Mutant huntingtin enhances activation of dendritic Kv4 K+ channels in striatal spiny projection neurons
Figures
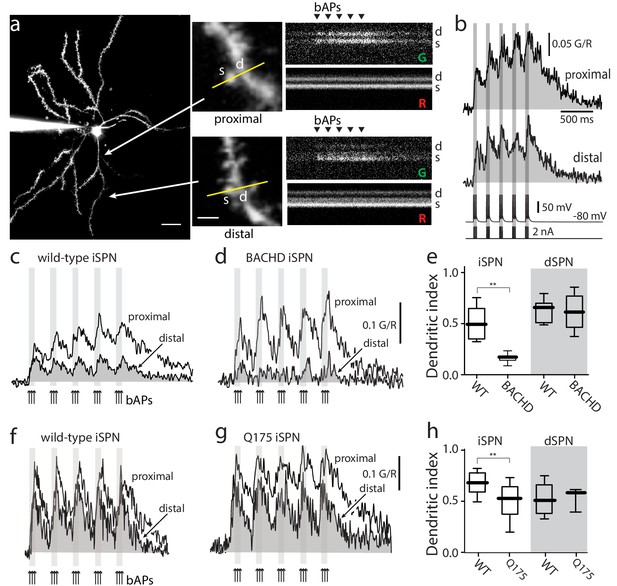
Distal dendritic excitability was reduced in iSPNs from HD models.
(a) Characterization of Ca2+ transients induced by bAPs in dendritic spines of SPNs. Left: Maximum intensity Z projection images of an iSPN filled with Alexa Fluor 568 (wild-type mouse >2 months; scale bar 20 μm). Middle: Line scans taken in proximal (~50 μm) and distal (~100 μm) spines (letter s) and dendrites (letter d) located in the same focal plane (scale bar 2 μm). Right: Calcium transients evoked by bAPs (triangles). (b) Quantification of the area under the curve from Ca2+ transients taken at proximal (first row) and distal (second row) head spines; similar transients were seen in dendritic shafts (Figure 1—figure supplement 1). Voltage responses (third row; 5 burst of 3 APs each) evoked by somatic current injection (fourth row; 2 nA amplitude; 2 ms duration; inter burst frequency: 5 Hz; intra burst frequency: 50 Hz). (c) Ca2+ transients evoked by bAPs (arrows) at proximal and distal dendritic sites in iSPNs from wild-type (WT) mice and (d) symptomatic BACHD mice. Here, the dendritic Ca2+ signal was derived from shafts; similar transients were seen in spines. (e) Dendritic index representing the ratio of the area under the curve between distal and proximal Ca2+ transients taken from dendritic spines and shafts in iSPNs and dSPNs demonstrates the reduction of dendritic excitability in iSPNs taken from symptomatic BACHD mice (left) (p=0.0008, Mann-Whitney U, Two-Tailed, n = 9). Dendritic index was not significantly different in dSPNs (right) from WT and BACHD mice (p=0.95216, Mann-Whitney U, Two-Tailed, n = 8). (f, g) Ca2+ transients from Q175 ±iSPNs and their WT littermates. (h) The boxplot shows that the dendritic index was reduced in Q175 ±iSPNs compared to WT littermates (left; p=0.0107, Mann-Whitney U, One-Tailed, n = 6). Dendritic index was not significantly different in dSPNs WT and Q175 ±mice (right; p=0.15; Mann-Whitney U, Two-tailed, n = 5 WT, n = 3 Q175+/-). See Figure 1—source data 1.
-
Figure 1—source data 1
Source data for Figure 1.
- https://doi.org/10.7554/eLife.40818.007
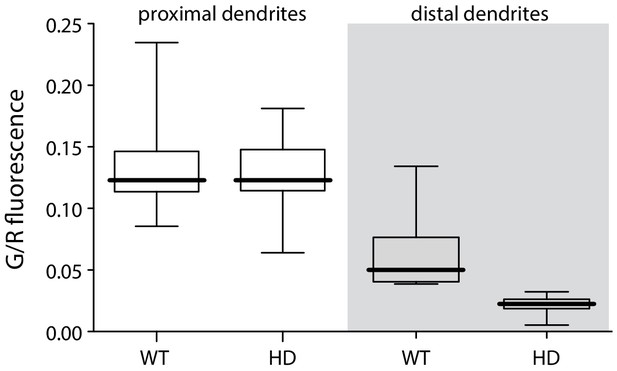
bAP-evoked distal but not proximal dendritic fluorescence measurements were diminished in BACHD iSPNs.
Left, boxplot of peak fluorescence measurements (G/R) in proximal dendrites of BACHD and WT iSPNs (p=1.0, Mann-Whitney U, Two-Tailed; n = 9); right, boxplot of peak fluorescent measurements in distal dendrites (p=0.00042, Mann-Whitney U, Two-Tailed; n = 9). See Figure 1—figure supplement 1—source data 1.
-
Figure 1—figure supplement 1—source data 1
Source data for Figure 1—figure supplement 1.
