NKB signaling in the posterodorsal medial amygdala stimulates gonadotropin release in a kisspeptin-independent manner in female mice
Figures
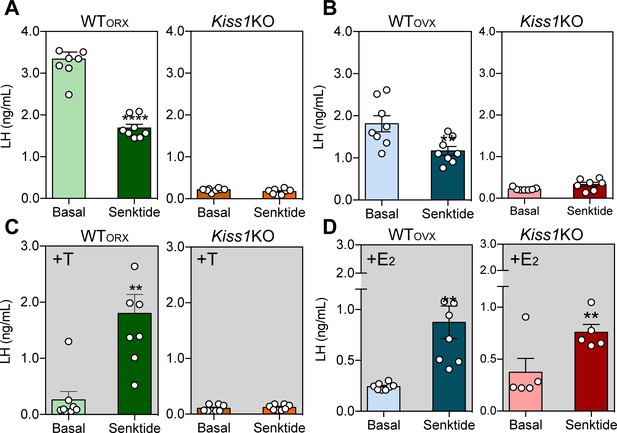
ICV injection of senktide stimulates LH release in female Kiss1 KO mice supplemented with estradiol.
Mean ± SEM LH concentrations (ng/ml) in male (A) and (C) and female (B) and (D) adult WT and homozygous Kiss1Cre/Cre (i.e., Kiss1 KO) mice. Blood samples were collected before (basal) and 25 min after ICV injection of senktide (an NK3R-specific agonist; 600 pmol diluted in 5 µl 0.9% NaCl). (A, B) Mean ± SEM LH concentrations (ng/ml) in adult WT male and female mice gonadectomized (WTORX or WTOVX, respectively) and studied in parallel to hypogonadal (with low sex steroid levels) Kiss1Cre/Cre (i.e., Kiss1 KO) littermates (n = 7–10/group). (C, D) Mean ± SEM LH concentrations (ng/ml) in adult gonadectomized WT and Kiss1Cre/Cre (i.e., Kiss1 KO) male and female mice supplemented with testosterone or estradiol, respectively (WTORX+T, WTOVX+E2, Kiss1 KO+T, Kiss1 KO+E2; n = 5–10/group). Basal versus after senktide injection LH concentrations were compared with a paired t-test ****p < 0.0001, **p < 0.007. T = testosterone, E2 = estradiol.
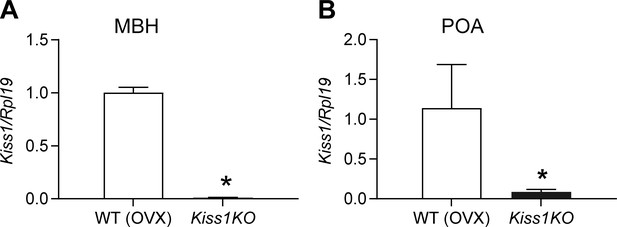
Expression profile of (A) Kiss1 gene in the mediobasal hypothalamus (MBH), (B) Kiss1 gene in the preoptic area (POA), of ovariectomized (OVX) WT and hypogonadal Kiss1 KO female mice.
Comparison between groups was carried out with a student’s t-test (*p < 0.001). The data were normalized using L19 primers as an internal control.
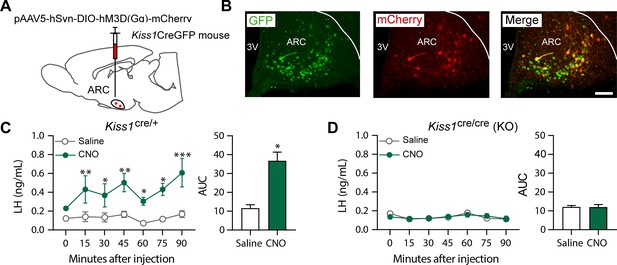
Kiss1ARC (KNDy) neuron stimulates LH release in Kiss1Cre/+but not Kiss1Cre/Cre (i.e., Kiss1 KO) mice supplemented with estradiol.
