BUB-1 promotes amphitelic chromosome biorientation via multiple activities at the kinetochore
Figures
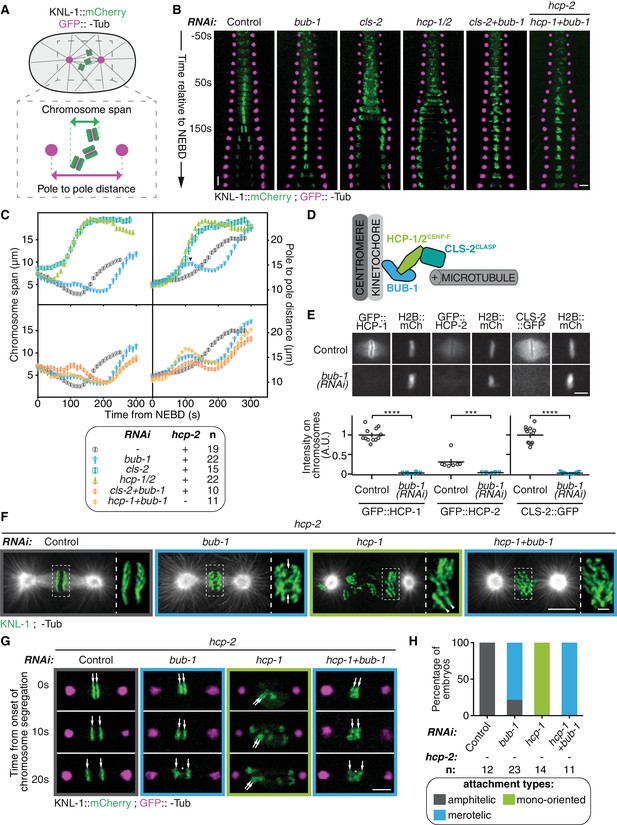
BUB-1 inhibits chromosome biorientation.
(A) Assay for kinetochore-microtubule attachment formation and chromosome congression. GFP::γ-Tub is used to measure the pole to pole distance, and KNL-1::mCherry is used to measure the chromosome span in the spindle pole axis. (B) Kymographs generated from embryos expressing GFP::γ-Tub and KNL-1::mCherry, for the different indicated conditions. Horizontal scale bar, 5 μm; Vertical scale bar, 20 s. (C) Chromosome span and pole to pole distance as functions of time after NEBD for the indicated conditions. Top right corner: Arrowhead, spindle pole bump following BUB-1 depletion. (D) Schematics of BUB-1 at kinetochores recruiting its downstream partners HCP-1/2CENP-F and CLS-2CLASP. (E) Top: Representative images from time-lapse movies showing BUB-1 dependent localisations of GFP::HCP-1CENP-F, GFP::HCP-2CENP-F and CLS-2CLASP::GFP on chromosomes (H2B::mCherry), at metaphase. Bottom: Quantification of the GFP signal on chromosomes at metaphase. Mann Whitney tests were used to determine significance (GFP::HCP-1 p < 0.0001, GFP::HCP-2 p = 0.0003, CLS-2::GFP p < 0.0001). Scale bar, 5 μm. (F) Immunofluorescent staining of kinetochores (KNL-1) and microtubules (DM1α) in Δhcp-2 zygotes at metaphase in the indicated conditions. Scale bar, 5 μm. Magnifications of the kinetochore region (highlighted by a dashed rectangle) are shown on the right of each panel. Arrows point to bent merotelic kinetochores in the BUB-1-depleted zygote. Arrowheads show a mono-oriented chromosome in the HCP-1CENP-F-depleted zygote. Scale bar, 1 μm. (G) Representative images of kinetochores (KNL-1::mCherry, green) and spindle poles (GFP::γ-Tub, magenta), at different times from the onset of chromosome segregation, for the indicated conditions. White arrows point towards sister kinetochores. White asterisks indicate the presence of kinetochore stretches. Scale bar, 5 μm. (H) Quantification of the percentage of embryos with chromosomes engaged in amphitelic, merotelic and mono-oriented attachments, in the indicated conditions. Error bars represent the SEM.
-
Figure 1—source data 1
Chromosome span and pole to pole distance as functions of time after NEBD for the indicated conditions.
- https://doi.org/10.7554/eLife.40690.008
-
Figure 1—source data 2
GFP::HCP-1, GFP::HCP-2, and CLS-2::GFP signals on chromosomes at metaphase.
- https://doi.org/10.7554/eLife.40690.009
-
Figure 1—source data 3
Percentage of embryos with chromosomes engaged in amphitelic, merotelic and mono-oriented attachments, in the indicated conditions.
