A β-carotene-binding protein carrying a red pigment regulates body-color transition between green and black in locusts
Figures
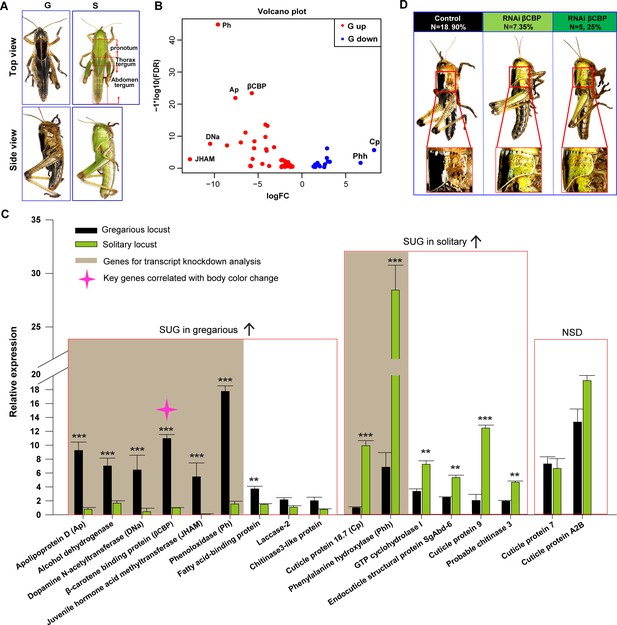
Identification of β-carotene-binding protein (βCBP) as a target associated with body color changes between gregarious and solitary locusts.
(A) Body colors of typical gregarious and solitary nymphs. The tergums include pronotum, thorax tergum and abdomen tergum. (B) Logarithmic fold alterations of 68 differentially expressed genes (DEGs) associated with animal coloration between gregarious and solitary locusts are shown in the volcano plot diagram generated from genome-wide RNA-Seq. Red and blue dots indicate up- and down regulated genes, respectively, in the gregarious locusts (n = 2 samples, 10 integuments/sample). (C) The top 17 DEGs (Log FC >2.8, FDR < 1e−5) among the 68 genes from the volcano plot diagram were confirmed in the gregarious and solitary integuments via qPCR (n = 6 samples; eight integuments/sample; Student’s t-test; red boxes indicate significantly upregulated genes (SUG) in gregarious/solitary locusts and no significant difference between gregarious and solitary locusts; *p < 0.05; **p < 0.01; ***p < 0.001). Brown shades denote the eight genes used for transcript knockdown analysis. The red star in the histogram indicate the identified target gene that correlates with body color change in the phase transition of locusts. (D) βCBP fosters the body color change from the gregarious pattern to the solitary pattern. Effects of βCBP dsRNA treatment on the body color of gregarious locusts were studied two stadiums after injection. Each second-instar nymph was injected with 3 μg of dsRNA three times on day three for the second-instar nymphs and on days 1 and 5 for the third-instar nymphs. Control nymphs were injected with equivalent volumes of dsGFP alone. .
-
Figure 1—source data 1
Numerical data that are represented as graphs in Figure 1C.