(A) Schematic representation of the site used to inject a pAAV encoding a Cre-dependent hM3Dq DREADD tagged to mCherry (pAAV5/hSyn-DIO-hm3Dq:mCherry; titer 3 × 1012 genome copies per ml; 1 µl per hemisphere) in heterozygous Kiss1Cre/+ (n = 10) and homozygous Kiss1Cre/Cre (i.e., Kiss1 KO) female mice (n = 20). (B) Representative photomicrograph of a coronal brain section stained for green fluorescent protein (GFP; green), mCherry (red) and merged GFP and mCherry immunoreactivity in the ARC of a homozygous Kiss1Cre/Cre (i.e., Kiss1 KO) female mouse >3 weeks after hM3Dq:mCherry injection (Scale bar 50 µm). In this animal model Cre and GFP have been targeted to the Kiss1 locus and are, therefore, expressed solely in Kiss1 neurons. Colocalization of GFP and m-Cherry immunoreactivity represents Kiss1ARC cells that have been infected with the pAAV encoding a Cre-dependent hM3Dq DREADD. (C, D) Mean ± SEM LH concentrations (ng/ml) and area under the curve (AUC) after an i.p. injection of saline (grey line-empty bar) or CNO (10 mg/kg dissolved in saline; green line-green bar) three weeks after the hM3Dq DREADD-injection in (C) heterozygous Kiss1Cre/+ and (D) homozygous Kiss1Cre/Cre (i.e., Kiss1 KO supplemented with estradiol) female mice (n = 5–8/group). Blood samples were collected just before saline or CNO i.p. injection (0) and then every 15 min for 90 min. 3V: third ventricle, ARC: arcuate nucleus. Repeated LH concentrations at multiple time-points and between treatments were compared using a 2-WAY ANOVA and a Fishers posthoc test when appropriate. Area under the curve was compared with 2-tailed student t tests. *p < 0.035, **p < 0.006, ***p < 0.0001.
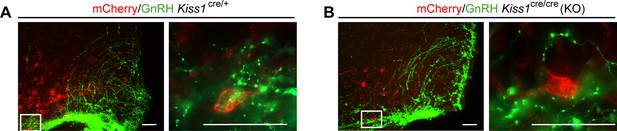
Representative merged images of mCherry (red) and GnRH (green) immunoreactivity in the ARC of Kiss1Cre/+ (A) and Kiss1Cre/Cre (B).
Right panels are higher magnifications (Scale bar 20 µm) of boxed areas from left panels (Scale bar 50 µm).
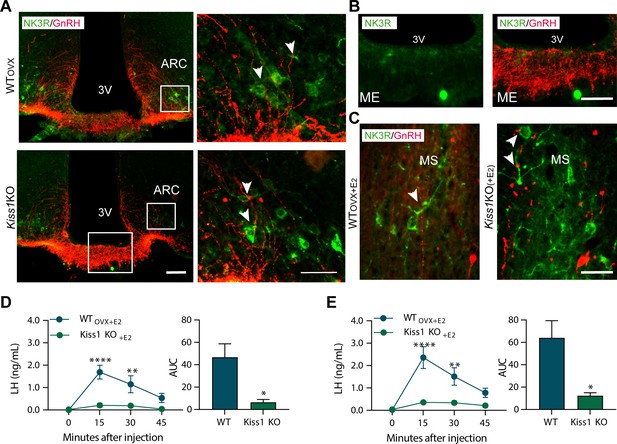
Senktide administration directly in to the arcuate nucleus or preoptic area (at the level of the medial septum) stimulates LH release in adult WT but not Kiss1Cre/Cre (i.e., Kiss1 KO) female mice supplemented with estradiol.
(A, B) Representative photomicrographs depicting dual label detection of NK3R (green) and GnRH (red) in the ARC (A) and ME (B) of ovariectomised WT (WTovx) and homozygous Kiss1Cre/Cre (i.e., Kiss1 KO animals). (A) Panels on the right are enlarged images (scale bar: 20 µm) from boxed areas on the left (scale bar: 100 µm) showing infrequent close appositions (arrowheads) between NK3R and GnRH in the ARC. (B) Enlarged images of the boxed area from the Kiss1 KO animal in (A) showing intense GnRH but lack of NK3R staining in the ME. (C) Dual-label detection of NK3R (green) and GnRH (red) in the POA and specifically the level of the MS of ovariectomized WT and homozygous Kiss1Cre/Cre (i.e., Kiss1 KO) female mice supplemented with estradiol [WTOVX+E2 and Kiss1 KO+E2, respectively; scale bar: 50 µm]. Arrowheads indicate infrequent sites of close apposition. (D, E) Mean ±SEM LH concentrations (ng/ml) and area under the curve (AUC) after an injection of senktide into the ARC (D) or POA (E) of ovariectomized WT (WTOVX+E2; blue line-blue bar) and homozygous Kiss1Cre/Cre (i.e., Kiss1 KO+E2; green line-green bar) female mice supplemented with estradiol (n = 5/group). 3V: third ventricle, ARC: arcuate nucleus, ME: median eminence, POA: preoptic area, MS: medial septum. Repeated LH concentrations at multiple time-points and between treatments were compared using a 2-WAY ANOVA and a Fishers posthoc test when appropriate. Area under the curve was compared with 2-tailed student t tests. **p < 0.0015, ****p < 0.0001.