- https://doi.org/10.7554/eLife.40818.004
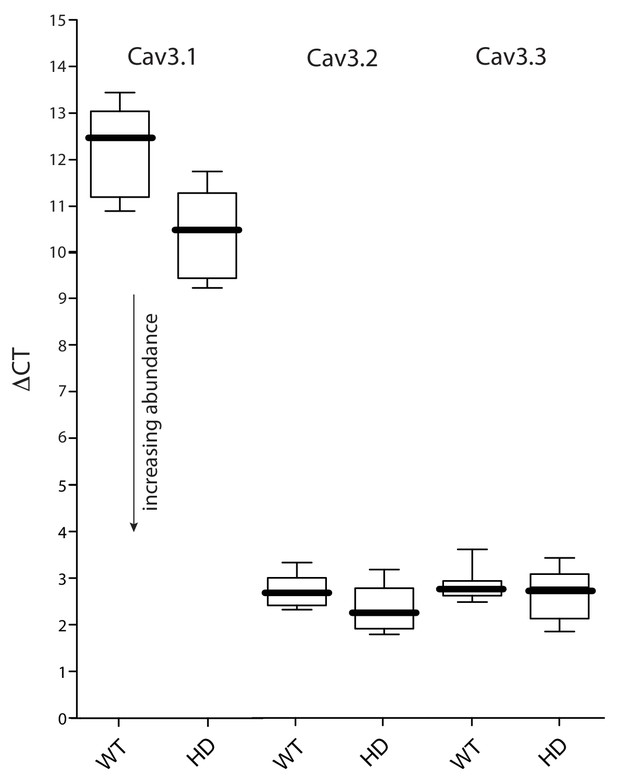
Expression of Cav3 mRNAs was not significantly altered in HD iSPNs.
(a FACS-qPCR was performed to determine transcript expression in Q175 ±iSPNs relative to their WT controls. Data are presented as ΔCT. A modest elevation in Cav3.1 expression (lower ΔCT) was observed in the Q175 iSPNs compared to their WT controls (p=0.0492, Mann-Whitney U test; n = 11–13 mice). However, Cav3.1 transcripts were very low in abundance. No alterations in the expression of Cav3.2 or Cav3.3 was observed (p>0.05, Mann-Whitney U, Two-Tailed; n = 11–13 mice). See Figure 1—figure supplement 2—source data 1.
-
Figure 1—figure supplement 2—source data 1
Source data for Figure 1—figure supplement 2.
- https://doi.org/10.7554/eLife.40818.006
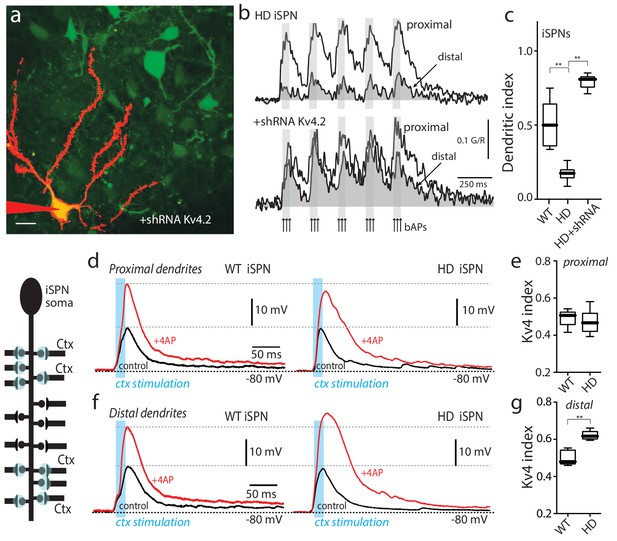
Upregulation of Kv4 channel activity suppressed dendritic excitability in HD iSPNs.
(a) Viral delivery of Kv4.2 shRNA into the striatum of BACHD mice. Z projection images of an iSPN filled with Alexa Fluor 568 and transfected with Kv4.2 shRNA for 4 weeks. Infected neurons are labeled by eGFP, note the presence of the label in the soma and along the dendrites of SPNs (scale bar 10 μm). (b) Calcium transients in response to bAPs (arrows) at proximal and distal dendritic spines in iSPNs from BACHD mice; the loss of dendritic excitability observed in non-transfected neurons (top) was rescued by knocking down Kv4.2 channels (bottom). (c) The dendritic index was significantly smaller in non-infected neurons than in infected iSPNs (p=0.0002, Mann-Whitney U, Two-Tailed, n = 6). (d) Voltage responses to optogenetic stimulation of corticostriatal terminals at proximal dendrites in WT (left) and HD (right) iSPNs before and after application of 4-AP (2 mM). (e) Kv4 index reflecting the engagement of Kv4.2 potassium channels is not significantly different in proximal dendrites of WT compared with BACHD mice (p=0.3401, Mann-Whitney U, Two-Tailed, n = 9). (f) Voltage responses to optogenetic stimulation of corticostriatal terminals at distal dendrites in WT (left) and BACHD (right) iSPNs before and after 4-AP (2 mM) application. (g) Kv4 index was larger in distal dendrites of HD iSPNs than in WT iSPNs (p=0.0007, Mann-Whitney U, Two-Tailed, n = 9). See Figure 2—source data 1.
-
Figure 2—source data 1
Source data for Figure 2.
