Coding of whisker motion across the mouse face
Figures
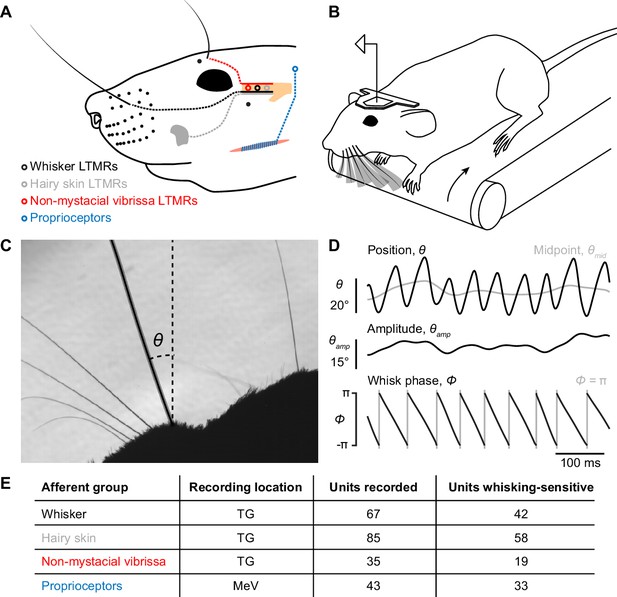
Recording whisking and facial mechanosensory afferent spiking.
(A) Schematic of types of afferents (open circles with dotted lines) recorded, grouped by type of receptive field: trigeminal ganglion (TG, beige) low threshold mechanoreceptors (LTMRs) with receptive fields localized to (1) a mystacial whisker follicle (filled black dots; e.g. black whisker), (2) hairy skin (e.g. gray patch on cheek), or (3) a non-mystacial vibrissa (red dots; e.g. black supraorbital vibrissa), and trigeminal mesencephalic nucleus (MeV) proprioceptors innervating facial muscles. (B) Schematic of experimental setup. A head-fixed mouse ran on a treadmill and whisked in air. Single neurons were recorded simultaneously with high-speed (500 Hz) video of the whiskers. (C) Example video frame, capturing the silhouette of the whiskers and profile of the mouse face, and illustrating measurement of the angular position of a whisker (θ) relative to the mediolateral axis. (D) Example traces showing one second of whisker angular position (θ), whisking midpoint (θmid), amplitude (θamp; bottom of scale bar indicates 0°), and phase (Φ; gray vertical lines, times when the whisker is fully retracted, Φ = π). (E) Overview of dataset, including the number of units recorded and the number of each type that were whisking-sensitive (Glossary).
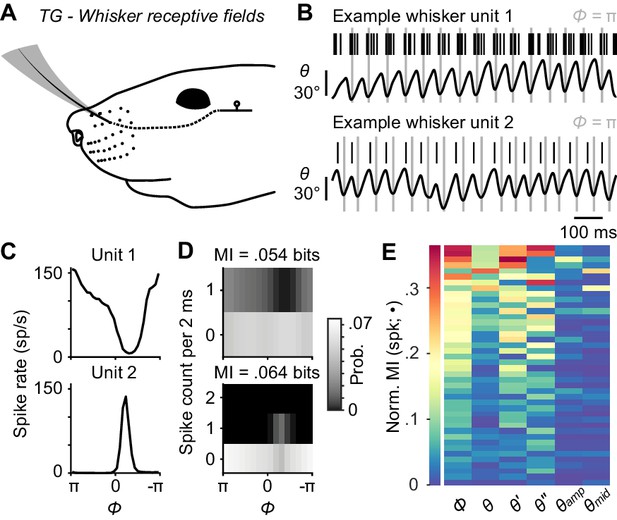
Self-motion responses from mechanoreceptors innervating whisker follicles.
(A) Schematic of a unit with whisker receptive field. (B) Spike times (black ticks) for two example whisker afferent units, each aligned with whisker position traces (gray lines: fully retracted positions). Unit 1 (top) responded during protracting phases. Unit 2 (bottom) responded during retracting phases. (C) Phase tuning curves (mean ± SEM; SEM here and in some subsequent panels narrower than line width) for unit 1 and unit 2. (D) Joint probability distributions for spike count and whisk phase (Φ), obtained from 2 ms periods corresponding to individual video frames, for unit 1 (top) and unit 2 (bottom). Mutual information (MI) between spike count and phase for each unit is shown at the top of each panel. Per 2 ms period, unit 1 spiked up to once and unit 2 up to twice. (E) Heatmap of normalized mutual information values for all whisking-sensitive whisker mechanoreceptors (n = 42), measured between spike count and each kinematic quantity (•): phase (Φ), position (θ), velocity (θ'), acceleration (θ''), amplitude (θamp), and midpoint (θmid). Units (rows) are sorted by increasing normalized MI averaged across the kinematic quantities. A subset of whisker afferent recordings was previously reported (Severson et al., 2017) and is reanalyzed here (see Supplementary file 1 for details). Data for panel E are given in Figure 2—source data 1.