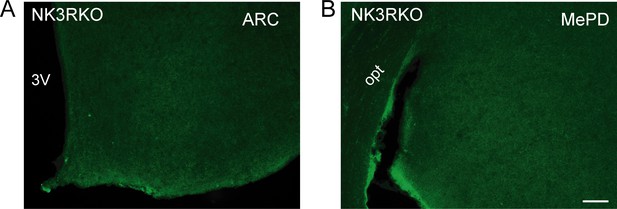
NK3R antibody validation.
Representative photomicrographs of sections processed for immunofluorescent detection of NK3R in NK3R KO female mice. The tissue was derived from females that were either OVX or OVX and E2-treated known to induce maximal NK3R expression in the ARC and MePD, respectively. (A) Complete absence of staining in the ARC of an OVX NK3R KO female mouse. (B) Complete absence of staining in the MePD of an OVX and E2-treated NK3R KO female mouse. Scale bar: 100 µm.
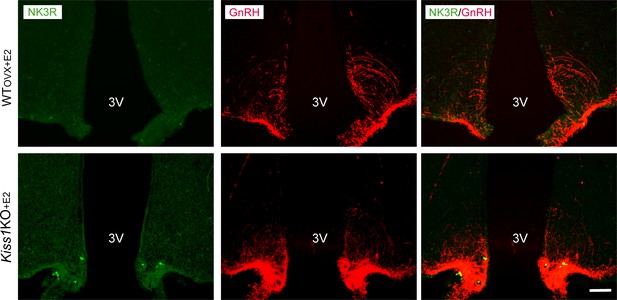
Representative photomicrographs depicting dual label detection of NK3R (green) and GnRH (red) in the ARC of WT(OVX+E2) and Kiss1 KO+E2 animals.
Scale bar: 100 µm. Note the lack of NK3R staining as opposed to the absence of E2 in the ARC (Figure 3).
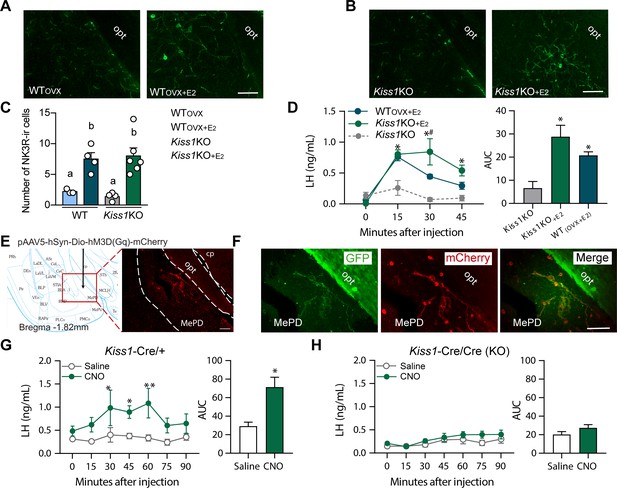
Senktide administration into the posterodorsal medial amygdala stimulates LH release in WT and Kiss1 KO female mice supplemented with estradiol.
(A, B) Representative photomicrographs depicting NK3R-immunoreactive cell bodies and fibers in the MePD of (A) ovariectomized WT (WTOVX; left panel) or ovariectomized and estradiol-supplemented WT (WTOVX+E2; right panel) and (B) homozygous Kiss1Cre/Cre (i.e., Kiss1 KO; left panel) or Kiss1 KO supplemented with estradiol (Kiss1 KO+E2; right panel) female mice. (C) Mean ±SEM number of NK3R-immunoreactive cells per 30 µm section in the MePD of WTOVX, WTOVX+E2, Kiss1 KO and Kiss1 KO+E2 female mice (as quantified from 6 to 8 sections from 4 to 5 animals/group). (D) Mean ± SEM LH concentrations (ng/ml) and area under the curve (AUC) after an injection of senktide into the MePD of ovariectomized WT (WTOVX+E2; blue line-blue bar) or homozygous Kiss1Cre/Cre (i.e., Kiss1 KO+E2; green line-green bar) supplemented with estradiol or hypogonadal (with low sex steroids) Kiss1 KO female mice (n = 5/group). (E) Schematic representation of the site of injection of an AAV encoding a Cre-dependent hM3Dq:mCherry. The left panel is a schematic representation of the location of the MePD injection site and its anatomical relationship to the optic tract (−1.82 mm from bregma). The right panel is a higher magnification of the boxed area on the left showing MePD kisspeptin neurons tagged with mCherry (red fluorescence), which indicates hM3Dq receptor expressing kisspeptin neurones. (F) Representative photomicrograph of a coronal brain section stained for GFP (green), mCherry (red) and merged GFP and mCherry immunoreactivity in the MePD of a Kiss1 KO female mouse >3 weeks after hM3Dq:mCherry injection (Scale bar 50 µm). In this animal model Cre and GFP have been targeted to the Kiss1 locus and are, therefore, expressed solely in Kiss1 neurons. Thus, colocalization of GFP and m-Cherry immunoreactivity represents Kiss1ARC cells that have been infected with the pAAV encoding a Cre-dependent hM3Dq DREADD. (G, H) Mean ± SEM LH responses and area under the curve (AUC) to an injection of saline (grey line-empty bar) or CNO (green line-green bar) of hM3Dq DREADD injected Kiss1Cre/+ (G) and Kiss1Cre/Cre (KO; H) female mice (n = 5/group). opt: optic tract. Repeated LH concentrations at multiple time-points and between treatments were compared using a 2-WAY ANOVA and a Fishers posthoc test when appropriate. Area under the curve was compared with 2-tailed student t tests. *p < 0.025, **p < 0.0014.