- https://doi.org/10.7554/eLife.40818.014
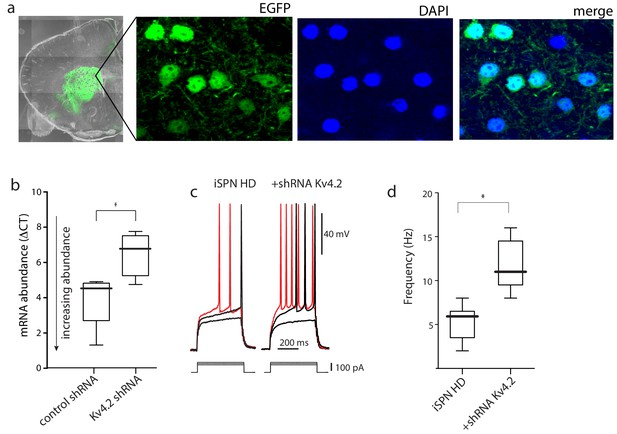
AAV Kv4.2-shRNA mediated knockdown in striatum increased iSPN excitability.
(a) Confocal images of a coronal brain section 4 weeks after stereotaxic delivery of AAV Kv4.2 shRNA-eGFP expression construct to the striatum. DAPI was used to stain nuclei. (b) The knockdown efficiency of Kv4.2-shRNA-eGFP was tested by qPCR from striatal tissue dissected from the injection site. AAV delivery of the Kv4.2 shRNA resulted in a significant reduction in Kv4.2 mRNA abundance (p=0.039, Mann-Whitney U, Two-Tailed; n = 4–5 mice). (c) The evoked responses in BACHD iSPNs in ex vivo slices from mice injected with the Kv4.2 shRNA; on the left the responses to increasing current steps of an uninfected iSPN and on the right the responses in an infected iSPN; note the stronger responses in the infected iSPNs. (d) Boxplots showing spike rates in uninfected and infected iSPNs from BACHD mice in response to a current step of 150 pA (p=0.0059, Mann-Whitney U, Two-Tailed; n = 6,6 cells). See Figure 2—figure supplement 1—source data 1.
-
Figure 2—figure supplement 1—source data 1
Source data for Figure 2—figure supplement 1.
- https://doi.org/10.7554/eLife.40818.010
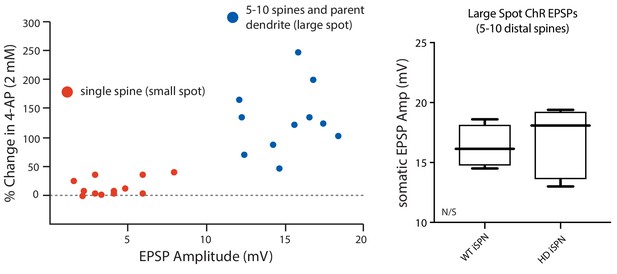
4-AP (2 mM) increased EPSP amplitude only when 5–10 spines were stimulated.
(a) MNI-Glu was uncaged directly adjacent to individual spine heads (small spot, red dots) or by optogenetically stimulating release of Glu onto a group of 5–10 spines (large spot, blue dots) before and after bath application of 2 mM 4-AP to assess the contribution of Kv4 potassium channels. The data suggests that these channels may be primarily localized to dendritic shafts due to the lack of enhancement of the uEPSP amplitude following Kv4 blockade in many of the spines assayed. (b) Summary plots show that there is no difference in the amplitude of ePSPs evoked from Q175 ±and their WT litter mates further suggesting similar expression of Kv4 channels in the two groups (p=0.15, Mann-Whitney U, Two-Tailed; n = 5–6 mice, data not shown). See Figure 2—figure supplement 2—source data 1.
-
Figure 2—figure supplement 2—source data 1
Source data for Figure 2—figure supplement 2.
- https://doi.org/10.7554/eLife.40818.012
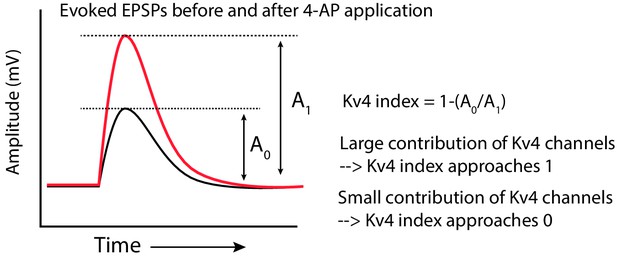
Computation of the Kv4-index.
Schematic showing how the Kv4 index was computed. The amplitude of the EPSP before (A0) and after (A1) increasing 4-AP concentration was measured. The ratio (A0/A1) was subtracted from one to get the index.
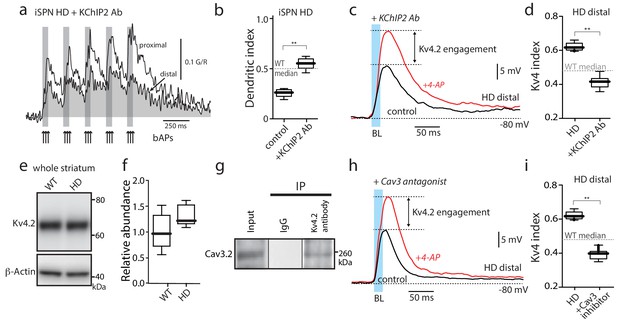
Functional upregulation of Kv4 channels depended upon KChIPs.