-
Figure 2—source data 1
MATLAB R2016b ‘table’ data structure with Normalized MI values shown in Figure 2E.
- https://doi.org/10.7554/eLife.41535.005
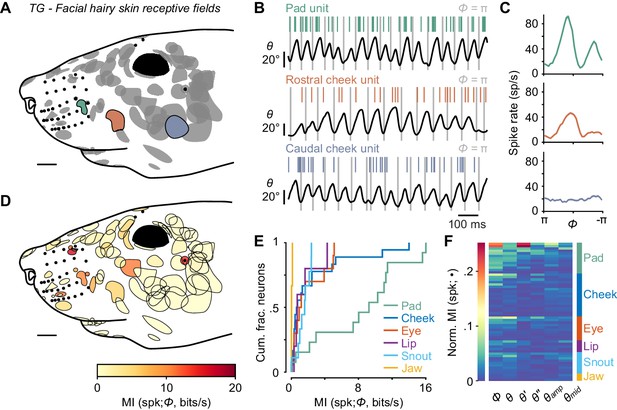
Self-motion responses from mechanoreceptors innervating facial hairy skin.
(A) Receptive fields on facial hairy skin (n = 85). Approximate size, shape, and location of receptive fields (gray) were compiled onto a template image of a mouse face (scale bar: 2 mm). Colored receptive fields show examples from whisker pad (green), rostral cheek (orange), and caudal cheek (blue). Whisker follicles and non-mystacial vibrissae (filled black dots) are included as fiducial marks. (B) Example one second traces showing spike times (colored ticks) aligned with whisker position, from recordings corresponding to the examples in (A). (C) Phase tuning curves (mean ± SEM) for example pad unit (green, top), rostral cheek unit (orange, middle), and caudal cheek unit (blue, bottom). Units with similar mean spike rates during whisking can differ in their phase modulation. (D) Mutual information rate between spike count and phase overlaid on outlines of receptive fields (scale bar: 2 mm). Many but not all receptive fields with large MI rates were located near whiskers. (E) Cumulative histograms of MI rate between spike count and phase for whisking-sensitive neurons with receptive fields in each region of the face, including whisker pad (n = 13), cheek (n = 18), eye (n = 10), lip (n = 5), snout (n = 9), and jaw (n = 3). (F) Heatmap of normalized MI values for all whisking-sensitive facial hairy skin units (n = 58), measured between spike count and each kinematic quantity (•, columns): phase (Φ), position (θ), velocity (θ'), acceleration (θ''), amplitude (θamp), and midpoint (θmid). Units (rows) are sorted by receptive field location (labeled at right) and within each face region by increasing normalized MI averaged across the kinematic quantities. Data for panels E, F are given in Figure 3—source data 1.
-
Figure 3—source data 1
MATLAB R2016b ‘table’ data structure with MI and Normalized MI values shown in Figure 3E,F.
- https://doi.org/10.7554/eLife.41535.008
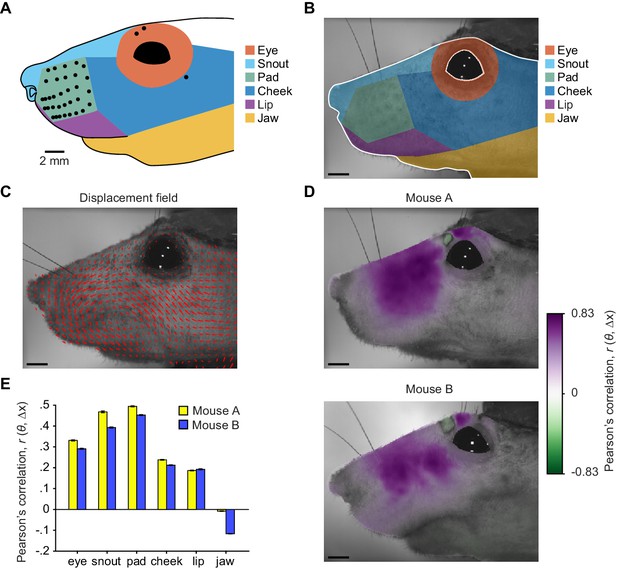
Widespread facial movement correlated with whisker motion.
