Valence-encoding in the lateral habenula arises from the entopeduncular region
Figures
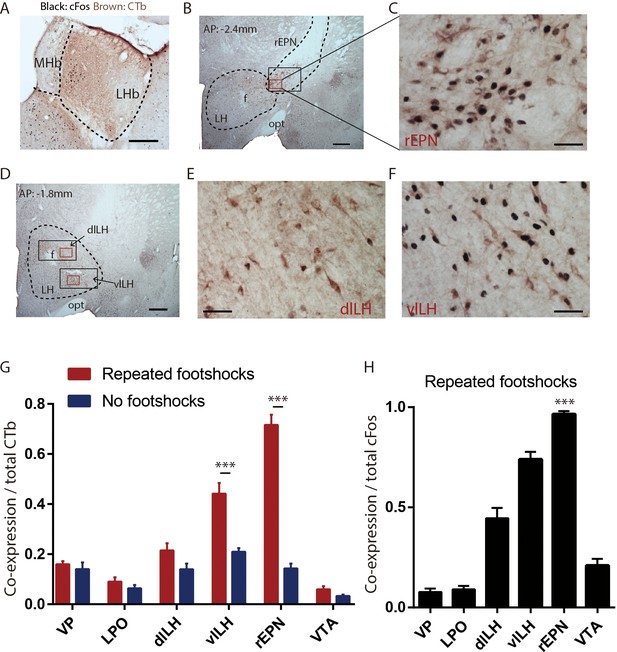
LHb-projecting neurons in the rEPN and the LH are significantly activated by repeated footshocks.
(A) Representative photos of CTb injection site in LHb (brown immunoreactive product). Representative photos of CTb immunostaining (brown) and shock-induced cFos (black) in the rEPN (B, C) and the LH (D–F). Black squares: areas for cFos counting. Red squares: areas enlarged in representative images. f: fornix; opt: optic tract; MHb: medial habenula. (G) Proportion of CTb-labeled neurons expressing cFos in the VTA, rEPN, dlLH, vlLH, LPO, and VP, showing the largest shock-induced increases in the rEPN and LH. (E) Proportion of cFos neurons expressing CTb in these same regions. Scalebars 250 µm (A), 500 µm (B, D), 100 µm (C, E and F).
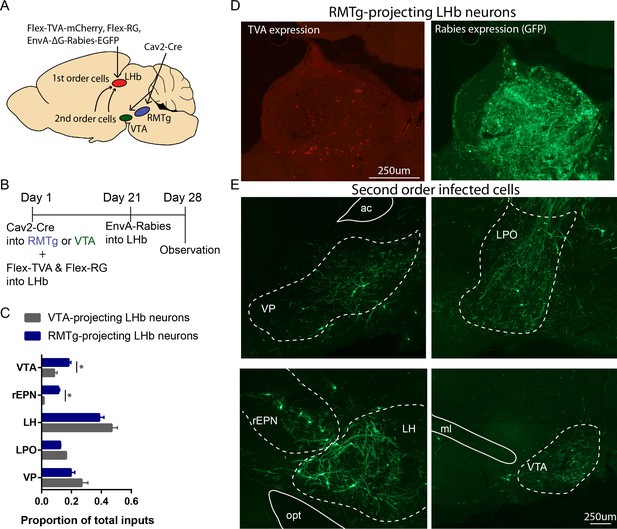
rEPN neurons innervate LHb neurons projecting to the RMTg.
(A, B) Schematics of procedure in which retrogradely transported virus expressing Cre-recombinase was injected into the RMTg or VTA and viruses expressing flox-stopped TVA and RG were injected into the LHb. EnvA-ΔG-rabies virus was injected into the LHb 3 weeks later. Animals were perfused at the end of the forth week. (C) Neurons in the rEPN and the VTA preferentially projected to RMTg-projecting LHb neurons over VTA-projecting LHb neurons. (D) Representative pictures of RMTg-projecting TVA expressing cells and first-order rabies infected cells in the LHb. (E) Representative pictures of second-order rabies infected cells in the VP, LPO, rEPN, LH, and VTA after Cav2-Cre injection into RMTg, indicating neurons disynaptically connected to RMTg.