- https://cdn.elifesciences.org/articles/41362/elife-41362-fig1-data1-v2.xlsx
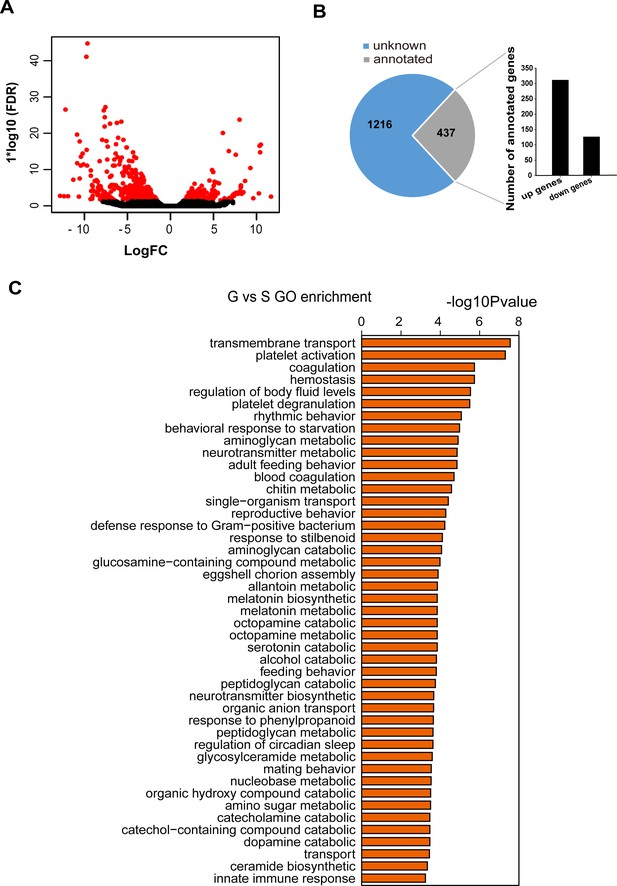
Transcriptomic profiles of locust pronotum integuments revealed by strand-specific RNA-seq.
(A) Logarithmic fold alteration of DEGs in the integument transcriptomes between the gregarious and solitary locusts. (B) Numbers of unknown genes and annotated genes among DEGs, and numbers of up- and downregulated genes in the annotated genes. (C) Molecular function analysis of DEGs in the transcriptome. G, pronotum integuments of gregarious locusts; S, pronotum integuments of solitary locusts.
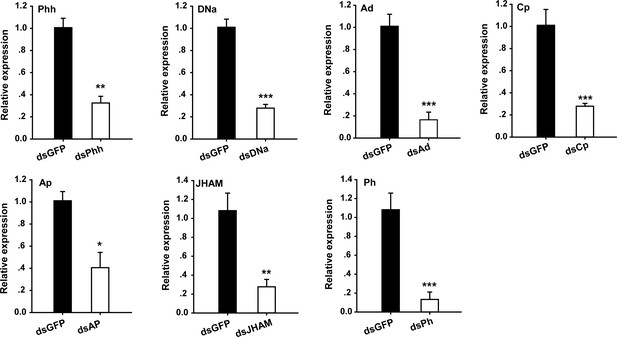
Analysis of interference efficiency of the other seven genes associated with animal coloration.
The mRNA expression of seven genes in the pronotum integument was quantified using qRT–PCR two stadiums after they were silenced in gregarious locusts, respectively. Phh, phenylalanine hydroxylase; DNa, Dopamine N-acetyltransferase; Ad, Alcohol dehydrogenase; Cp, Cuticle protein 18.7; Ap, Apolipoprotein D; JHAM, Juvenile hormone acid methyltransferase; Ph, Phenoloxidase; qPCR data are shown as the means ± SEM (n = 4). *p < 0.05; **p < 0.01; ***p < 0.001.
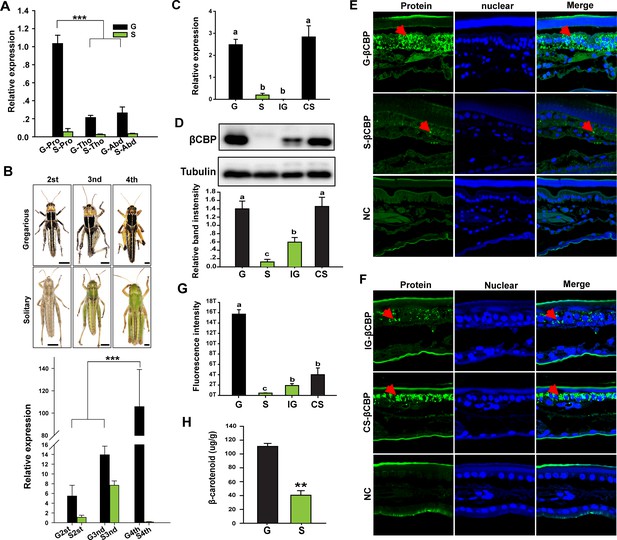
Expression or distribution of βCBP and its binding β-carotene shows phase-specific patterns.