(A) Six facial regions on template mouse face were used for categorizing afferent receptive fields. Zones were drawn based on fiducial marks, including the mystacial whiskers (filled circles). Locations of non-mystacial vibrissa follicles (open circles) are also indicated. Receptive fields were scaled to the template image. (B) ‘Fixed’ template image for image registration method of tracking facial motion. Overlaid are facial regions drawn using image fiducial marks. Pixels outside the edge of the face and within the eye, outlined in white, were effectively excluded from image registration by setting their values to zero. (C) Example ‘moving’ frame overlaid with a subset of the estimated x- and y-displacements, plotted as vectors (red arrows), that would align it to the fixed template image. (D) Template images from each of two mice overlaid with color scale showing Pearson’s correlation coefficients (r) between the time series of each pixel’s x-displacement (Δx) and the A1 whisker position (θ). (E) Pearson’s r values averaged (± SEM) across all pixels within each facial region, separately for two mice. (B–D) Scale bars: 2 mm.
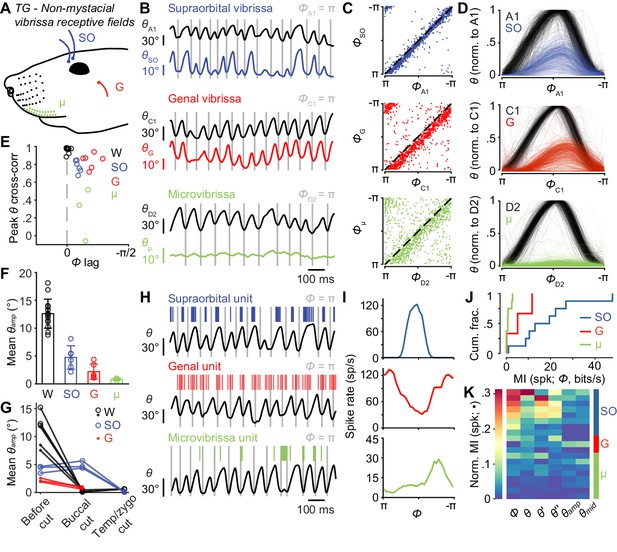
Non-mystacial vibrissae move in phase with whiskers, and their mechanoreceptors encode motion.
(A) Schematic of non-mystacial vibrissae included in these experiments: supraorbital (SO, blue), genal (G, red), and microvibrissae (μ, green) on the upper lip. (B) Correlated motion between whiskers and non-mystacial vibrissae. Example one second traces showing angular positions of a supraorbital vibrissa (top), genal vibrissa (middle) or microvibrissa (bottom), each with simultaneously tracked whisker angle. (C) Scatter plot of phase for whisker vs. non-mystacial vibrissae (n = 1000 random frames; top, SO; middle, G; bottom, μ; dashed lines: unity). (D) Example whisk cycles for whisker and non-mystacial vibrissae pairs, normalized based on whisker angle (n = 500 random whisks; top, A1 and SO; middle, C1 and G; bottom, D2 and μ). (E) Peak cross-correlation (Pearson’s r) and phase lag (open circles) between angular positions of a tracked whisker and either an adjacent whisker (W, n = 14 whisker pairs from 12 mice), a supraorbital vibrissa (SO, n = 6 recordings from six mice), a genal vibrissa (G, n = 6 recordings from six mice), or microvibrissa (μ, n = 3 recordings from three mice). (F) Mean whisk amplitude (± SD across recordings) for whisker, supraorbital, genal, or microvibrissa. (G) Result of unilateral facial nerve lesions on whisk amplitude for whiskers and non-mystacial vibrissae (n = 3 mice). Open circles: mean θamp for whiskers and supraorbital vibrissa during whisking (determined based on contralateral whiskers) before and after facial nerve cuts, performed sequentially at the buccal and then temporal/zygomatic branches. Closed circles: mean θamp obtained from separate videographic sessions for whiskers and genal vibrissae. (H) Example one second traces showing spike times from SO (top), G (middle), and μ units (bottom), each aligned with position of a tracked whisker (θ). (I) Phase tuning curves (mean ± SEM) for the example units in (H): top, SO; middle, G; bottom, μ. (J) Histograms of mutual information rate between spike count and phase for whisking-sensitive SO (n = 8), G (n = 3), and μ (n = 8) units. (K) Heatmap of normalized mutual information for all whisking-sensitive non-mystacial vibrissae units (n = 19). Conventions as in Figure 3F. Data for panels E, F are given in Figure 4—source data 1. Data for panel G are given in Figure 4—source data 2. Data for panels J, K are given in Figure 4—source data 3.