- https://doi.org/10.7554/eLife.40690.010
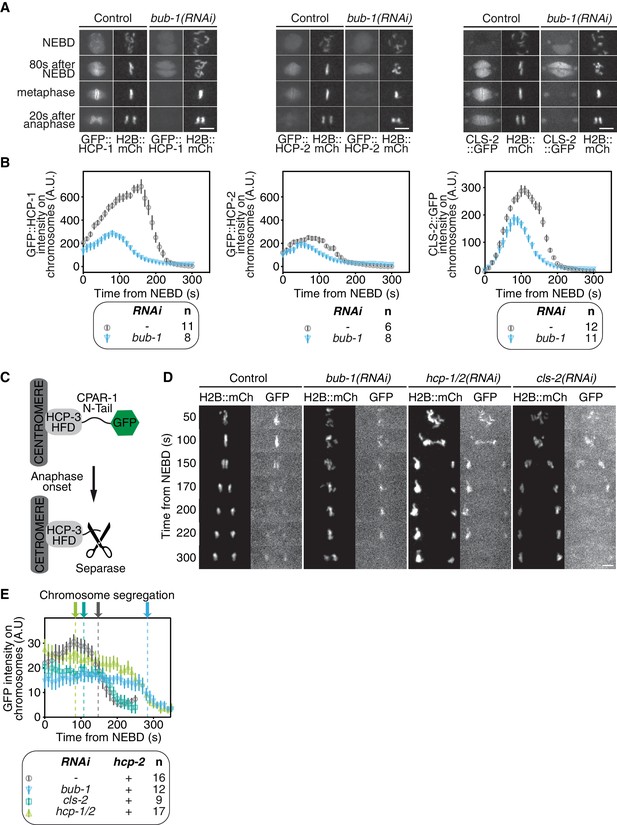
HCP-1/2CENP-F and CLS-2CLASP downstream of BUB-1 prevent premature chromosome segregation.
(A) Representative images from time-lapse movies showing BUB-1 dependent localizations of GFP::HCP-1CENP-F, GFP::HCP-2CENP-F and CLS-2CLASP::GFP on chromosomes (H2B::mCherry), at different times relative to NEBD and Anaphase onset. (B) Quantifications of the integrated GFP::HCP-1CENP-F, GFP::HCP-2CENP-F, and CLS-2CLASP::GFP signals measured on chromosomes as a function of time from NEBD, for the indicated conditions. (C) Schematic of the separase-sensor allowing detection of anaphase onset by the loss of GFP signal from centromeres. (D) Representative images from time-lapse movies of embryos expressing H2B::mCherry and the GFP-tagged anaphase sensor, in the indicated conditions. (E) Quantifications of the integrated GFP signal measured on chromosomes as a function of time from NEBD, for the indicated conditions. Arrows indicate the time of chromosome segregation onset for the different conditions. Error bars represent the SEM. Scale bars, 5 μm.
-
Figure 1—figure supplement 1—source data 1
GFP::HCP-1, GFP::HCP-2, and CLS-2::GFP signals on chromosomes over time.
- https://doi.org/10.7554/eLife.40690.004
-
Figure 1—figure supplement 1—source data 2
Quantifications of the integrated anaphase sensor GFP signal measured on chromosomes over time.
- https://doi.org/10.7554/eLife.40690.005
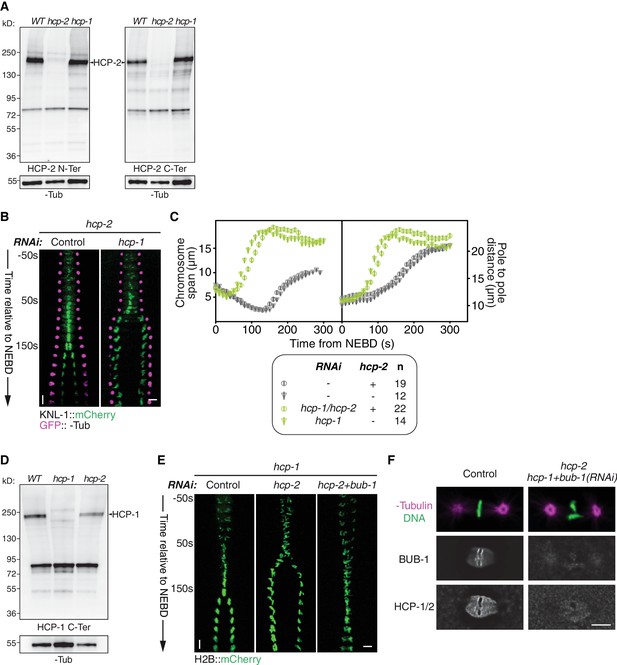
Characterization of the Δhcp-1 and Δhcp-2 alleles.
(A) Western blot against HCP-2CENP-F, in the indicated strains, using polyclonal antibodies targeting the HCP-2CENP-F N-Terminal or C-Terminal region. α-Tubulin is used as a loading control. (B) Kymographs generated from embryos expressing GFP::γ-Tub and KNL-1::mCherry for the indicated conditions. (C) Chromosome span and pole to pole distance as functions of time after NEBD for the indicated conditions. (D) Western blot against HCP-1CENP-F, in the indicated strains, using polyclonal antibodies targeting HCP-1CENP-F C-Terminal region. α-Tubulin is used as a loading control. (E) Kymographs generated from embryos expressing H2B::mCherry, for the different indicated conditions. (F) Representative fixed zygotes in metaphase, stained for DNA, α-Tubulin, BUB-1, and HCP-1/2CENP-F for the indicated conditions. Error bars represent the SEM. Horizontal scale bars, 5 μm; Vertical scale bars, 20 s.
-
Figure 1—figure supplement 2—source data 1
Chromosome span and pole to pole distance as functions of time after NEBD for the indicated conditions.