(a) Ca2+ transients in response to bAPs (arrows) at proximal and distal dendritic spines in iSPNs from BACHD mice with the internal perfusion of KChIP2 antibody (1:50); perfusion rescued dendritic excitability in HD iSPNs. (b) The dendritic index was significantly smaller with internal perfusion of the denatured KChIP2 antibody (boiled) (p=0.0022, Mann-Whitney U, Two-Tailed, n = 6). (c) Voltage responses to optogenetic stimulation of corticostriatal terminals at distal dendrites in iSPNs from BACHD mice with internal perfusion of KChIP2 antibody before and after 4-AP (2 mM). (d) The Kv4 index was significantly reduced by antibody perfusion (p=0.0006, Mann-Whitney U, Two-Tailed, n = 7). In all the dialysis experiments, recordings were taken >30 min after entering whole cell mode. (e) Western blot of Kv4.2 from striatum homogenates. (f) Kv4.2 protein levels show no significant difference between WT and Q175 ±mice (p=0.061, Mann-Whitney U, Two-Tailed, n = 5. (g) Co-immunoprecipitation of Cav3.2 channels with Kv4.2 channels from mouse striatum (Kv4.2 pulldown). (h) Voltage responses to optogenetic stimulation of corticostriatal terminals at distal dendrites of iSPNs from BACHD mice in the presence of the Cav3 Ca2+ channel blocker mibefradil (1 μM) before and after 4-AP (2 mM). (i) Changes in the Kv4 index were consistent with the loss of dendritic excitability in iSPNs being mediated by the interaction of Cav3 Ca2+ channels with Kv4.2 channels through KChIPs (p=0.0006, Mann-Whitney U, Two-Tailed, n = 7. See Figure 3—source data 1.
-
Figure 3—source data 1
Source data for Figure 3.
- https://doi.org/10.7554/eLife.40818.023
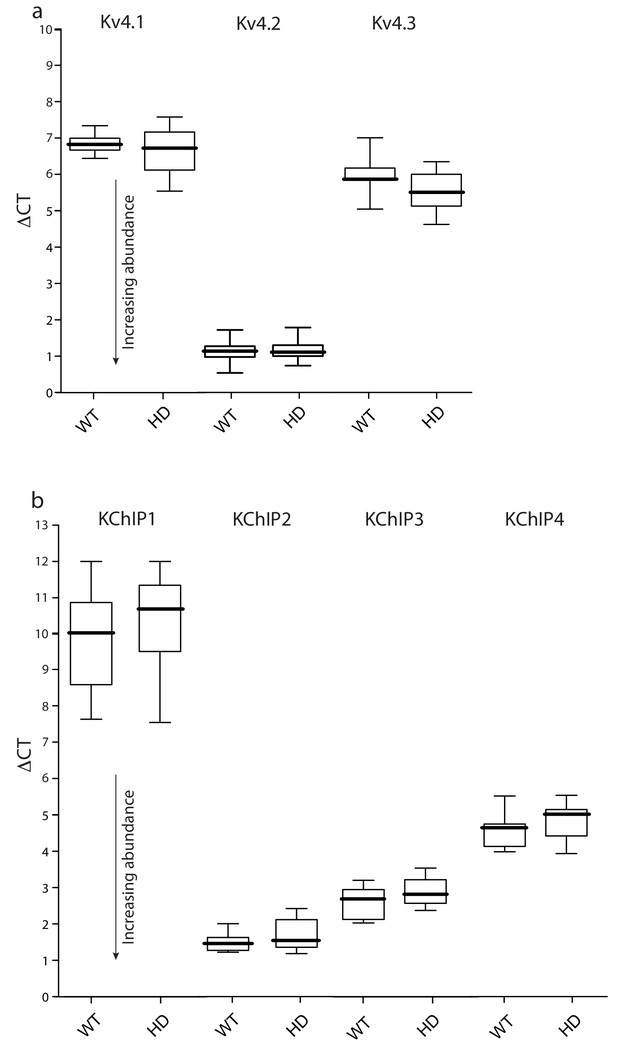
Expression of Kv4 and KChIP mRNAs was unchanged in HD iSPNs.
FACS-qPCR was performed to isolate and profile iSPNs. Data are presented as boxplots of ΔCT where smaller numbers reflect higher transcript abundance (see Materials and methods). (a) Boxplots of ΔCT values of Kv4.1–3 mRNAs in Q175 ±and WT iSPNs; all three transcripts were of similar abundance in the WT and Q175 ±iSPNs (p>0.05, Mann-Whitney U, Two-Tailed; n = 11–13 mice). A similar test of BACHD and WT iSPNs showed; again, there were no significant differences (p>0.05, Mann-Whitney U, Two-Tailed; n = 6–7 mice, data not shown). (b) KChIP1-4 abundance was not significantly different in WT and Q175 ±iSPNs (p>0.05, Mann-Whitney U, Two-Tailed; n = 11–13 mice). See Figure 3—figure supplement 1—source data 1.
-
Figure 3—figure supplement 1—source data 1
Source data for Figure 3—figure supplement 1.
