyltransferase; MB-COMT, membrane-binding form of COMT. (G), (H) No significant change was detected in the dopaminergic neurons clustered in the midbrain (VTA and SNC). (I), (J) In vivo microdialysis and HPLC data suggested that norepinephrine and dopamine levels were significantly increased in Th-Cre;Erbb4loxp/loxp mice. Standard curves are presented in Figure 2—figure supplement 2. n = 6 mice (control); n = 6 mice (Th-Cre;Erbb4loxp/loxp). Unpaired two-tailed Student’s t-test. Data are expressed as means ± s.e.m. *p<0.05.
-
Figure 2—source data 1
Statistical reporting of Figure 2.
- https://doi.org/10.7554/eLife.39907.011
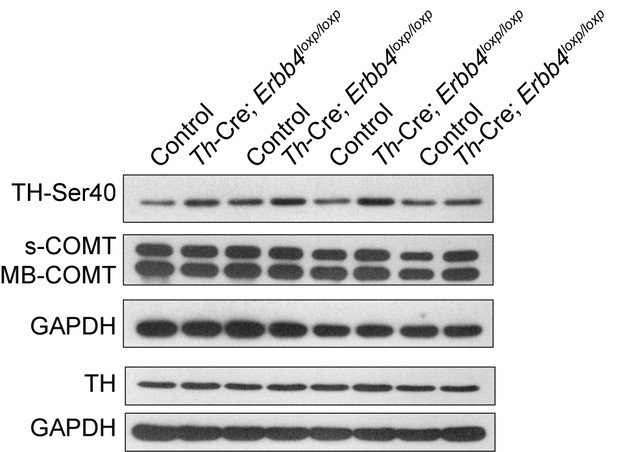
Representative Western blots of TH and COMT in the LC of control and Th-Cre;Erbb4loxp/loxp mice.
Ser40 phosphorylation of tyrosine hydroxylase (TH-Ser40), tyrosine hydroxylase (TH), soluble catechol-o-methyltransferase (s-COMT), membrane-bound form of COMT (MB-COMT). n = 4 (control); n = 4 (Th-Cre;Erbb4loxp/loxp).
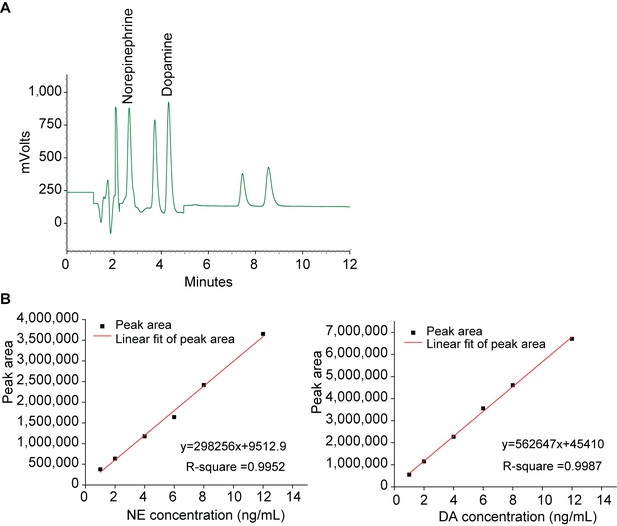
HPLC analysis of norepinephrine and dopamine.
(A) Representative graphs of main peaks in HPLC chromatograms. Norepinephrine; Dopamine. X-axis, retention time. (B) Standard curves of norepinephrine (NE) and dopamine (DA).
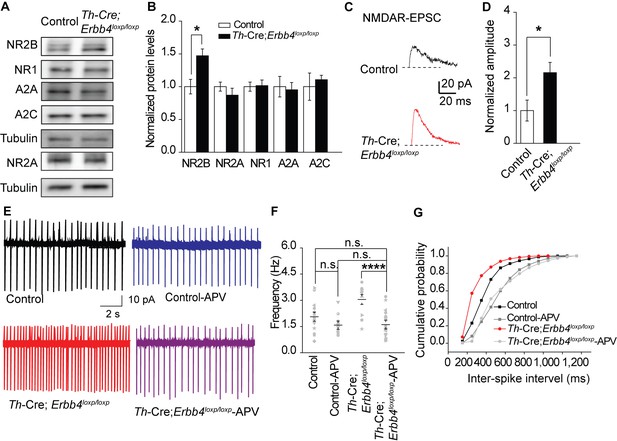
NMDA receptor mediates hyperexcitability of LC-NE neurons in Th-Cre;Erbb4loxp/loxp mice.