- https://doi.org/10.7554/eLife.40690.007
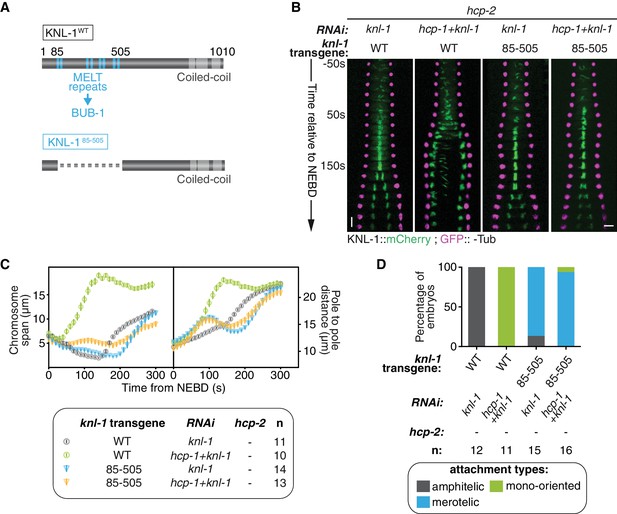
Biorientation inhibition requires BUB-1 localisation at the kinetochore.
(A) Schematics of WT KNL-1 and of the ∆85–505 mutant that leads to loss of BUB-1 from kinetochores. (B) Kymographs generated from embryos expressing GFP::γ-Tub and KNL-1::mCherry, for the indicated conditions. (C) Chromosome span and pole to pole distance as functions of time after NEBD for the indicated conditions. (D) Quantification of the percentage of embryos with chromosomes engaged in amphitelic, merotelic and mono-oriented attachments in the indicated conditions. Error bars represent the SEM. Horizontal scale bar, 5 μm; Vertical scale bar, 20 s.
-
Figure 2—source data 1
Chromosome span and pole to pole distance as functions of time after NEBD for the indicated conditions.
- https://doi.org/10.7554/eLife.40690.015
-
Figure 2—source data 2
Percentage of embryos with chromosomes engaged in amphitelic, merotelic and mono-oriented attachments, in the indicated conditions.
- https://doi.org/10.7554/eLife.40690.016
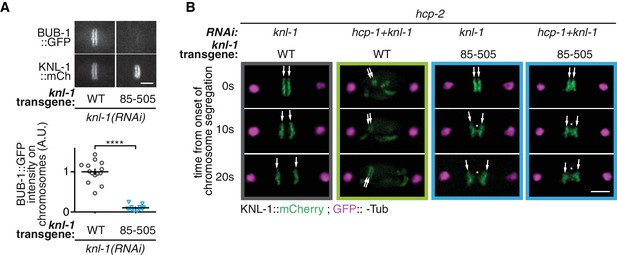
Biorientation inhibition requires BUB-1 localization at the kinetochore.
(A) Top: Representative images showing BUB-1::GFP recruitment to kinetochores (KNL-1::mCHerry) in presence of different KNL1 transgenes at metaphase. Bottom: Quantification of the BUB-1::GFP signal measured on kinetochores at metaphase in the indicated conditions. A Mann Whitney test was used to determine significance (p < 0.0001). (B) Representative images of kinetochores (KNL-1::mCherry, green) and spindle poles (GFP::γ-Tub, magenta), at different times from the onset of chromosome segregation for the indicated conditions. White arrows point towards sister kinetochores. White asterisks indicate the presence of kinetochore stretches. Scale bars, 5 μm.
-
Figure 2—figure supplement 1—source data 1
BUB-1::GFP signal measured on kinetochores at metaphase in the indicated conditions.
- https://doi.org/10.7554/eLife.40690.014
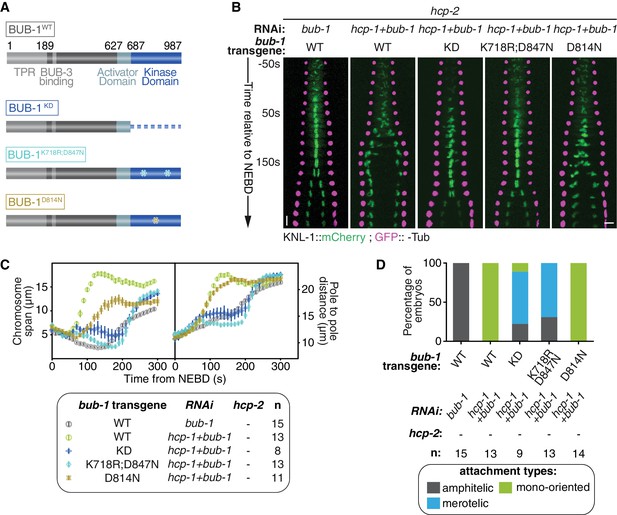
BUB-1 kinase domain inhibits biorientation independently of its kinase activity.
(A) Schematics of wild-type (WT) BUB-1 and of the different BUB-1 mutants. (B) Kymographs generated from embryos expressing GFP::γ-Tub and KNL-1::mCherry, for different indicated conditions. (C) Chromosome span and pole to pole distance as functions of time after NEBD for the indicated conditions. (D) Quantification of the percentage of embryos with chromosomes engaged in amphitelic, merotelic and mono-oriented attachments in the indicated conditions. Error bars represent the SEM. Horizontal scale bar, 5 μm; Vertical scale bar, 20 s.