- https://doi.org/10.7554/eLife.40818.017
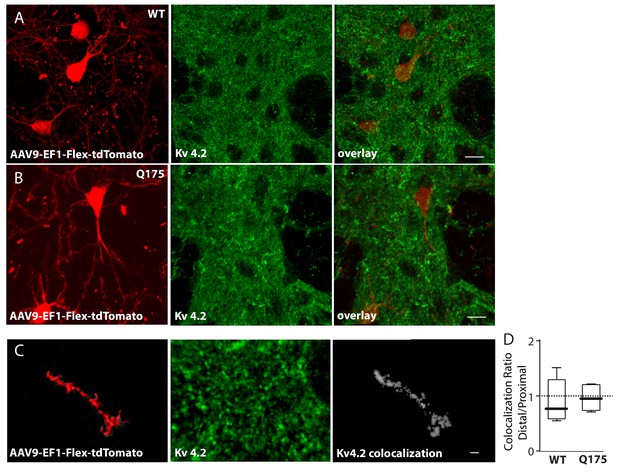
Colocalization of Kv 4.2 immunoreactivity and sparse-labeled ISPN dendrites.
(a) Red labeled ISPNs after striatal injection of AAV-EF1-Flex-tdTomato in the wild type and (b) Q175 mouse. Middle panels show Kv 4.2 immunoreactivity in the striatum and right panels are overlays (scale bars 10 µm). (c) High magnification images of Imaris created surface over sparse labeled distal dendrite (left), Kv 4.2 immunoreactivity (middle), and colocalization channel (right) created with ImarisColoc (scale bar 2 µm). (d) Boxplots showing no significant difference in colocalization ratio of distal/proximal dendrite between wild type and Q175 mice. (p=0.64, Mann-Whitney, Two-tailed, n = 5 WT, n = 6 Q175). See Figure 3—figure supplement 2—source data 1.
-
Figure 3—figure supplement 2—source data 1
Source data for Figure 3—figure supplement 2.
- https://doi.org/10.7554/eLife.40818.019
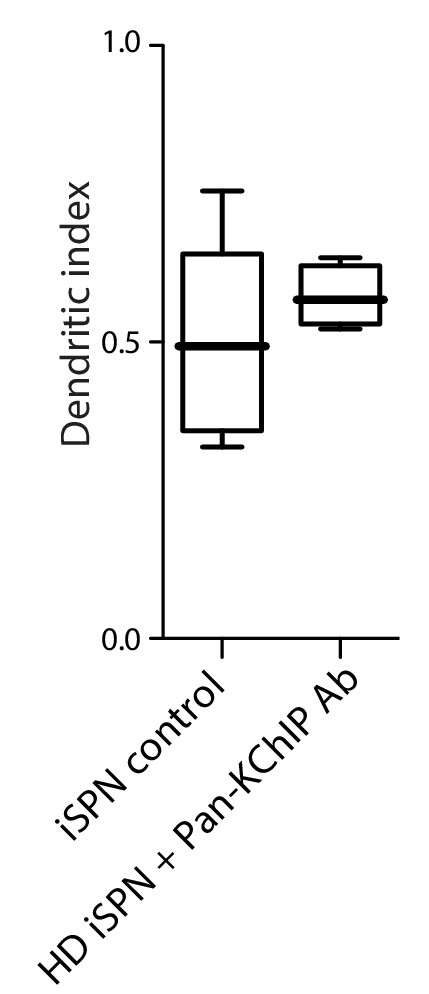
Pan-KChIP antibody normalized HD iSPN dendritic excitability.
Boxplot of the dendritic index in wild-type iSPN dendrites and BACHD iSPN dendrites after dialysis with a pan-KChIP antibody (p=0.49, Mann-Whitney U, Two tailed; n = 14 control, n = 4 BACHD). Measurements taken 30 min after the initiation of dialysis. See Figure 3—figure supplement 3—source data 1.
-
Figure 3—figure supplement 3—source data 1
Source data for Figure 3—figure supplement 3.
- https://doi.org/10.7554/eLife.40818.021
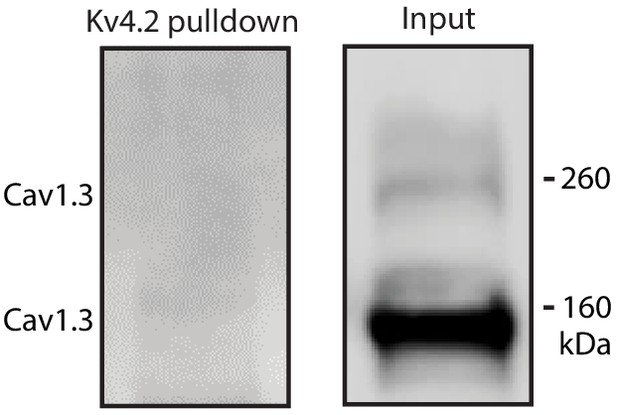
Cav1.3 channel was not associated with Kv4.2.
Kv4.2 antibody was unable to pulldown Cav1.3 in mouse striatum lysate.