(A), (B) Protein levels of NMDA receptor subunit NR2B were increased in the LC of Th-Cre;Erbb4loxp/loxp mice. Unpaired two-tailed Student’s t-test. Data are expressed as means ± s.e.m. *p<0.05. (C) Representative NMDAR-EPSC current traces from LC-NE neurons in Ai9;Th-Cre (control) and Ai9; Th-Cre;Erbb4loxp/loxp mice. (D) Amplitude of NMDAR current recorded in LC-NE neurons is significantly increased in ErbB4-deficient mice compared with control mice. n = 7 from three mice (control); n = 8 from three mice (Th-Cre;Erbb4loxp/loxp). (E) Representative firing of LC-NE neurons from control and Th-Cre;Erbb4loxp/loxp mice untreated or treated with APV (50 μM), an NMDA receptor antagonist. (F) Spontaneous firing frequency of LC-NE neurons was rescued by APV (50 μM) in Th-Cre;Erbb4loxp/loxp mice. n = 13 from three mice (Th-Cre;Erbb4loxp/loxp); n = 20 from three mice (Th-Cre;Erbb4loxp/loxp + APV). Two-way ANOVA. Data are expressed as means ± s.e.m. *p<0.05. (G) Inter-spike intervals were significantly rescued by APV (50 μM) in Th-Cre;Erbb4loxp/loxp mice. n = 13 from three mice (Th-Cre;Erbb4loxp/loxp); n = 20 from three mice (Th-Cre;Erbb4loxp/loxp + APV). Two-sample Kolmogorov-Smirnov test and data in (G) are presented in a cumulative frequency plot. ***p<0.001.
-
Figure 3—source data 1
Statistical reporting of Figure 3.
- https://doi.org/10.7554/eLife.39907.015
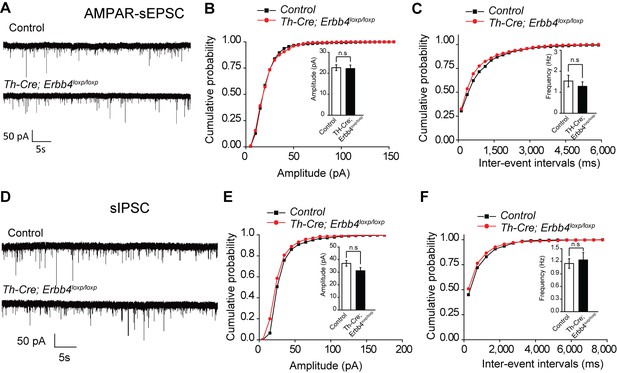
Spontaneous excitation and inhibition balance measured by sEPSC and sIPSC is not changed in LC-NE neurons of Th-Cre;Erbb4loxp/lox mice.
(A) Representative sEPSC traces of LC-NE neurons in control (Erbb4loxp/loxp) mice and Th-Cre;Erbb4loxp/loxp mice. (B), (C) No significant change in the amplitude (B) and inter-event intervals (C) of LC-NE neuron sEPSC in control mice and Th-Cre;Erbb4loxp/lox mice. n = 8 from three mice (control); n = 10 from three mice (Th-Cre;Erbb4loxp/loxp). (D) Representative sIPSC traces of LC-NE neurons in control mice and Th-Cre;Erbb4loxp/lox mice. (E), (F) No significant change in the amplitude (E) and inter-event intervals (F) of LC-NE neuron sEPSC in control mice and Th-Cre;Erbb4loxp/lox mice. n = 13 from three mice (control); n = 11 from three mice (Th-Cre;Erbb4loxp/lox). Unpaired two-tailed Student’s t-test and Two-sample Kolmogorov-Smirnov test. Data are expressed as means ± s.e.m. n.s., not significant.
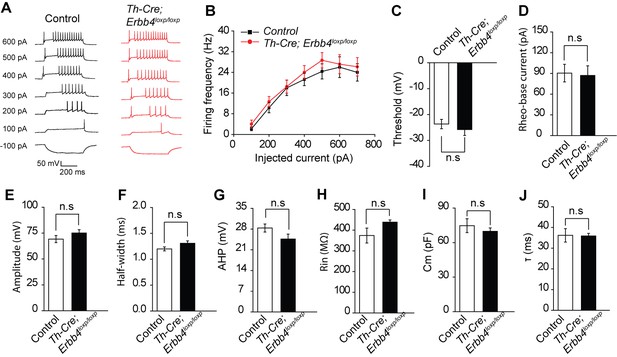
Intrinsic properties of LC-NE neurons are unchanged in Th-Cre; ErbB4loxp/loxp mice.