(A) βCBP expression in the tergums of different body segments of locusts in the gregarious (G) and solitary (S) phases. Pro: Pronotum; Tho: Thorax; Abd: Abdomen; (Tukey’s test, ***p < 0.001). (B) βCBP expression in the pronotums of different instars of gregarious (G) and solitary (S) nymphs. Body color phenotypes of typical gregarious and solitary nymphs in the second (2st), third (3nd), and fourth (4th) instar are shown. The scale is 2 mm for each nymph. mRNA expression of βCBP was quantified by using qRT–PCR. qPCR data are shown as the means ± SEM (n = 4, ***p < 0.001). (C, D) mRNA (C) and protein (D) expression levels of βCBP were determined in the integuments of fourth-instar gregarious (G) nymphs, solitary (S) nymphs, gregarious nymphs after isolation (IG), and solitary nymphs after crowding (CS) using qPCR and Western blot analyses. Western blot bands were quantified using densitometry, and the values are expressed as the mean ± SEM (n = 3). (E) Distribution analysis of CBP was conducted to determine the localization and differences in abundance of CBP in the integuments between the gregarious (G) and solitary (S) locusts via immunohistochemistry. The red arrows indicate the areas where βCBP was localized in the locust integuments. NC, negative control. Images were visualized using an LSM 710 confocal fluorescence microscope (Zeiss) at a magnification of 40×. (F) Expression signals for βCBP were analyzed in the integuments of gregarious nymphs after isolation (IG) and solitary nymphs after crowding (CS). (G) Fluorescence intensity was quantified using ZEN 2.1 software and expressed as the means ± SEM (n = 3). (H) β-carotene content in the gregarious and solitary locust integuments was evaluated using HPLC (n = 6, **p < 0.01). Double comparisons were evaluated using Student’s t-test and one-way ANOVA followed by Tukey’s test was used for multiple comparisons. The same letter indicates that the data are not significantly different.
-
Figure 2—source data 1
Numerical data that are represented as graphs in Figure 2A,B,C,D,G,H.
- https://cdn.elifesciences.org/articles/41362/elife-41362-fig2-data1-v2.xlsx
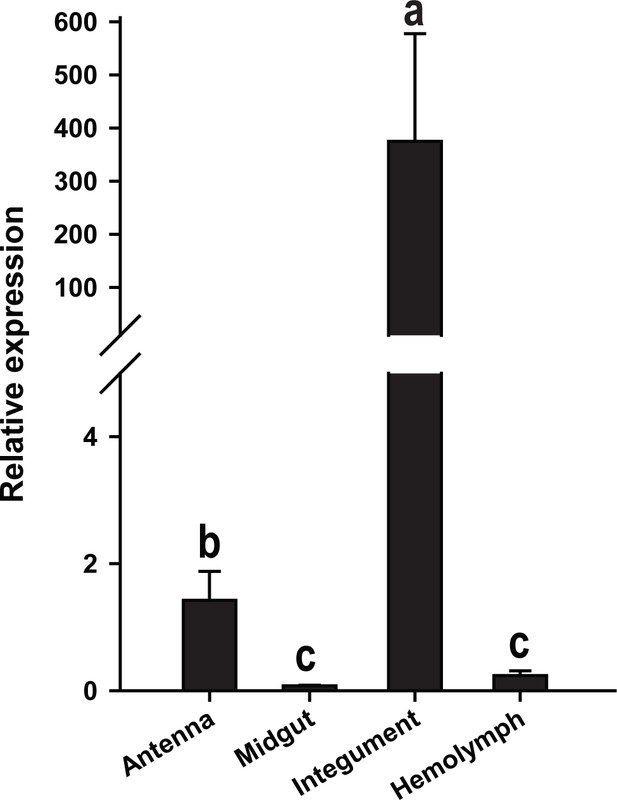
βCBP expression in the different tissues of gregarious locusts as determined by qRT–PCR.
Data are presented as the means ± SEM (n = 4); the same letter indicates that the data are not significantly different.