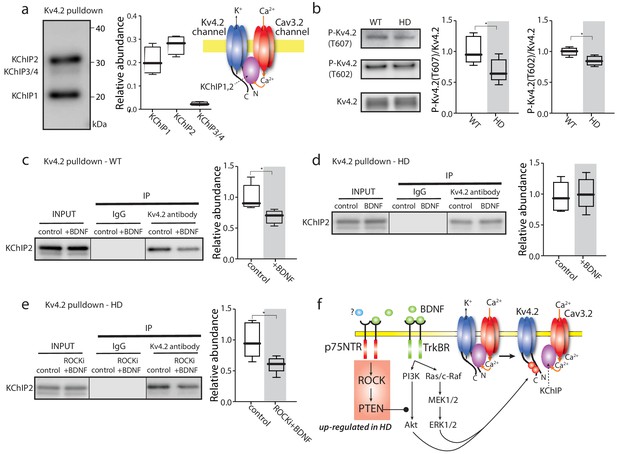
TrkBR signaling regulated Kv4 channel association with KChIPs.
(a) KChIPs co-immunoprecipitated with Kv4.2 in mice striata, KChIP1 and KChIP2 more robustly associated with Kv4.2 than KChIP3/4. To the right is a schematic of the Kv4.2/KChIP/Cav3.2 membrane complex. (b) Detection of Kv4.2 phosphorylation at Thr607 and Thr602, P-Kv4.2 levels were normalized to total Kv4.2; in Q175 ±mice, Kv4.2 phosphorylation was decreased at both Thr607 (p=0.03078, Mann-Whitney U, Two-Tailed, n = 6) and Thr602 (p=0.0226, Mann-Whitney U, Two-Tailed, n = 6). (c) Co-immunoprecipitation of KChIP2 with Kv4.2 in WT striata after incubation with BDNF or with vehicle control; association of KChIP2 was decreased (p=0.01208, Mann-Whitney U, Two-Tailed, n = 5). (d) Co-immunoprecipitation of KChIP2 with Kv4.2 in Q175 ±striata after incubation with BDNF or with vehicle control; no difference in Kv4.2 association with KChIP2 (p=0.83366, Mann-Whitney U, Two-Tailed, n = 5). (e) Co-immunoprecipitation of KChIP2 with Kv4.2 in Q175 ±striata after incubation with ROCKi +BDNF or with vehicle control; association of KChIP2 with Kv4.2 was decreased (p=0.03662, Mann-Whitney U, Two-Tailed, n = 5). (f) Diagram of the proposed mechanism showing how TrkBR and p75NTR signaling modulate KChIP association with Kv4.2 and Kv4.2 channel gating. See Figure 4—source data 1.
-
Figure 4—source data 1
Source data for Figure 4.
- https://doi.org/10.7554/eLife.40818.027
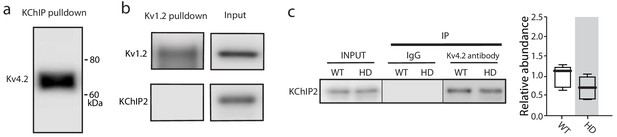
The co-immunoprecipitation experiments detected specific proteins.
(a) Kv4.2 was pulled-down by KChIP antibody, further verification of Kv4.2-KChIP interaction in mouse striatum. (b) Other similar Kv channels such as Kv1.2 did not interact with KChIPs, as Kv1.2 antibody was unable to co-immnuoprecipitate KChIP2. (c) Co-immunoprecipitation of KChIP2 with Kv4.2 in WT and Q175 ±mice striata; there was no significant change in the association of KChIP2 with Kv4.2 channels (p=0.1443, Mann-Whitney U, Two-Tailed, n = 5). See Figure 4—figure supplement 1—source data 1.
-
Figure 4—figure supplement 1—source data 1
Source data for Figure 4—figure supplement 1.
- https://doi.org/10.7554/eLife.40818.026
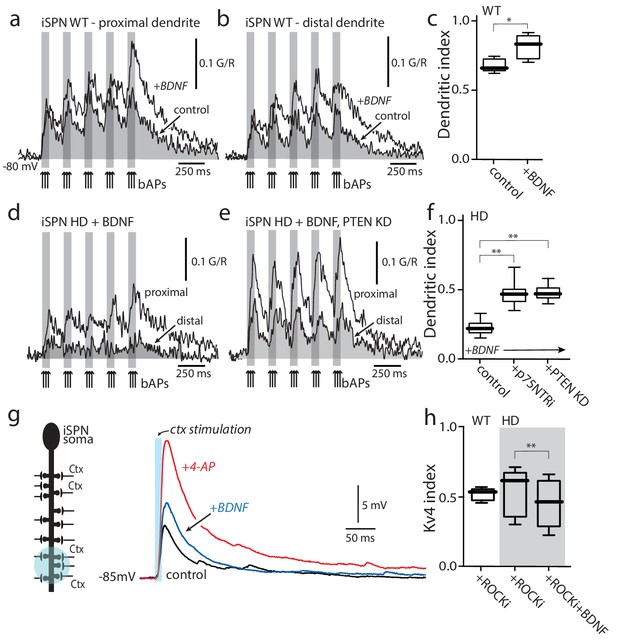
Enabling TrkBR signaling restored dendritic excitability.