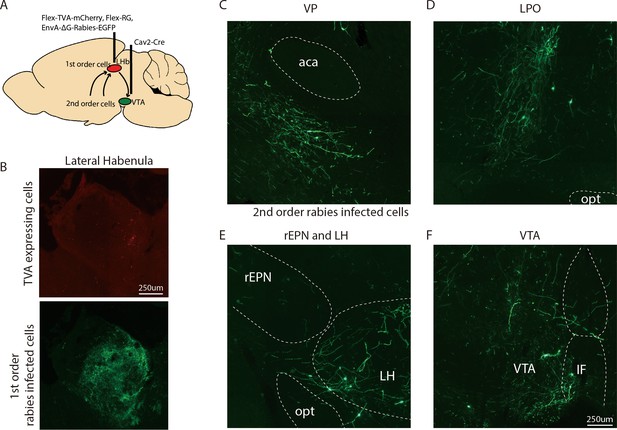
Representative images of neurons innervating VTA-projecting LHb neurons.
(A) Schematics of procedure for viral tracer experiments. (B) Representative pictures of VTA-projecting TVA expressing cells (red label) and first-order rabies infected cells (green label) in the LHb. (C–F) Representative pictures of second-order rabies infected cells in the VP, LPO, rEPN, LH, and VTA after Cav2-Cre injection into VTA.
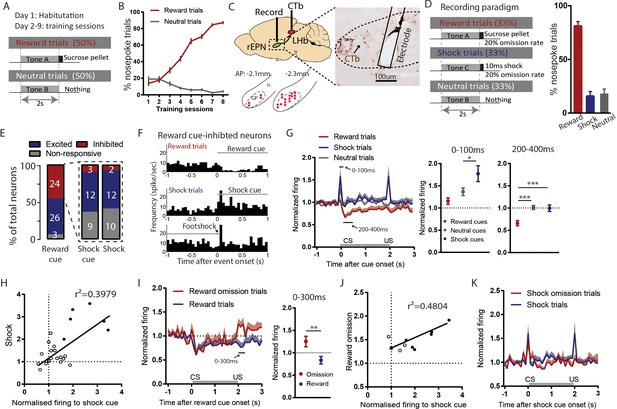
Reward cue-inhibited rEPN neurons encode valence.
(A) Schematic of training paradigm. (B) After eight training sessions, rats show clear discrimination between reward and neutral cues, as measured by the probability of animals making nose pokes during the 2 s cue presentation. (C) We recorded in the rEPN, as delineated by retrograde labeling of CTb from the LHb and absence of parvalbumin immunoreactivity. (D) Schematic of recording paradigm, in which three different auditory tones signaled delivery of sucrose pellets, mild footshocks, or nothing. Animals exhibited ~80% accuracy in discrimination between different cues. (E) rEPN neurons were divided roughly equally between reward cue-inhibited and –excited responses. Amongst reward cue-inhibited neurons, the most common response was excitation to footshocks and shock cues. (F) Representative histograms of individual rEPN neuron responses to reward and shock trials, analyzed in 50 ms bins. (G) Responses of reward cue-inhibited subpopulation to reward, shock, and neutral trials, again in 50 ms bins. Excitation to shock cue was most prominent in first two bins (0–100 ms, upper solid black line), while inhibition to reward cue was most prominent 200–400 ms after stimulus onset (lower solid black line). Average firing rates from these two windows are shown in adjacent graphs. (H) In reward cue-inhibited rEPN neurons, responses to shock cues were positively correlated with individual neuron’s responses to footshocks. (I) Reward cue-inhibited neurons also showed excitation to reward omission (0–200 ms window) (J) Responses to reward omissions were positively correlated with responses to shock cues in omission-activated neurons. (K) Reward cue-inhibited neurons did not respond to shock omissions. Solid circles indicate neurons significantly excited by both stimuli.