(A) Representative voltage response of LC-NE neurons to injected currents ranging from −100 pA to 600 pA at 100 pA step size. (B) Summary histogram of LC-NE neuron voltage response of control (Erbb4loxp/loxp) mice and Th-Cre; ErbB4loxp/loxp mice. (C–G) Positive membrane properties, including threshold (C) rheo-base current (D) amplitude (E) half-width (F) and afterhyperpolarization (AHP) (G) show no significant difference in the mutants compared with control mice. (H–J) Normal input resistance (H) membrane capacitance (I) and time constant (J) in LC-NE neurons in Th-Cre; ErbB4loxp/loxp mice. n = 6 from three mice (control); n = 8 from three mice (Th-Cre; ErbB4loxp/loxp). Unpaired two-tailed Student’s t-test. Data are expressed as means ± s.e.m. n.s., not significant.
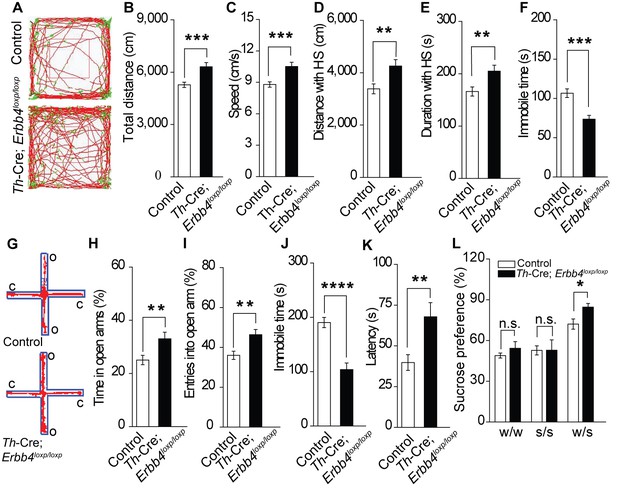
Th-Cre;Erbb4loxp/loxp mice show mania-like behaviors.
(A) Representative trajectories of control (Erbb4loxp/loxp) and Th-Cre;Erbb4loxp/loxp mice. We defined high speed (red line) as >10 cm/s, immobility as <2 cm/s, and low speed (green line) as 2 – 10 cm/s. (B), (C) Locomotor activity (B) and speed (C) of control and Th-Cre;Erbb4loxp/loxp mice in open field tests. n = 24 (control); n = 22 (Th-Cre;Erbb4loxp/loxp). (D), (E) Distance (D) and duration (E) traveled at high speed (HS). n = 17 (control); n = 13 (Th-Cre;Erbb4loxp/loxp). (F) Immobility time during open field tests decreased in Th-Cre;Erbb4loxp/loxp mice. (G) Examples of the performance of control and Th-Cre;Erbb4loxp/loxp mice in the EPM test. C, closed arm; O, open arm. (H), (I) Performance of control and Th-Cre;Erbb4loxp/loxp mice in the EPM test. n = 34 (control); n = 24 (Th-Cre;Erbb4loxp/loxp). (J), (K) Immobility time (J) and latency to first surrender (K) in the forced swim test. n = 22 (control); n = 13 (Th-Cre;Erbb4loxp/loxp). (L) Sucrose preference of control and Th-Cre;Erbb4loxp/loxp mice. Water (w). Sucrose (s). n = 19 (control); n = 14 (Th-Cre;Erbb4loxp/loxp). Unpaired two-tailed Student’s t-test. Data are expressed as means ± s.e.m. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001. n.s., not significant.
-
Figure 4—source data 1
Statistical reporting of Figure 4.
- https://doi.org/10.7554/eLife.39907.019
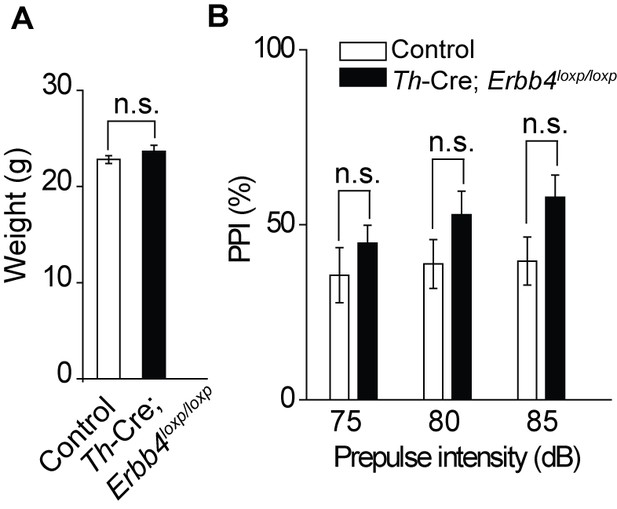
There was no significant difference in body weight between control and Th-Cre;Erbb4loxp/loxp mice and no deficit of Th-Cre;Erbb4loxp/loxp mice in the prepulse inhibition experiment.