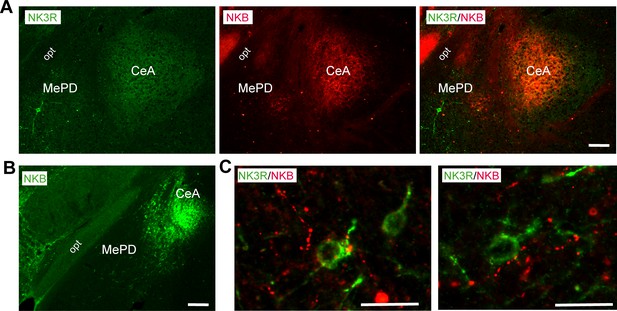
Representative photomicrographs of a coronal section stained for NK3R (green), NKB (red) and merged NK3R and NKB immunoreactivity in the amygdala of (A) WT(OVX+E2) and NKB (green) of (B) WT(OVX) female mouse.
Scale bar: 150 µm (C) Enlarged images depicting NKB fibers (presumably from the CeA) in close contact to NK3R-immunoreactive cell bodies in a WTOVX+E2 (left panel) and Kiss1 KO+E2 (right panel). Scale bar: 20 µm. opt: optic tract, MePD: posterodorsal medial amygdala, CeA: central amygdala.
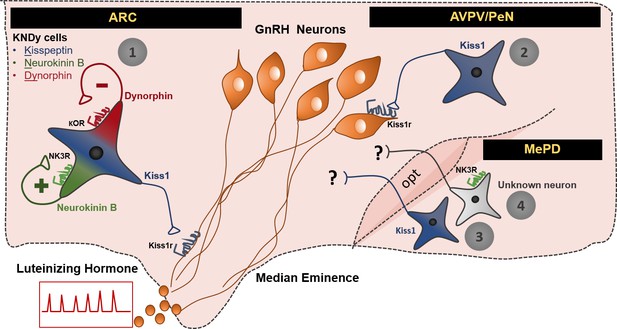
Schematic representation of the neuronal pathways known to stimulate GnRH/LH release in the female mouse.
The first pathway involves Kiss1 neurons located in the arcuate nucleus (ARC), which also express neurokinin B and dynorphin (thus, often referred to as KNDy neurons). These cells have been mainly implicated in the regulation of GnRH/LH pulsatile secretion in males and females. The second GnRH/LH stimulating mechanism, originates from Kiss1 cells located in the anteroventral periventricular/periventricular preoptic nucleus (AVPV/PeN) and is mainly implicated in the generation of the GnRH/LH surge, which leads to ovulation, and is specific to females. The third and fourth stimulating mechanisms are described in this study and originate from the posterodorsal medial amygdala (MePD). Thus, the third mechanism known to stimulate GnRH/LH release involves Kiss1 neurons and the fourth NK3R expressing neurons of unknown phenotype. The latter are activated only in the presence of estradiol in a mechanism that is female specific. The precise neuronal pathway linking these MePD cells with the stimulation of GnRH/LH release, as well as the physiological relevance of this stimulation, remains to be elucidated. ARC: Arcuate Nucleus, AVPV/PeN: anteroventral periventricular/periventricular preoptic nucleus, MePD: posterodorsal medial amygdala, NK3R: neurokinin receptor 3, κOR: κ opioid receptor, Kiss1r: kiss1 receptor, opt: optic tract.
Additional files
-
Transparent reporting form
- https://doi.org/10.7554/eLife.40476.012