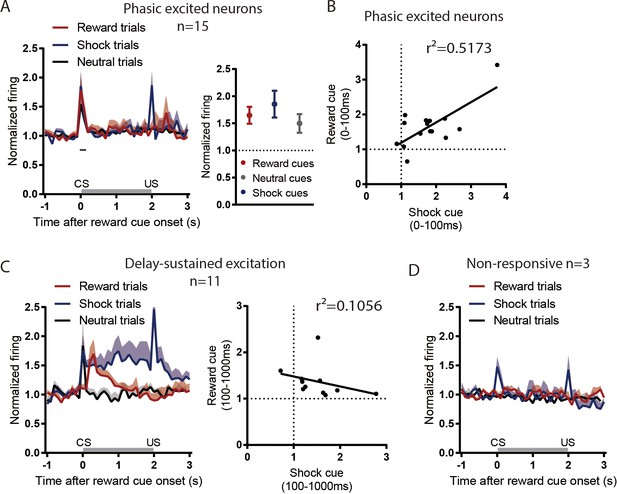
rEPN neurons not inhibited by reward cues do not show valence-like encoding patterns.
24 of 53 rEPN neurons were reward cue-inhibited, leaving 29 that were not. Among the non-inhibited neurons, 15, 11, and three neurons showed reward cue phasic excitation (0–100 ms) (A-B), delayed excitation (100–1000 ms) (C), and non-response (D), respectively, to reward cues. Phasically excited neurons did not discriminate between cue types (A), while neurons showing delay-sustained excitation showed sustained responses to both reward-predictive and shock-predictive cues, but not neutral cues, consistent with salience-like encoding (B). Neurons lacking responses to reward cues nonetheless showed phasic excitations to shock-predictive cues but not neutral cues (C).
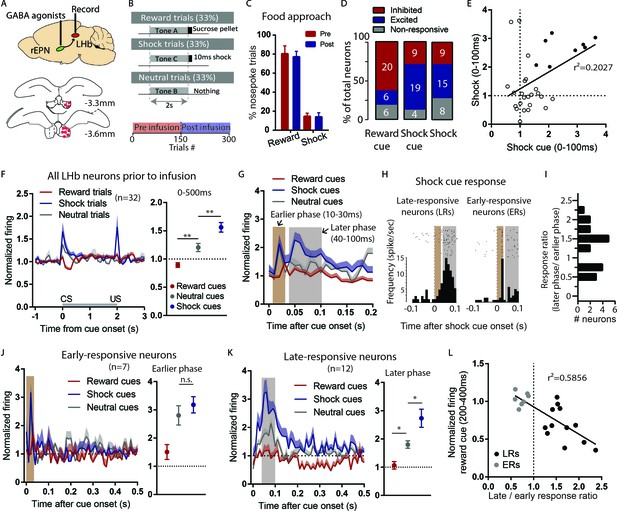
LHb responses to shock cues are encoded in two distinct subpopulations.
(A) Schematic of recording from the LHb while inactivating ipsilateral rEPN with GABA agonists. (B) Schematic of inactivation behavioral paradigm in which reward and shock were delivered at 100% probability. (C) Ipsilateral rEPN inactivaiton did not affect behavioral response (food tray approach) in reward trials. (D) A majority of LHb neurons are inhibited by reward cue and activated by shock and shock cues. (E) Responses to shock cues were positively correlated with individual neuron’s responses to footshocks. Solid circles indicate neurons significantly excited by both shock cues and footshocks. (F) LHb neurons on average showed inhibition to reward cues and excitation to shock cues during 0–500 ms post-stimulus window, suggesting a valence encoding pattern. (G) Excitation to shock cues contained two phases: an ‘earlier’ phase from 10 to 30 ms post-stimulus and a ‘later’ phase from 40 to 100 ms post-stimulus (brown and gray shaded bars, respectively). (H) Rasterplots and histograms of representative early- and late-responsive LHb neurons. (I) Histogram of all shock cue-activated LHb neurons shows bimodal distribution of ratio late to early response components. (J) Early-responsive neurons do not differentiate between shock cues and neutral cues. (K) In contrast, late-responsive neurons exhibited greater responses to shock cues compared to neutral cues and reward cues. Shaded bar indiactes analysis windows for ajacent panels. (L) Late shock cue-responsive showed strongly inhibitions to reward cues than early shock cue-responsive neurons.