(A) No significant differences in body weight were detected between control and Th-Cre;Erbb4loxp/loxp mice. n = 7 (control); n = 8 (Th-Cre;Erbb4loxp/loxp). (B) No prepulse inhibition deficit was observed in Th-Cre;Erbb4loxp/loxp mice. n = 10 (control); n = 10 (Th-Cre; Erbb4loxp/loxp). Unpaired two-tailed Student’s t-test. Data are expressed as means ± s.e.m. *p<0.05. n.s., not significant.
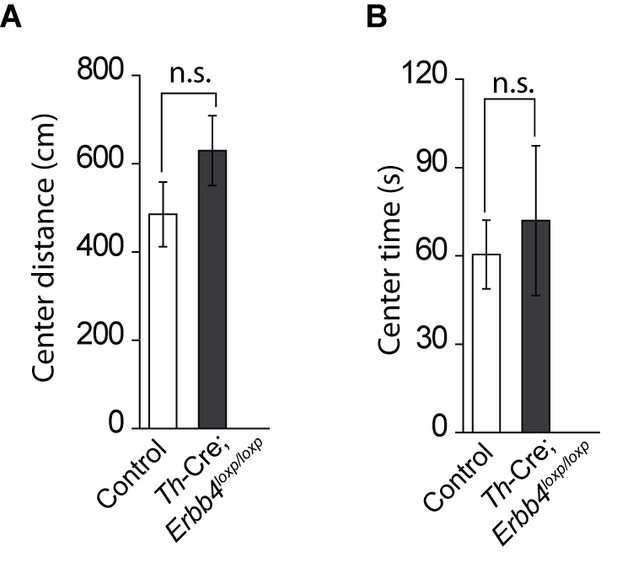
No significant change in the distance travelled in center and time spent in center area between control (Erbb4loxp/loxp) mice and Th-Cre; Erbb4loxp/loxp mice in open field test.
(A) Distance travelled in center area of control mice and Th-Cre; Erbb4loxp/loxp mice in open field test. n = 8 (control); n = 8 (Th-Cre; Erbb4loxp/loxp). (B) Time spend in center area in open field test. n = 11 (control); n = 11 (Th-Cre; Erbb4loxp/loxp). Unpaired two-tailed Student’s t-test. Data are expressed as means ± s.e.m. n.s., not significant.
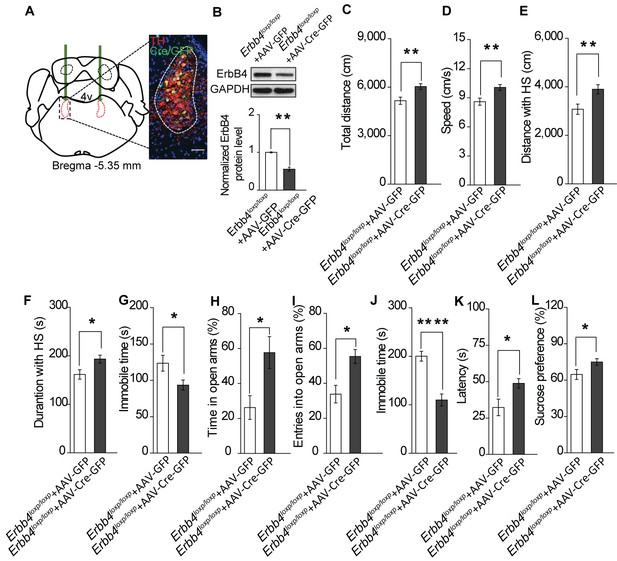
Specific ablation of ErbB4 in the LC is sufficient to cause mania-like behaviors.