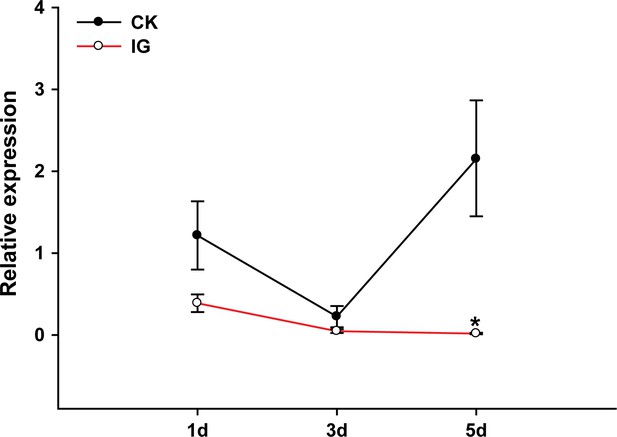
βCBP expression in the time course of gregarious locusts.
The expression levels of βCBP were determined by qPCR in the pronotum integuments of fourth-instar gregarious nymphs after isolation (IG) for 1, 3, and 5 days. qPCR data are shown as the means ± SEM (n = 4). *p < 0.05.
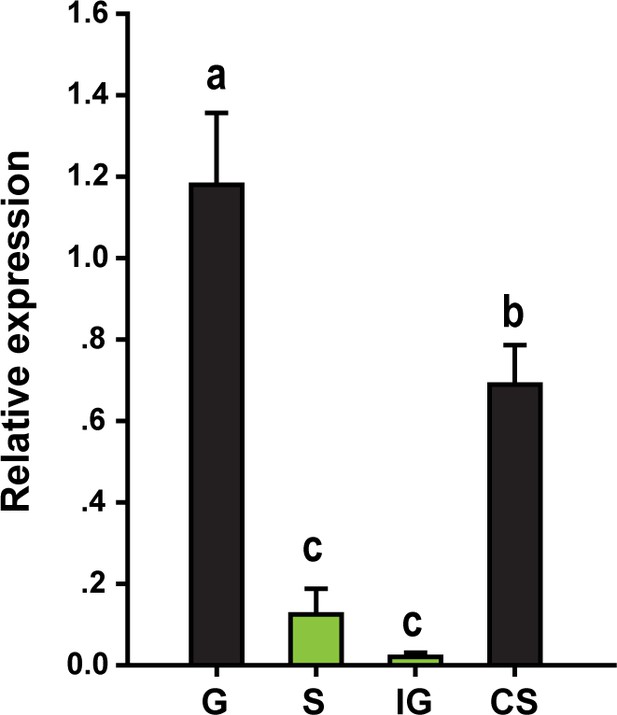
βCBP expression in the tergum of the thorax and abdomen of locusts.
The mRNA expression levels of βCBP were determined in the tergum of the thorax and abdomen of fourth-instar gregarious (G) nymphs, solitary (S) nymphs, gregarious nymphs after isolation (IG) and solitary nymphs after crowding (CS) by performing qPCR analyses. qPCR data are shown as the means ± SEM (n = 4).
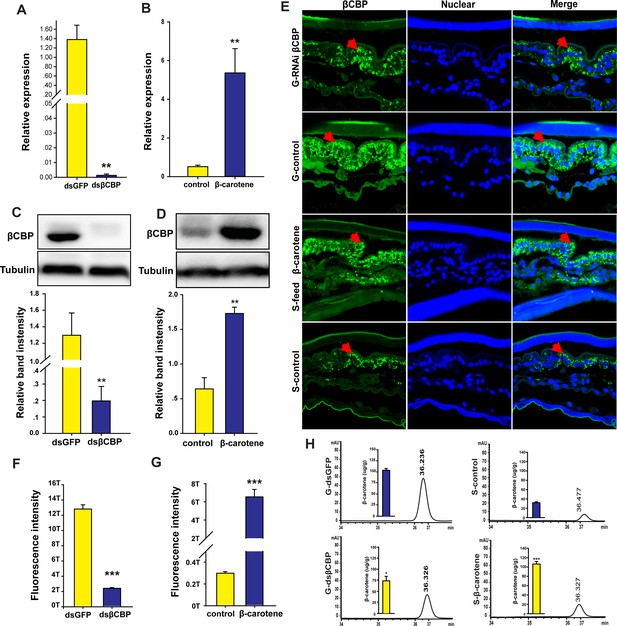
βCBP accumulates β-carotene pigment to affect pigmentation in the phase transition of locusts.