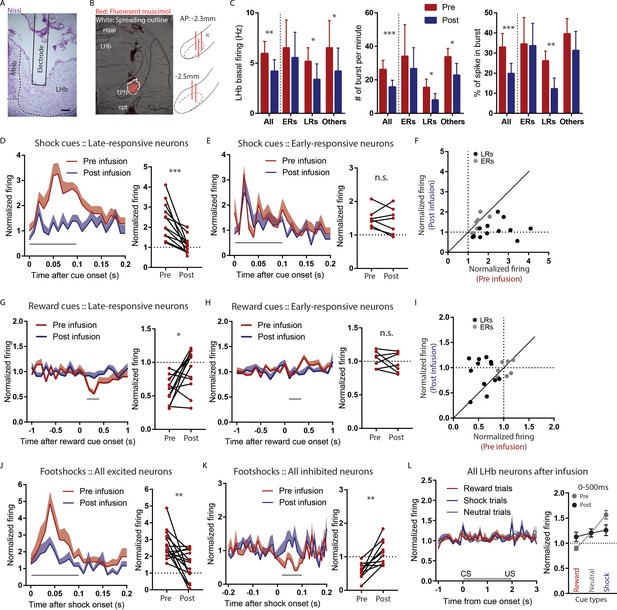
Temporary inactivation of the rEPN reduces LHb basal firings and preferentially diminishes LHb responses to negative motivational stimuli.
(A) Representative photomicrograph of recording electrode track in the LHb. Scalebar: 100 µm. (B) Representative photo of muscimol spread over 30 min and cannula placements in the EPN. (C) rEPN inactivation decreased basal firing, number of bursts per minute, and percentage of spikes in bursts in LHb neurons. (D–F) Responses of late- but not early-responsive LHb neurons to shock cues were eliminated by rEPN inactivation. (G–I) Responses of late- but not early-responsive LHb neurons to reward cues were eliminated by rEPN inactivation. (J, K) rEPN inactivation greatly diminished both excitotory and inhibitory LHb responses to footshocks. Black bars under time course indicate analysis windows for adjacent panels. (L) After rEPN inactivation, the population average of LHb responses only showed weak responses to cues that did not discriminate between different cue types (compared with robust responses in Figure 4F). Adjacent panel on right shows loss of monotonic responses to shock cue vs neutral cue vs reward cue (black symbols), relative to pre-infusion responses (grey symbols, adapted from Figure 4F).
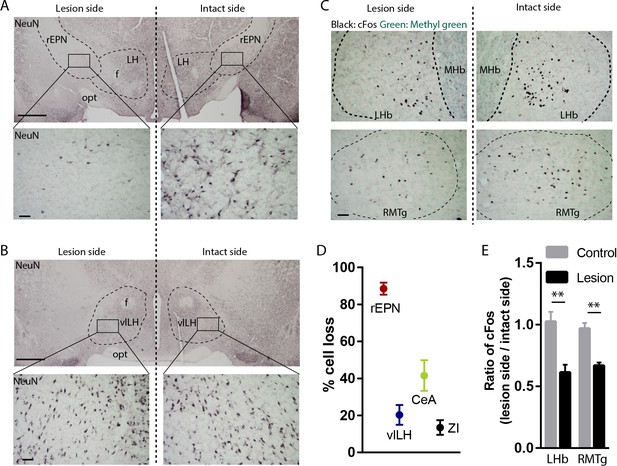
Excitotoxic lesion of the rEPN reduces cFos induced by unsignaled footshocks in LHb and RMTg.
(A, B) Representative photomicrographs of the ipsilateral (lesioned) and contralateral (intact) rEPN and vlLH immunostained for the neuronal marker NeuN (black label). (C) Representative photographs of footshock-induced cFos (black label) in the LHb and RMTg with methyl green counterstain. (D) Number of cells dramatically decreased in the entire rEPN, on the lesioned side, with smaller reductions in vlHL, CeA and ZI. (E) rEPN lesion reduced cFos expression induced by unsignaled footshocks in the ipsilateral LHb and RMTg, compared with contralateral (intact) side. Scalebars are 1 mm in top panels for A), (B), and 100 µm in all other panels.
Additional files
-
Transparent reporting form
- https://doi.org/10.7554/eLife.41223.010