(A) Illustration of bilateral viral injection of AAV-Cre-GFP in the mouse LC. LC sections were examined for Cre/GFP (green) 5 weeks after stereotaxic microinjection of AAV-Cre-GFP into the LC of Erbb4loxp/loxp mice; antibody staining for TH is shown in red. Scale bars, 50 μm. Cartogram is presented in Figure 5—figure supplement 1 . (B) ErbB4 expression detected by immunoblotting was significantly decreased in LC protein lysates from Erbb4loxp/loxp mice after AAV-Cre-GFP injection. n = 4 (AAV-GFP); n = 4 (AAV-Cre-GFP). (C, D) Locomotor activity (C) and speed (D) of mice injected with AAV-GFP or AAV-Cre-GFP in the open field test. n = 14 (AAV-GFP); n = 12 (AAV-Cre-GFP). (E–G) Distance (E) and duration (F) traveled at HS and immobility time (G) of mice in the open field test after viral injection. n = 14 (AAV-GFP); n = 12 (AAV-Cre-GFP). (H, I) Percentage of time (H) and entries (I) into the open arms by mice injected with AAV-GFP or AAV-Cre-GFP in the EPM test. n = 10 (AAV-GFP); n = 18 (AAV-Cre-GFP). (J, K) Immobility time (J) and latency to first surrender (K) in the forced swim test with AAV-GFP or AAV-Cre-GFP injection. n = 11 (AAV-GFP); n = 18 (AAV-Cre-GFP). (L) Sucrose preference of mice injected with AAV-GFP or AAV-Cre-GFP. n = 10 (AAV-GFP); n = 13 (AAV-Cre-GFP). Unpaired two-tailed Student’s t-test. Data are expressed as means ± s.e.m. *p<0.05, **p<0.01, ****p<0.0001.
-
Figure 5—source data 1
Statistical reporting of Figure 5.
- https://doi.org/10.7554/eLife.39907.025
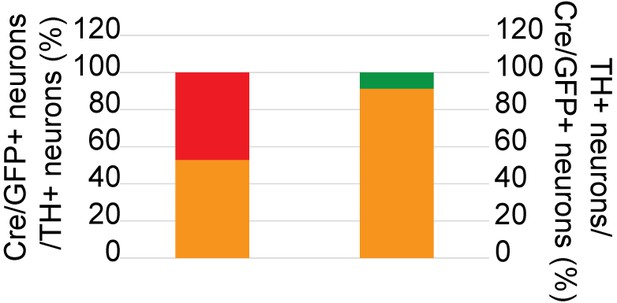
Cartogram of the colocalization of Cre/GFP-positive (Cre/GFP+) and NE neurons (TH+) in the LC 5 weeks after viral injection.
Percentages of Cre/GFP+ neurons among NE neurons of the LC and of LC-NE neurons among Cre/GFP+ neurons in Erbb4loxp/loxp mice after virus injection. Three mice were studied, with three slices for each mouse.
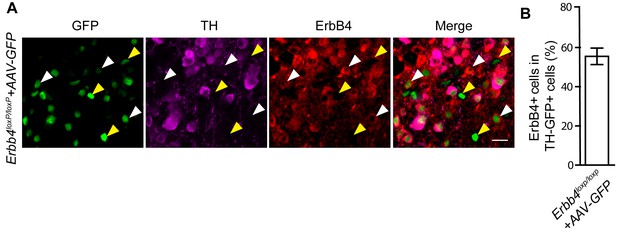
55.5% of TH-GFP + neurons in AAV-injected Erbb4loxp/loxp mice are ErbB4+.
(A) Representative image of GFP, TH, and ErbB4 colocalization in immunostained brain slice from Erbb4loxp/loxp mice injected with AAV-GFP in the LC. (B) Quantification of percentage of ErbB4 + cells in TH-GFP+ cells. White arrowhead: ErbB4 + TH-GFP+cells; Yellow arrowhead: ErbB4-TH-GFP + cells. n = 22 slices from three mice. Scale bar, 20 µm.
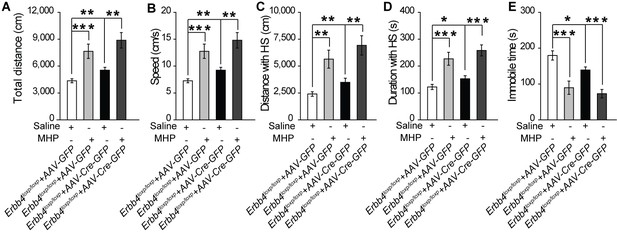
ErbB4-deficient mice are not an ADHD model.