(a) Ca2+ transients evoked by bAPs (arrows) at proximal and (b) distal dendritic spines in iSPNs from WT mouse in the presence of BDNF (40 ng/ml). (c) Dendritic index was significantly increased after BDNF application (p=0.00286, Mann-Whitney U, One-Tailed, n = 4). (d) Calcium transients in response to bAPs (arrows) in iSPNs from BACHD mouse in the presence of BDNF (40 ng/ml). (e) Ca2+transients in response to bAPs (arrows) at proximal and distal dendritic spines in iSPNs from BACHD mouse injected with shRNA PTEN. Note that BDNF is able to rescue the loss of dendritic excitability. (f) Dendritic index demonstrates that the loss of dendritic excitability observed in iSPNs taken from BACHD mice can be attributed to the abnormal activity of p75 (BDNF (20 ng/ml) in the presence of p75 inhibitor pep5 (1 μM) and PTEN (traces not shown) (p=0.0012, Kruskal-Wallis three groups, n = 7). Note that in these experimental conditions BDNF is able to increase Ca2+ transients in distal dendrites of iSPNs. (g) EPSPs evoked by optogenetic stimulation of distal dendrites of Q175 ±mice and their WT litter mates during bath application of the ROCK2 inhibitor (200 nM, black control trace), following 5 min BDNF (blue trace), and then in 2 mM 4-AP (red trace). (h) The boxplot shows a reduction in the Kv4 index in HD iSPNs following 5 min BDNF (50 nM) as estimated by Kv4 index (p=0.0245, Mann-Whitney U, One-Tailed, n = 9). See Figure 5—source data 1.
-
Figure 5—source data 1
Source data for Figure 5.
- https://doi.org/10.7554/eLife.40818.029
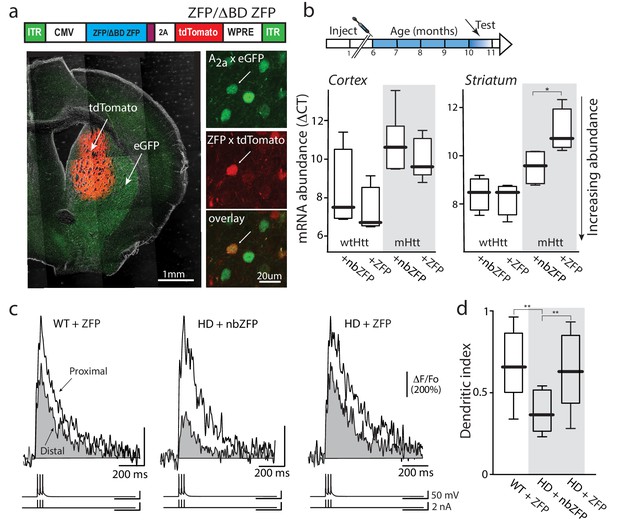
Suppression of mHtt normalized iSPN excitability.
(a) ZFP vector map 1646 bp (EcoR1/HindIII) inserts from pVAX-30645 (Sigma) sub-cloned into pAAV-CMV-SV40-WPRE vector and packaged by Virovek. The expression of N-term NLS and C-term FLAG tagged human mutant Htt-repressor ZFP and tdTomato is bridged by viral 2A cleavage peptide. The vector shown here lacking the binding domain sequence (ΔBD, non-binding) was used as a vector control. The coronal slice images demonstrate both the coverage and restriction to the striatum of the stereotaxically injected AAV carrying ZFP and tdTomato genes (stereotaxic injection coordinates: ML = −1.7, AP = −0.98, DV = −3.6). High magnification images show that both iSPNs (A2A eGFP X Q175) and ZFP tdTomato infected cells can be clearly identified (center panel). These Q175 ±mice were injected at 6 months and tested at 10–11 months (cartoon). (b) mRNA abundance (ΔCT) levels were determined by qPCR in striata and cerebral cortex from Q175 ±mice 4 months after striatal delivery of AAV-ZFP. Striatal ZFP expression repressed mHtt gene expression in striatal but not cortical tissue (n = 4–5 mice; striatum: p=0.028, Mann-Whitney U, Two-tailed; cortex: p=0.73, Mann-Whitney U, Two-tailed). (c) 2PLSM line scans of bAP-evoked Ca2+ transients, taken at proximal and distal locations within the same dendrite; shaft Ca2+ transients were used to calculate the dendritic index (as previously defined). Left: Ca2+ transients evoked in an iSPN from a WT mouse injected with AAV ZFP at 6 months and tested at 10 months; Center: Q175 ±iSPN Ca2+ transients following striatal injection of nbZFP AAV; Right: Q175 ±iSPN Ca2+ transients following striatal injection of ZFP AAV. (d) Summary boxplots showing the lack of effect of the nbZFP AAV on Q175 ±iSPN dendrites and restoration of normal excitability in Q175 ±iSPNs following ZFP AAV injection (WT littermates with ZFP: n = 5 mice, 10 cells. Q175 ±with nbZFP: n = 5 mice, 7 cells; p=0.0055, Mann-Whitney U, Two-tailed. Q175 ±with ZFP: n = 5 mice, 12 cells; p=0.0202, Mann-Whitney U, Two-tailed). See Figure 6—source data 1.
-
Figure 6—source data 1
Source data for Figure 6.