-
Figure 3—source data 1
Chromosome span and pole to pole distance as functions of time after NEBD for the indicated conditions.
- https://doi.org/10.7554/eLife.40690.023
-
Figure 3—source data 2
Percentage of embryos with chromosomes engaged in amphitelic, merotelic and mono-oriented attachments, in the indicated conditions.
- https://doi.org/10.7554/eLife.40690.024
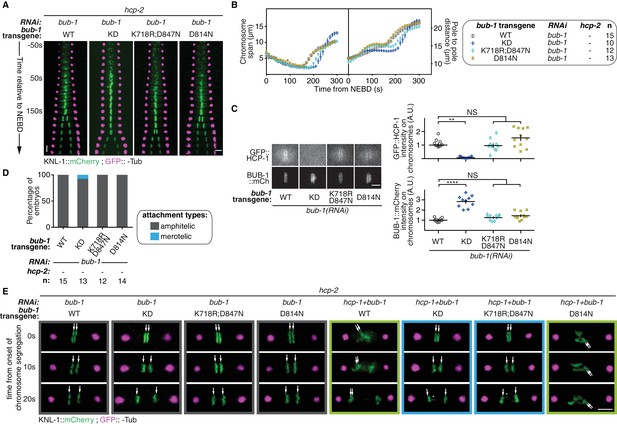
BUB-1 kinase domain inhibits biorientation independently of its kinase activity.
(A) Kymographs generated from embryos expressing GFP::γ-Tub and KNL-1::mCherry, for the different indicated conditions. (B) Chromosome span and pole to pole distance as functions of time after NEBD for the indicated conditions. (C) Representative images showing the kinetochore recruitment of GFP::HCP-1CENP-F and the different BUB-1::mCherry transgenes at metaphase. Quantification of the BUB-1::mCherry and GFP::HCP-1CENP-F signals measured on kinetochores at metaphase in the indicated conditions. Kruskall Wallis tests with Dunn’s correction for multiplicity were used to assess significance (GFP::HCP-1CENP-F ∆KD p = 0,0029, GFP::HCP-1CENP-F K718R;D847N p > 0,9999, GFP::HCP-1CENP-F D814N p = 0,4366, BUB-1::mCherry ∆KD p < 0,0001, BUB-1::mCherry K718R;D847N p = 0,5369, BUB-1::mCherry D814N p = 0,1915). (D) Quantification of the percentage of embryos with chromosomes engaged in amphitelic and merotelic attachments in the indicated conditions. (E) Representative images of kinetochores (KNL-1::mCherry, green) and spindle poles (GFP::γ-Tub, magenta), at different times from the onset of chromosome segregation for the indicated conditions. White arrows point towards sister kinetochores. White asterisks indicate the presence of kinetochore stretches. Error bars represent the SEM. Horizontal scale bars, 5 μm; Vertical scale bar, 20 s.
-
Figure 3—figure supplement 1—source data 1
Chromosome span and pole to pole distance as functions of time after NEBD for the indicated conditions.
- https://doi.org/10.7554/eLife.40690.020
-
Figure 3—figure supplement 1—source data 2
BUB-1::mCherry and GFP::HCP-1CENP-F signals measured on kinetochores at metaphase in the indicated conditions.
- https://doi.org/10.7554/eLife.40690.021
-
Figure 3—figure supplement 1—source data 3
Percentage of embryos with chromosomes engaged in amphitelic and merotelic, in the indicated conditions.
- https://doi.org/10.7554/eLife.40690.022
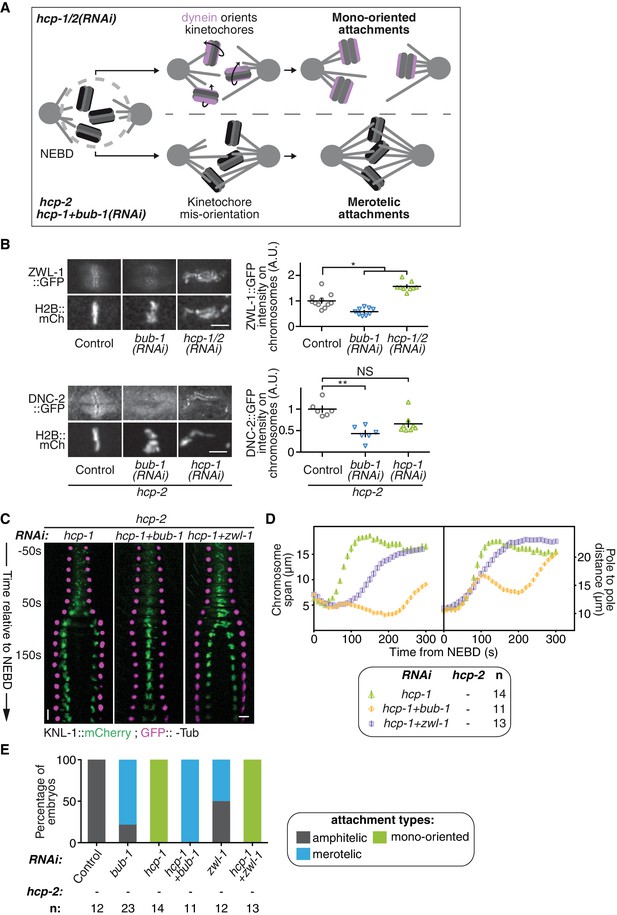
BUB-1 accelerates non-merotelic end-on attachments via dynein, independently of the inhibitory effect on biorientation.