(A), (B) Total distance and speed in open field test are significantly increased in control (Erbb4loxp/loxp) mice and Th-Cre; Erbb4loxp/loxp mice after MHP treatment. (C–E) Distance (C) and duration (D) traveled at HS and immobility time (E) after MHP treatment of MHP-treated control mice and Th-Cre; Erbb4loxp/loxp mice in open field test. n = 10 (control + Saline); n = 7 (Th-Cre; Erbb4loxp/loxp + Saline); n = 11 (control +MHP); n = 12 (Th-Cre; Erbb4loxp/loxp + MHP). Two-way ANOVA. Data are expressed as means ± s.e.m. *p<0.05, **p<0.01, ***p<0.001. n.s., not significant.
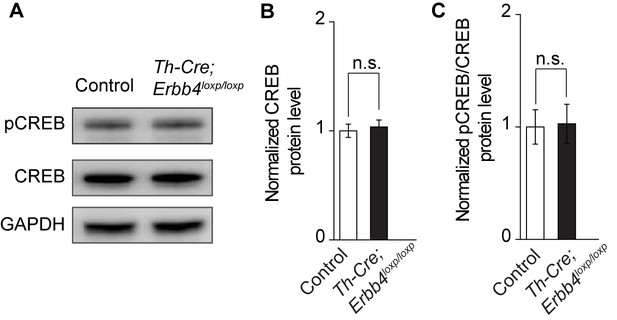
Normal CREB signaling activity in Th-Cre;ErbB4loxp/loxp mice.
(A) Representative blot of pCREB, CREB, and loading control GAPDH from LC protein lysates of ErbB4loxp/loxp (Control) and Th-Cre;ErbB4loxp/loxp mice. (B), (C) Quantification of relative ratio of total CREB to GAPDH (B) and pCREB to total CREB (pCREB/CREB) (C) in the LC of control and mutant mice. n = 6 (control); n = 6 (Th-Cre; ErbB4loxp/loxp). Unpaired two-tailed Student’s t-test. Data are expressed as means ± s.e.m. n.s., not significant.
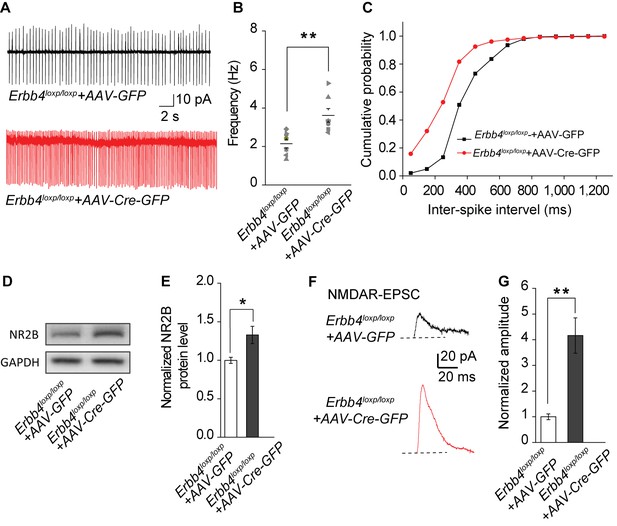
The spontaneous excitability, NR2B expression, and NMDAR current of LC-NE neurons are increased in ErbB4loxp/loxp mice bilaterally injected with AAV-Cre-GFP virus into the LC.
(A) Representative spontaneous firing traces of LC-NE neurons in ErbB4loxp/loxp mice bilaterally injected with AAV- GFP or AAV-Cre-GFP virus. (B), (C) Quantification of firing frequency (B) and cumulative histogram of inter-spike interval (C) of spontaneous firing. n = 8 (ErbB4loxp/loxp-AAV-GFP); n = 7 (ErbB4loxp/loxp-AAV-Cre-GFP). (D), (E) Representative blot (D) and quantification (E) of NR2B protein level from LC protein lysates of ErbB4loxp/loxp mice received AAV-GFP or AAV-Cre-GFP injection. n = 3 (control); n = 3 (Th-Cre; ErbB4loxp/loxp). (F), (G) NMDAR current is enhanced in ErbB4loxp/loxp mice bilaterally injected with AAV-Cre-GFP. n = 3 (ErbB4loxp/loxp-AAV-GFP); n = 3 (ErbB4loxp/loxp-AAV-Cre-GFP). Unpaired two-tailed Student’s t-test and Two-sample Kolmogorov-Smirnov test. Data are expressed as means ± s.e.m. *p<0.05, **p<0.01.
-
Figure 6—source data 1
Statistical reporting of Figure 6.