(A, C) mRNA and protein expression of βCBP in the pronotum integument was quantified using qRT–PCR (A) and western blot analyses (C) two stadiums after βCBP silencing and isolating in gregarious locusts. (B, D) mRNA and protein expression levels of βCBP in the pronotum integument were determined two stadiums after feeding with β-carotene and crowding in solitary locusts using qPCR (B) and western blot analyses (D). (E) Fluorescence signals of βCBP were determined in gregarious (G) locusts after βCBP silencing coupled with isolation and in solitary (S) locusts after feeding with β-carotene diet coupled with crowding. (F, G) Fluorescence signal intensity of βCBP was quantified using ZEN 2.1 software and expressed as the means ± SEM (n = 3). (H) β-carotene content was evaluated in gregarious locusts after βCBP silencing coupled with isolation and in solitary locusts after feeding with β-carotene diet coupled with crowding by using HPLC. qPCR and HPLC data are shown as the means ± SEM (n = 6). Western blot bands were quantified using densitometry, and the values are expressed as the means ± SEM (n = 3). All the double comparisons were evaluated using Student’s t-test, *p < 0.05; **p < 0.01; ***p < 0.001.
-
Figure 3—source data 1
Numerical data that are represented as graphs in Figure 3A,B,C,D,F,G,H.
- https://cdn.elifesciences.org/articles/41362/elife-41362-fig3-data1-v2.xlsx
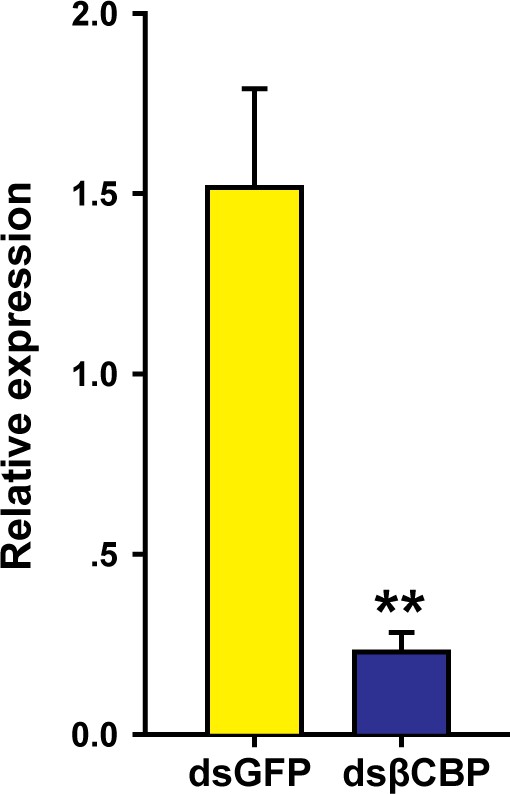
βCBP expression in the tergum of the thorax and abdomen of locusts after RNAi βCBP.
mRNA expression of βCBP was quantified by using qRT–PCR two stadiums after treatment of gregarious nymphs with 3 μg of dsRNA. qPCR data are shown as the means ± SEM (n = 6). **p < 0.01.

βCBP expression in the tergum of the thorax and abdomen of solitary locusts.
(A, B) mRNA (A) and protein (B) expression levels of βCBP were determined in solitary nymphs after feeding with β-carotene followed by crowding. Controls were fed with a regular diet without β-carotene and were crowded afterward. qPCR data are shown as the means ± SEM (n = 6). Western blot bands were quantified using densitometry, and the values are expressed as the means ± SEM (n = 3); *p < 0.05.