- https://doi.org/10.7554/eLife.40818.033
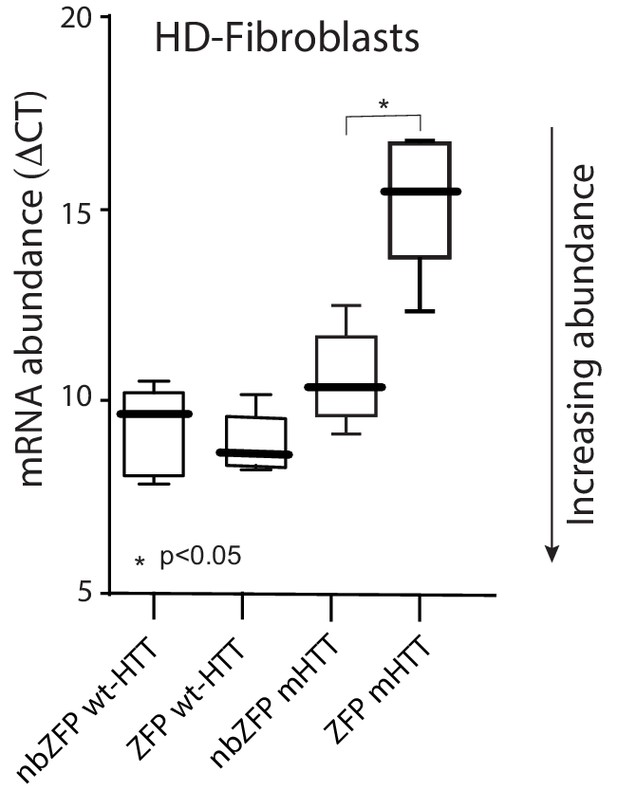
ZFP-30645 construct selectively suppressed mHtt transcription in fibroblasts.
A striatal derived cell line ST HDH Q7/111 (Coriell, CH00096 1) from a knock-in transgenic mouse containing heterozygous Huntingtin (Htt) loci with a humanized Exon 1 with 111 polyglutamine repeats is used to test the ZFP mediated repression of mHtt expression. Cells were plated in 12 well plates and then infected with AAV carrying ZFP-30645 or nonbinding ZFP; cells were harvested for RNA isolation 72 hr after infection. ZFP transcription factors (DNA binding proteins) selectively repressed mHtt gene expression without effecting wt-Htt mRNA expression (n = 5; p=0.0159, Mann-Whitney U, two-tailed). See Figure 6—figure supplement 1—source data 1.
-
Figure 6—figure supplement 1—source data 1
Source data for Figure 6—figure supplement 1.
- https://doi.org/10.7554/eLife.40818.032
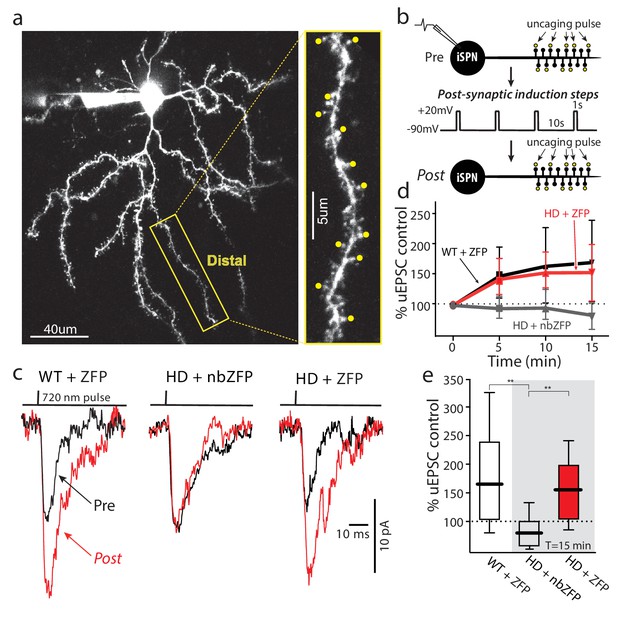
Suppression of mHtt restored TrkB receptor-mediated spine LTP in distal iSPN dendrites.
(a) Maximum projection image of ZFP +Q175±iSPN with a high magnification image of a distal dendrite where 2PLSM spot uncaging of MNI-Glu was conducted (uEPSCs, yellow dots). (b) Schematic of the LTP induction protocol. (c) Traces (uEPSCs) showing individual uEPSCs before (pre, black) and after (post, red) the post-synaptic induction steps were evoked. (d) Time course showing the evolution of the TrkBR-mediated LTP normalized to pre-induction EPSC amplitude for ZFP +Q175+/- (red line), nbZFP +Q175+/- (grey line), and their ZFP +WT littermates (black line). (e) Summary boxplots constructed from the 15 min post-induction time point shows first, the loss of spine LTP in Q175 ±that received the nbZFP compared to their WT littermates that received the functional ZFP vector; and then second, the recovery of the spine LTP in Q175 ±that received the functional ZFP: ZFP +WT littermate (n = 4 mice, 4 cells, 32 spines), nbZFP +Q175+/- (n = 5 mice, 5 cells, 40 spines), and ZFP +Q175+/- (n = 5 mice, 6 cells, 48 spines); p=0.0001, Mann-Whitney U, Two-tailed. See Figure 7—source data 1.
-
Figure 7—source data 1
Source data for Figure 7.
- https://doi.org/10.7554/eLife.40818.035
Additional files
-
Transparent reporting form
- https://doi.org/10.7554/eLife.40818.036