- https://doi.org/10.7554/eLife.39907.027
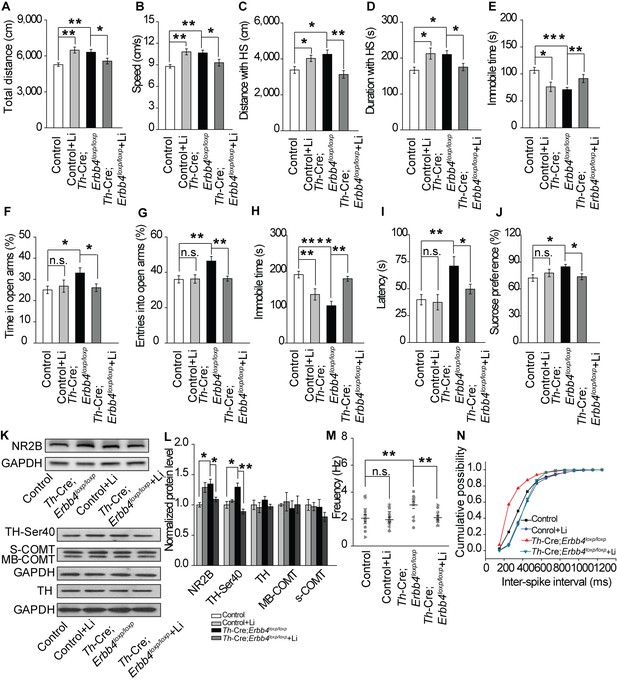
Lithium rescued the behavioral, molecular, and electrophysiological phenotypes of Th-Cre;Erbb4loxp/loxp mice.
(A), (B) Locomotor activity (A) and speed (B) in the open field test. n = 24 (Erbb4loxp/loxp), n = 10 (Erbb4loxp/loxp + lithium), n = 22 (Th-Cre;Erbb4loxp/loxp), n = 13 (Th-Cre;Erbb4loxp/loxp + lithium). (C–E) Distance (C) and duration (D) traveled at HS and immobility time (E) after lithium treatment. (F), (G) Percentage of time (F) and entries (G) into open arms by Th-Cre;Erbb4loxp/loxp mice with and without lithium in the EPM test. n = 23 (Erbb4loxp/loxp); n = 8 (Erbb4loxp/loxp + lithium); n = 24 (Th-Cre;Erbb4loxp/loxp); n = 18 (Th-Cre;Erbb4loxp/loxp + lithium). (H), (I) Immobility time (H) and latency to first surrender (I) in forced swim test. n = 20 (Erbb4loxp/loxp); n = 8 (Erbb4loxp/loxp + lithium); n = 16 (Th-Cre;Erbb4loxp/loxp); n = 20 (Th-Cre;Erbb4loxp/loxp + lithium). (J) Sucrose preference of Th-Cre;Erbb4loxp/loxp mice treated with lithium. n = 19 (Erbb4loxp/loxp); n = 12 (Erbb4loxp/loxp + lithium); n = 13 (Th-Cre;Erbb4loxp/loxp); n = 16 (Th-Cre;Erbb4loxp/loxp + lithium). (K) Western blots of LC samples from Th-Cre;Erbb4loxp/loxp mice with and without lithium treatment. (L) Protein level of NR2B and TH-Ser40 in the LC after lithium treatment. Protein levels of TH, s-COMT, and MB-COMT were not significantly changed in the LC after lithium treatment. TH, tyrosine hydroxylase; S-COMT, soluble catechol-o-methyltransferase; MB-COMT, membrane-binding form of COMT. n = 4 (Erbb4loxp/loxp); n = 4 (Erbb4loxp/loxp + lithium); n = 4 (Th-Cre;Erbb4loxp/loxp); n = 4 (Th-Cre;Erbb4loxp/loxp + lithium). (M), Spontaneous firing of LC-NE neurons after lithium treatment. n = 13 from three mice (Th-Cre;Erbb4loxp/loxp); n = 15 from three mice (Th-Cre;Erbb4loxp/loxp + lithium). (N) Interspike intervals after lithium treatment. n = 13 from three mice (Th-Cre;Erbb4loxp/loxp); n = 15 from three (Th-Cre;Erbb4loxp/loxp + lithium). Two-way ANOVA. Data are expressed as means ± s.e.m. Two-sample Kolmogorov-Smirnov test and data in (N) are presented as a cumulative frequency plot. *p<0.05, **p<0.01, ****p<0.0001. n.s., not significant.
-
Figure 7—source data 1
Statistical reporting of Figure 7.