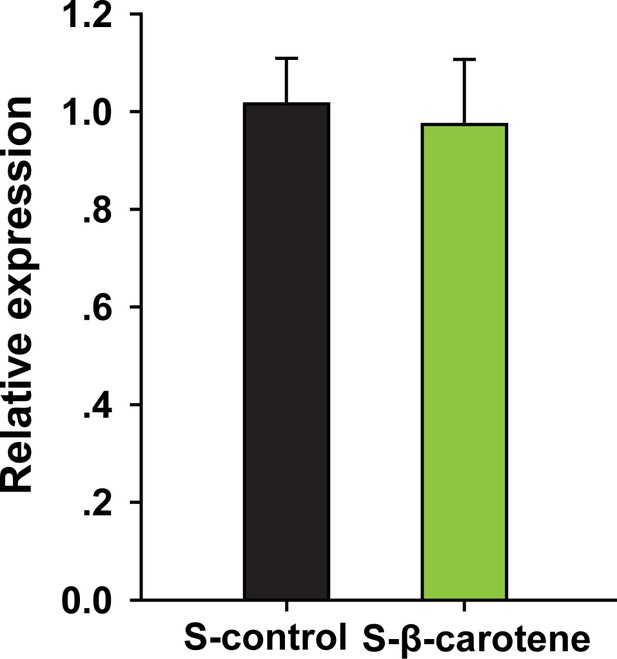
βCBP expression in the pronotum of solitary locusts only pre-fed with β-carotene but no crowding by qRT–PCR.
qPCR data are shown as the means ± SEM (n = 6).
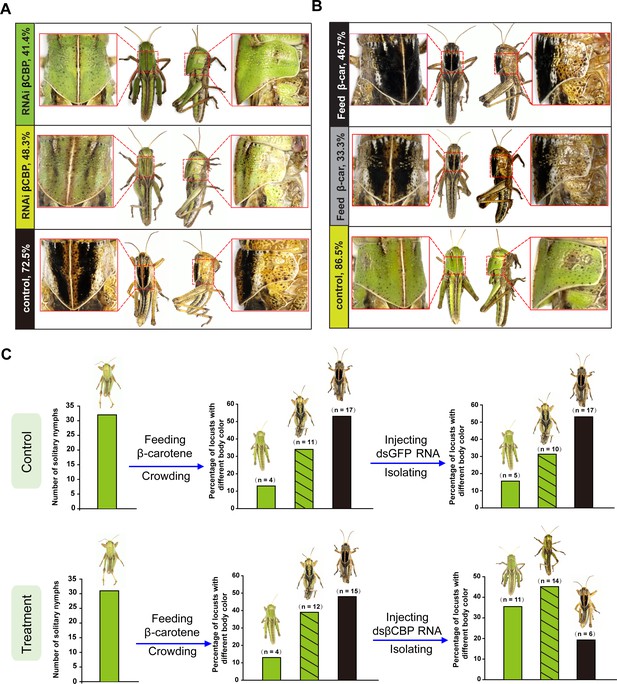
βCBP and its binding of β-carotene fosters the body color phase transition of the locust.
(A) Effects of βCBP dsRNA treatment on the body color of gregarious locusts. Each second-instar nymph was injected with 3 μg of dsRNA thrice and exposed to isolation. Control nymphs were injected with equivalent volumes of dsGFP and then isolated. (B) Effects of β-carotene feeding on the body color of solitary locusts. Solitary nymphs were fed a synthetic diet containing β-carotene and were then subjected to crowding. Control insects were fed a regular diet without β-carotene and were then subjected to crowding. (C) A body color rescue experiment in solitary locusts was performed by injecting dsRNA against βCBP at day three in third-instar nymphs (N3D3) that had been pretreated with β-carotene feeding at day one as second-instar nymphs (N2D1). The control group was injected with dsGFP as third-instar nymphs that had been pretreated with β-carotene feeding as second-instar nymphs.