(A) Schematics of the potential mechanism for chromosome biorientation inhibition by BUB-1 in absence of HCP-1/2CENP-F. By orienting kinetochores relative to spindle poles, BUB-1 downstream partners RZZ and dynein-dynactin connect chromosomes in a mono-oriented conformation to short non-dynamic microtubules, leading to premature chromosome segregation. In absence of BUB-1, kinetochore mis-orientation enables the establishment of bioriented merotelic connections capable of resisting cortical traction forces. (B) Left: Representative images from time-lapse movies showing localisations of ZWL-1Zwilch::GFP and DNC-2::GFP on chromosomes (H2B::mCherry) in the indicated conditions, 100 s after NEBD. Right: Quantifications of the GFP signal on chromosomes 100 s after NEBD. Kruskall Wallis tests with Dunn’s correction for multiplicity were used to assess significance (ZWL-1::GFP bub-1(RNAi) p = 0,0276, ZWL-1::GFP hcp-1/2(RNAi) p = 0,0341, DNC-2::GFP bub-1(RNAi) p = 0,0015, DNC-2::GFP hcp-1(RNAi) p = 0,0671). (C) Kymographs generated from embryos expressing GFP::γ-Tub and KNL-1::mCherry for the indicated conditions. (D) Chromosome span and pole to pole distance as functions of time after NEBD for the indicated conditions. (E) Quantification of the percentage of embryos with chromosomes engaged in amphitelic, merotelic and mono-oriented attachments in the indicated conditions. Error bars represent the SEM. Horizontal scale bars, 5 μm; Vertical scale bar, 20 s.
-
Figure 4—source data 1
ZWL-1Zwilch::GFP and DNC-2::GFP signals on chromosomes100safter NEBD.
- https://doi.org/10.7554/eLife.40690.031
-
Figure 4—source data 2
Chromosome span and pole to pole distance as functions of time after NEBD for the indicated conditions.
- https://doi.org/10.7554/eLife.40690.032
-
Figure 4—source data 3
Percentage of embryos with chromosomes engaged in amphitelic, merotelic and mono-oriented attachments, in the indicated conditions.
- https://doi.org/10.7554/eLife.40690.033
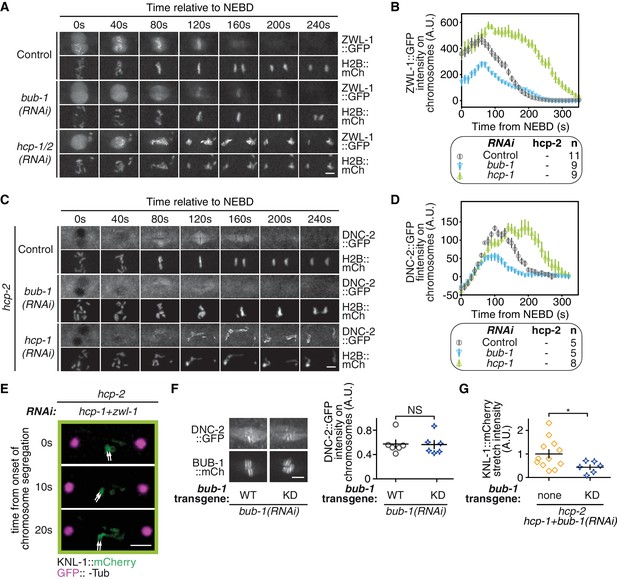
BUB-1 accelerates non-merotelic end-on attachments via dynein, independently of the inhibitory effect on biorientation.
(A) Representative images from time-lapse movies of embryos expressing ZWL-1Zwilch::GFP and H2B::mCherry in the indicated conditions. (B) Quantification of the integrated ZWL-1Zwilch::GFP signal on chromosomes as a function of time for the indicated conditions shown in (A). (C) Representative images from time-lapse movies of embryos expressing DNC-2::GFP and H2B::mCherry in the indicated conditions. (D) Quantification of the integrated DNC-2::GFP signal on chromosomes as a function of time for the indicated conditions shown in (C). (E) Representative images of kinetochores (KNL-1::mCherry, green) and spindle poles (GFP::γ-Tub, magenta), at different times from the onset of chromosome segregation for the indicated conditions. White arrows point towards sister kinetochores. (F) Left: Representative images showing the kinetochore recruitment of DNC-2::GFP and the different BUB-1::mCherry transgenes 100 s after NEBD. Right: Quantification of the DNC-2::GFP signal on kinetochores 100 s after NEBD in the indicated conditions. A Mann Whitney test was used to determine significance (p > 0,9999). (G) Quantification of the KNL-1::mCherry signal between segregating sister kinetochores 20 s after anaphase onset for the indicated conditions. A Mann Whitney test was used to determine significance (p = 0,0415). Error bars represent the SEM. Horizontal scale bars, 5 μm; Vertical scale bar, 20 s.