- https://doi.org/10.7554/eLife.39907.029
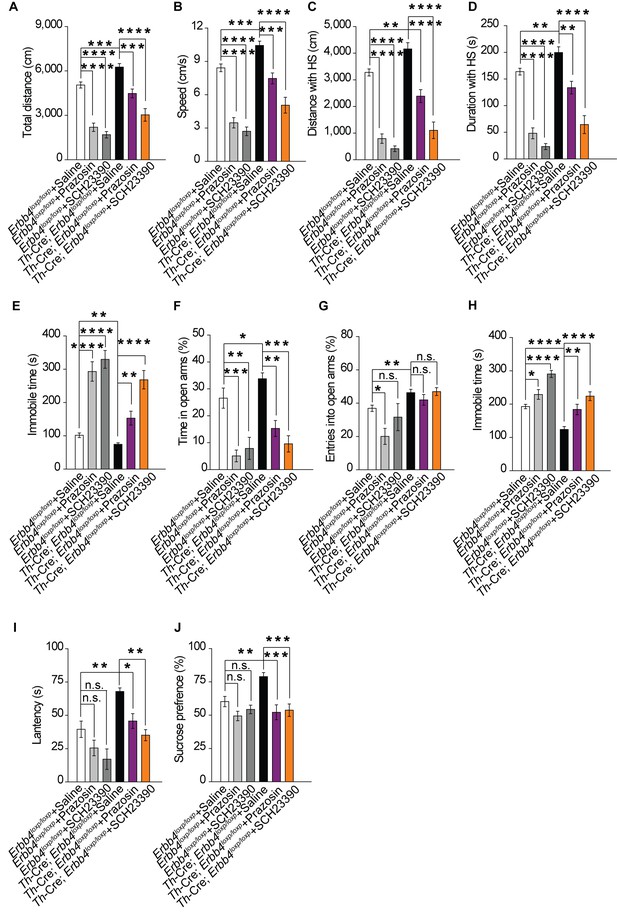
Increase in both norepinephrine and dopamine contribute to mania-like behaviors.
(A), (B) Locomotor activity (A) and speed (B) of Th-Cre;Erbb4loxp/loxp mice treated with saline (sal), prazosin, or SCH23390 in open field test. n = 17 (Erbb4loxp/loxp + sal); n = 12 (Erbb4loxp/loxp + prazosin); n = 11 (Erbb4loxp/loxp + SCH23390); n = 11 (Th-Cre;Erbb4loxp/loxp + sal); n = 10 (Th-Cre;Erbb4loxp/loxp + prazosin); n = 9 (Th-Cre;Erbb4loxp/loxp + SCH23390). (C), (D) Distance (C) and duration (D) at HS in open field test. (E) Immobility time in open field test. (F), (G) Time (F) and entries (G) in open arms in EPM test. n = 11 (Erbb4loxp/loxp + sal); n = 12 (Erbb4loxp/loxp + prazosin); n = 11 (Erbb4loxp/loxp + SCH23390); n = 14 (Th-Cre;Erbb4loxp/loxp + sal); n = 11 (Th-Cre;Erbb4loxp/loxp + prazosin); n = 8 (Th-Cre;Erbb4loxp/loxp + SCH23390). (H), (I) Immobility time (H) and latency to first surrender (I) in forced swim tests. n = 12 (Erbb4loxp/loxp + sal); n = 10 (Erbb4loxp/loxp + prazosin); n = 10 (Erbb4loxp/loxp + SCH23390); n = 16 (Th-Cre;Erbb4loxp/loxp + sal); n = 12 (Th-Cre;Erbb4loxp/loxp + prazosin); n = 12 (Th-Cre;Erbb4loxp/loxp + SCH23390). (J) Sucrose preference of Th-Cre;Erbb4loxp/loxp mice after prazosin or SCH23390 treatment. n = 11 (Erbb4loxp/loxp + sal); n = 9 (Erbb4loxp/loxp + prazosin); n = 10 (Erbb4loxp/loxp + SCH23390); n = 11 (Th-Cre;Erbb4loxp/loxp + sal); n = 12 (Th-Cre;Erbb4loxp/loxp + prazosin); n = 12 (Th-Cre;Erbb4loxp/loxp + SCH23390). One-way ANOVA and Tukey's multiple comparison test. Data are expressed as means ± s.e.m. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001. n.s., not significant.
-
Figure 8—source data 1
Statistical reporting of Figure 8.
- https://doi.org/10.7554/eLife.39907.031
Additional files
-
Transparent reporting form
- https://doi.org/10.7554/eLife.39907.032