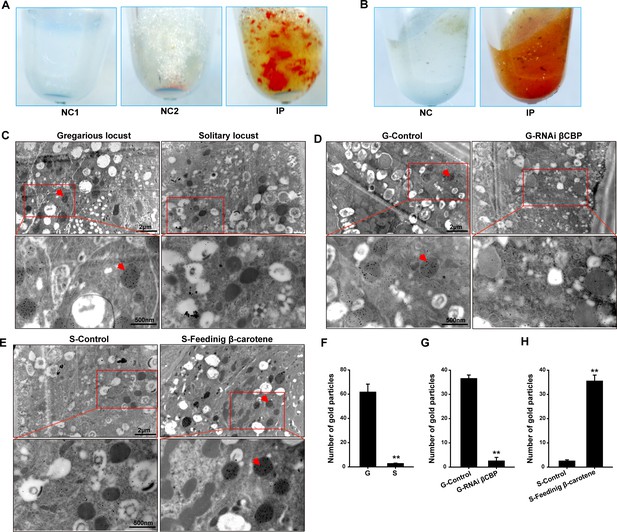
The red complex of βCBP located in the pigment granules of integument and βCBP binding of β-carotene directly contributes to the black back pattern of gregarious locusts.
(A) Red βCBP–pigment complex was confirmed by recombinant βCBP (rβCBP) incubation with β-carotene under immunoprecipitation using βCBP antibody conjugated to protein A-Sepharose. NC1, containing β-carotene without rβCBP; NC2, containing BSA and β-carotene; IP, containing β-carotene and rβCBP. (B) Red pigment accumulates in the precipitate of the locust pronotum when treated with anti-βCBP-immunoprecipitation compared with IgG-immunoprecipitation. NC, containing pronotums and IgG; IP, containing pronotums and βCBP antibody. (C) The subcellular distribution of βCBP was investigated in the integuments of gregarious and solitary locusts by immunoelectron microscopy. (D, E) Immunogold labeling signals of βCBP were comparatively analyzed in the integuments of gregarious locusts after injection with βCBP dsRNAs and subsequent isolation and (D) in the integuments of solitary integuments after feeding with β-carotene diet followed by crowding (E). (F, G, H) Average number of gold particles in three randomly selected pigment granules in sections from various treatments. Variation is calculated based on three biological replicates (n = 3, Student’s t-test, **p < 0.01). All sections were probed with anti-βCBP, followed by protein A-gold conjugate. Images outlined with red squares are magnified at 30,000 × using the Ruli H-750 TEM (Japan). The red arrow indicates the gold particles of βCBP located in the pigment granules.
-
Figure 5—source data 1
Numerical data that are represented as graphs in Figure 5F,G,H.
- https://cdn.elifesciences.org/articles/41362/elife-41362-fig5-data1-v2.xlsx
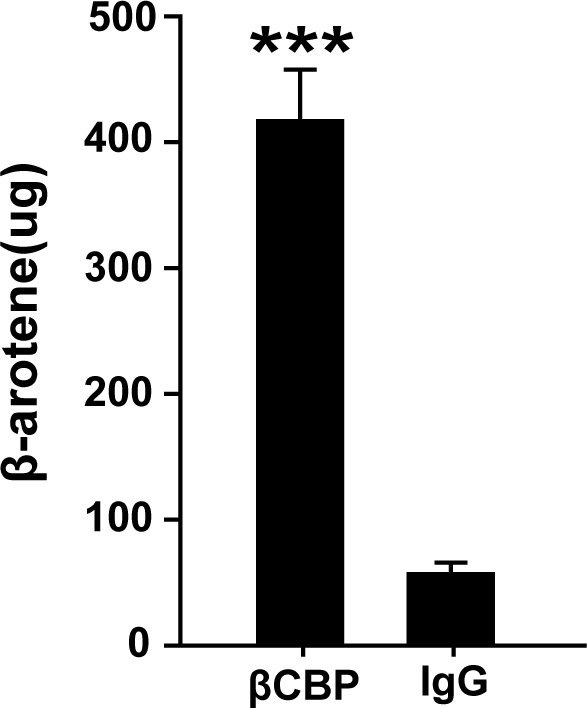
Confirmation of βCBP binding to β-carotene via HPLC.
β-carotene content was assayed in the precipitate of gregarious pronotums after pigment immunoprecipitation in vivo by using βCBP antibody vs. IgG. HPLC data are shown as the means ± SEM (n = 6). ***p < 0.001.