-
Figure 4—figure supplement 1—source data 1
Integrated ZWL-1::GFP signal on chromosomes as a function of time for the indicated conditions.
- https://doi.org/10.7554/eLife.40690.028
-
Figure 4—figure supplement 1—source data 2
Integrated DNC-2::GFP signal on chromosomes as a function of time for the indicated conditions.
- https://doi.org/10.7554/eLife.40690.029
-
Figure 4—figure supplement 1—source data 3
DNC-2::GFP signal on kinetochores100safter NEBD in the indicated conditions.
- https://doi.org/10.7554/eLife.40690.030
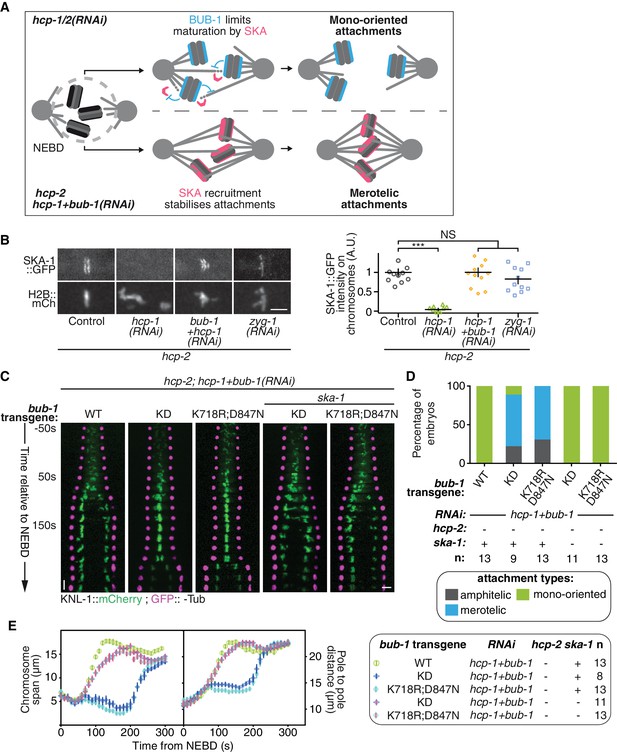
BUB-1 inhibits biorientation in absence of HCP-1/2CENP-F by preventing SKA complex recruitment.
(A) Schematics of the potential mechanism for chromosome biorientation inhibition by BUB-1 in absence of HCP-1/2CENP-F. BUB-1 limits attachment maturation by the SKA complex, leading to the incapacity for chromosomes to birorient when microtubules are short and non-dynamic. Co-depleting BUB-1 restores SKA complex recruitment, allowing the strengthening of attachments and therefore the establishment of biorientation even when microtubules are short and non-dynamic. (B) Left: Representative images from time-lapse movies showing the localization of SKA-1::GFP on chromosomes (H2B::mCherry) in the indicated conditions at metaphase. Right: Quantification of the GFP signal on chromosomes at metaphase. Kruskall Wallis tests with Dunn’s correction for multiplicity were used to assess significance (hcp-1(RNAi) p = 0,0006, hcp-1 +bub-1(RNAi) p > 0.9999, zyg-1(RNAi) p > 0,9999). (C) Kymographs generated from embryos expressing GFP::γ-Tub and KNL-1::mCherry for the indicated conditions. (D) Quantification of the percentage of embryos with chromosomes engaged in amphitelic, merotelic and mono-oriented attachments in the indicated conditions. (E) Chromosome span and pole to pole distance as functions of time after NEBD for the indicated conditions. Error bars represent the SEM. Horizontal scale bars, 5 μm; Vertical scale bar, 20 s.
-
Figure 5—source data 1
SKA-1::GFP signal on chromosomes at metaphase.
- https://doi.org/10.7554/eLife.40690.044
-
Figure 5—source data 2
Percentage of embryos with chromosomes engaged in amphitelic, merotelic and mono-oriented attachments, in the indicated conditions.
- https://doi.org/10.7554/eLife.40690.045
-
Figure 5—source data 3
Chromosome span and pole to pole distance as functions of time after NEBD for the indicated conditions.
- https://doi.org/10.7554/eLife.40690.046
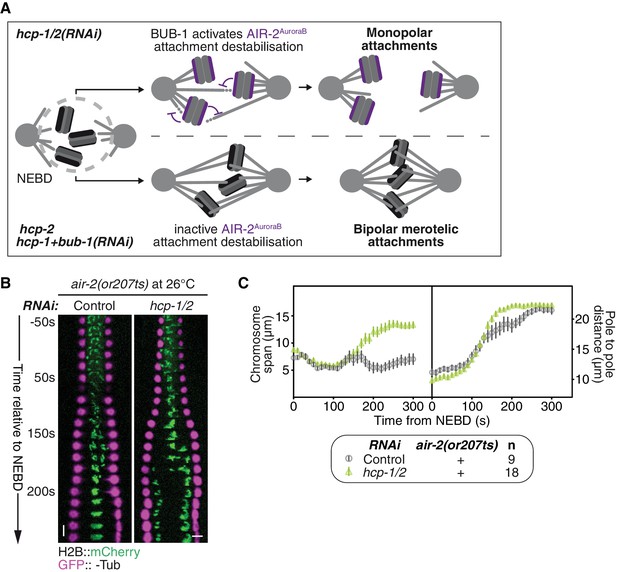
BUB-1 does not inhibit biorientation via AIR-2AuroraB.