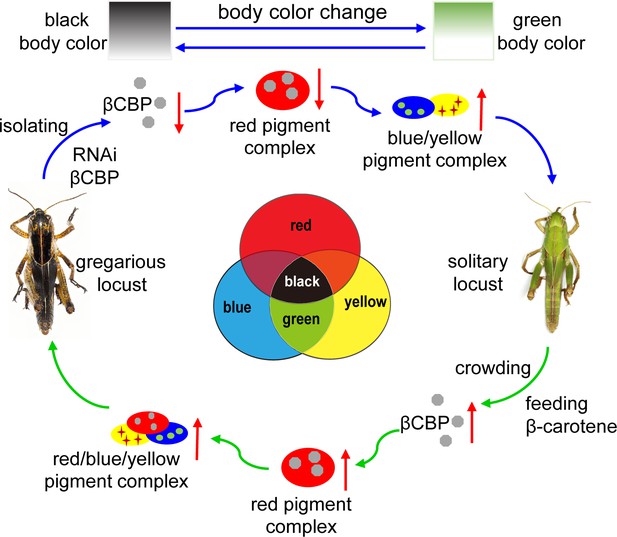
Model of a new mechanism in accordance with the physically trichromatic rule.
Body color change is mediated by the red βCBP–β-carotene complex, which has a superposition effect on the solitary green background and produces the black back pattern of gregarious locusts. Silencing βCBP in gregarious locusts reduced the red color pigment β-carotene complex, thereby leading to the green pattern (instead of black). By contrast, feeding the solitary locusts β-carotene induced βCBP expression and caused gregarious-like body coloration.
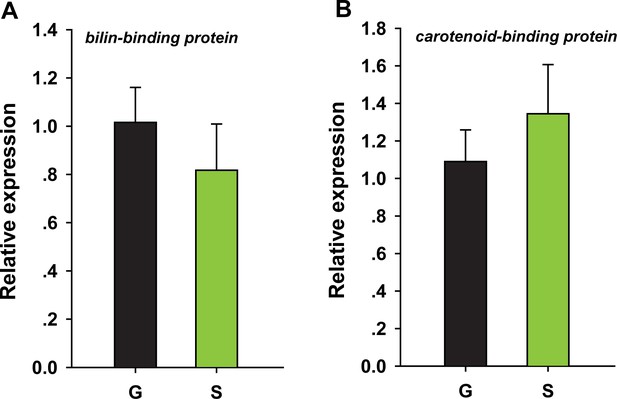
Biliverdin-binding protein and carotene-binding protein expression in the pronotum of gregarious and solitary locusts by qRT–PCR.
qPCR data are shown as the means ± SEM (n = 4). Additional files.
Additional files
-
Supplementary file 1
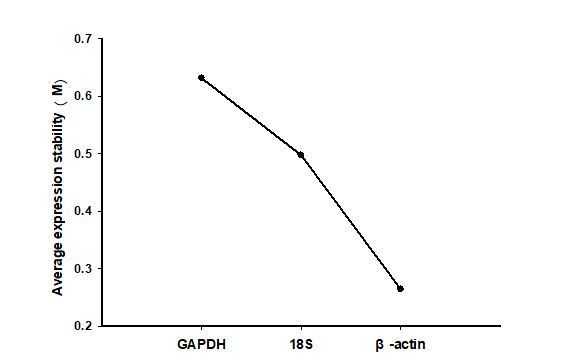
The gene-stability measure (M) values of three reference genes in different stage of gregarious and solitary locustsas as calculated by geNorm algorithms.
- https://cdn.elifesciences.org/articles/41362/elife-41362-supp1-v2.xlsx
-
Supplementary file 2
Primers used in the qPCR analysis of βCBP, and actin and GFP used for RNA interference.
- https://cdn.elifesciences.org/articles/41362/elife-41362-supp2-v2.xlsx
-
Supplementary file 3
Composition of the basic synthetic diet.
- https://cdn.elifesciences.org/articles/41362/elife-41362-supp3-v2.xlsx
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/41362/elife-41362-transrepform-v2.docx