(A) Schematics of the potential mechanism for chromosome biorientation inhibition by BUB-1 in absence of HCP-1/2CENP-F. BUB-1 promotes attachment destabilisation by AIR-2AuroraB, leading to the incapacity for chromosomes to biorient when microtubules are short and non-dynamic. Co-depleting BUB-1 limits AIR-2AuroraB-dependent attachment destabilization, allowing the strengthening of attachments and therefore the establishment of chromosome biorientation even when microtubules are short and non-dynamic. (B) Kymographs generated from embryos expressing GFP::β-Tub and H2B::mCherry for the indicated conditions. (C) Chromosome span and pole to pole distance as functions of time after NEBD for the indicated conditions. Error bars represent the SEM. Horizontal scale bar, 5 μm; Vertical scale bar, 20 s.
-
Figure 5—figure supplement 1—source data 1
Chromosome span and pole to pole distance as functions of time after NEBD for the indicated conditions.
- https://doi.org/10.7554/eLife.40690.037
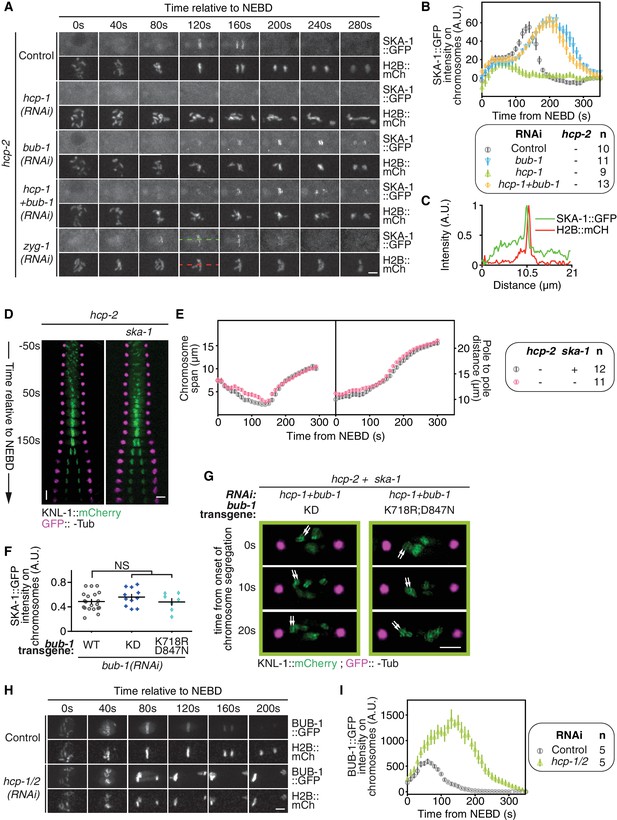
BUB-1 inhibits chromosome biorientation in absence of HCP-1/2CENP-F by preventing SKA complex recruitment.
(A) Representative images from time-lapse movies showing localizations of SKA-1::GFP on chromosomes (H2B::mCherry) in the indicated conditions at different times relative to NEBD. (B) Quantification of the integrated SKA-1::GFP signal measured on chromosomes as a function of time from NEBD for the indicated conditions. (C) Quantification of the SKA-1::GFP and H2B::mCherry intensities along a linescan depicted by the dotted lines in (A) at 120 s after NEBD. (D) Kymographs generated from embryos expressing GFP::γ-Tub and KNL-1::mCherry for the indicated conditions. (E) Chromosome span and pole to pole distance as functions of time after NEBD for the indicated conditions. (F) Quantification of the SKA-1::GFP signal on chromosomes at metaphase in the indicated conditions. Kruskall Wallis tests with Dunn’s correction for multiplicity were used to assess significance (∆KD p = 0,5079, K718R;D847N p > 0,9999. (G) Representative images of kinetochores (KNL-1::mCherry, green) and spindle poles (GFP::γ-Tub, magenta) at different times from the onset of chromosome segregation for the indicated conditions. White arrows point towards sister kinetochores. (H) Representative images from time-lapse movies showing localizations of BUB-1::GFP on chromosomes (H2B::mCherry) in the indicated conditions at different times relative to NEBD. (I) Quantification of the integrated BUB-1::GFP signal measured on chromosomes as a function of time from NEBD for the indicated conditions. Error bars represent the SEM. Horizontal scale bars, 5 μm; Vertical scale bar, 20 s.
-
Figure 5—figure supplement 2—source data 1
SKA-1::GFP signal measured on chromosomes as a function of time.
- https://doi.org/10.7554/eLife.40690.039
-
Figure 5—figure supplement 2—source data 2
SKA-1::GFP and H2B::mCherry intensities along a linescan.
- https://doi.org/10.7554/eLife.40690.040
-
Figure 5—figure supplement 2—source data 3
Chromosome span and pole to pole distance as functions of time after NEBD for the indicated conditions.
- https://doi.org/10.7554/eLife.40690.041
-
Figure 5—figure supplement 2—source data 4
SKA-1::GFP signal on chromosomes at metaphase in the indicated conditions.
- https://doi.org/10.7554/eLife.40690.042
-
Figure 5—figure supplement 2—source data 5
Integrated BUB-1::GFP signal measured on chromosomes as a function of time.
- https://doi.org/10.7554/eLife.40690.043
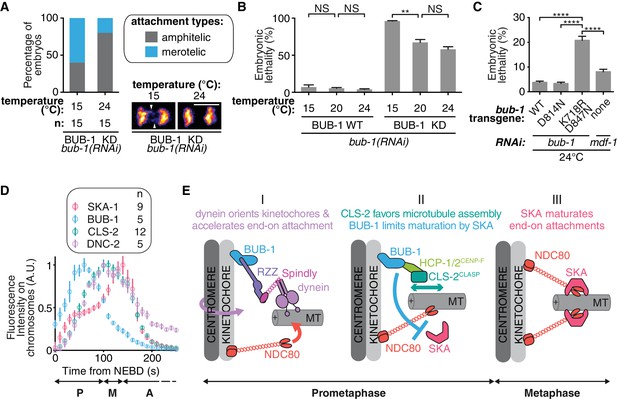
BUB-1 limits merotely by balancing kinetochore microtubule assembly and kinetochore attachment maturation.
(A) Quantification of the percentage of embryos with chromosomes engaged in amphitelic and merotelic attachments in the indicated conditions. Representative images of merotelic and non merotelic segregations are shown based on the KNL-1::mCherry signal 20 s after anaphase onset. White arrowheads point toward kinetochore stretches. (B) Embryonic lethality in the indicated conditions. Kruskall Wallis tests with Dunn’s correction for multiplicity were used to assess significance (WT 15°C (n = 252 embryos) vs 20°C (n = 1934) p > 0,9999, WT 20°C vs 24°C (n = 2107) p > 0,9999, ∆KD 15°C (n = 352) vs 20°C (n = 1104) p = 0,0012, ∆KD 20°C vs 24°C (n = 751) p = 0,4722). (C) Embryonic lethality in the indicated conditions. Kruskall Wallis tests with Dunn’s correction for multiplicity were used to assess significance (WT (n = 2107 embryos) vs K718R;D847N (n = 2663) p < 0,0001, D814N (n = 2181) vs K718R;D847N p < 0,0001, K718R;D847N vs mdf-1(RNAi) (n = 3410) p < 0,0001). (D) Quantifications of the integrated signals measured on chromosomes for different GFP-tagged proteins as function of time from NEBD. (E) (I) In prometaphase, BUB-1 localises to kinetochores and favours amphitely via two independent mechanisms. Downstream of BUB-1, the RZZ complex, Spindly and dynein-dynactin orient kinetochores and regulate NDC-80 activity, leading to the acceleration of end-on attachment in a non-merotelic conformation. (II) BUB-1 further contributes to establishing amphitelic attachments by promoting kinetochore microtubule assembly via HCP-1/2CENP-F and CLS-2CLASP recruitment, while limiting attachment maturation via the SKA complex. (III) In metaphase, BUB-1 leaves kinetochores allowing attachment maturation by the SKA complex. Scale bar, 5 μm.
-
Figure 6—source data 1
Percentage of embryos with chromosomes engaged in amphitelic and merotelic attachments in the indicated conditions.
- https://doi.org/10.7554/eLife.40690.049
-
Figure 6—source data 2
Percentage of embryonic lethality in the indicated conditions.
- https://doi.org/10.7554/eLife.40690.050
-
Figure 6—source data 3
Percentage of embryonic lethality in the indicated conditions.
- https://doi.org/10.7554/eLife.40690.051
-
Figure 6—source data 4
Integrated signals measured on chromosomes for the indicated GFP-tagged proteins as function of time.
- https://doi.org/10.7554/eLife.40690.052
Videos
One-cell C. elegans embryos in the indicated conditions.
10 s per frame. Magenta, γ-Tubulin::GFP (spindle poles); Green, KNL-1::mCherry (kinetochores).
One-cell C. elegans embryos in the indicated conditions.
10 s per frame. Magenta, γ-Tubulin::GFP (spindle poles); Green, KNL-1::mCherry (kinetochores).
One-cell C. elegans embryos in the indicated conditions.
10 s per frame. Magenta, γ-Tubulin::GFP (spindle poles); Green, KNL-1::mCherry (kinetochores).
One-cell C. elegans embryos in the indicated conditions.
10 s per frame. Magenta, γ-Tubulin::GFP (spindle poles); Green, KNL-1::mCherry (kinetochores).
One-cell C. elegans embryos in the indicated conditions.
10 s per frame. Magenta, γ-Tubulin::GFP (spindle poles); Green, KNL-1::mCherry (kinetochores).
Additional files
-
Supplementary file 1
(A) List of worm strains used in this study. (B) List of templates and primers used to synthesize double stranded RNA. (C) Targeted regions for CRISPR-Cas9 mediated hcp-1CENP-F and hcp-2CENP-F deletion mutant generation. (D) Images and datasets presented in different figures and panels.
- https://doi.org/10.7554/eLife.40690.053
-
Transparent reporting form
- https://doi.org/10.7554/eLife.40690.054