-
Figure 4—source data 1
MATLAB R2016b ‘table’ data structure with cross-correlation and mean amplitude values shown in Figure 4E,F.
- https://doi.org/10.7554/eLife.41535.012
-
Figure 4—source data 2
MATLAB R2016b ‘table’ data structure with mean amplitude values before and after nerve cuts shown in Figure 4G.
- https://doi.org/10.7554/eLife.41535.013
-
Figure 4—source data 3
MATLAB R2016b ‘table’ data structure with MI and Normalized MI values shown in Figure 4J,K.
- https://doi.org/10.7554/eLife.41535.014
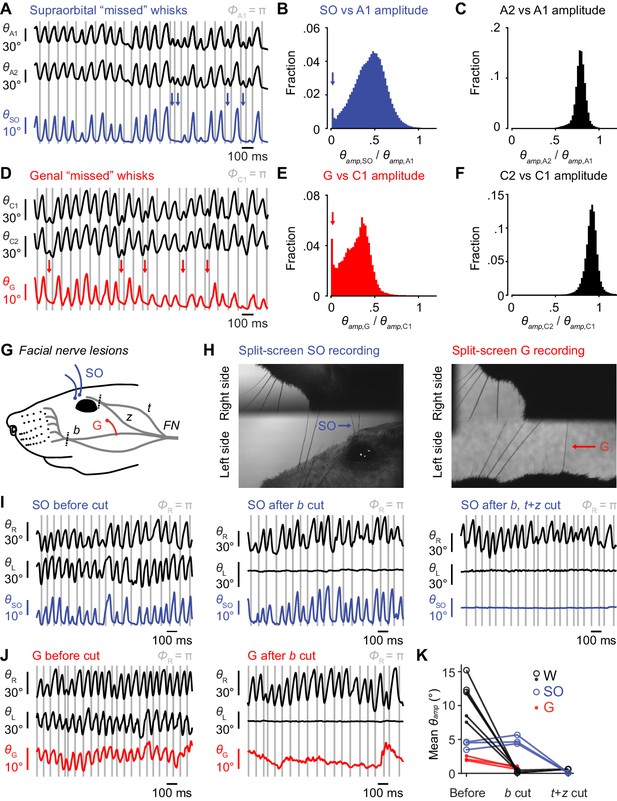
Non-mystacial vibrissae movement and its dependence on facial nerve innervation.
(A) Example traces of simultaneously recorded A1 whisker position (θA1, top black trace), A2 whisker position (θA2, bottom black trace) and supraorbital vibrissa position (θSO, blue; gray lines: fully retracted phase of A1). Whisks by A1 that were not matched by whisks of SO vibrissa (missed whisks) are marked by blue arrows. (B) Histogram of ratio of SO vibrissa amplitude (θamp,SO) over A1 whisker amplitude (θamp,A1) for whisking periods (330,085 frames) in the example recording in (A). Ratios above 1.2 are not shown for clarity (0.09% of frames). The peak with ratio near zero, indicated by blue arrow, indicates a substantial fraction of missed whisks in this example recording. (C) Same as (B) but for A2 vs A1 whiskers. There is no histogram peak indicative of missed whisks (0.16% of values are above the axis limit of 1.2). (D) Example traces of θC1 and θC2 (black traces) and θG (red). Missed whisks by the genal vibrissa are marked by red arrows. (E) Histogram of ratio of genal vibrissa amplitude (θamp,G) versus C1 whisker amplitude (θamp,C1) for whisking periods (96,429 frames) in the example recording in (D). Ratios above 1.2 not shown (0.36% of frames). Missed whisks are indicated by red arrow. (F) Same as (E) but for C2 vs C1 whiskers (0.75% of values above axis limit). (G) Diagram of facial nerve innervation of whisking muscles in mouse, adapted from Dörfl, 1985. Motor nerve lesions were performed by cutting the facial nerve (FN) sequentially at the buccolabialis branch (b), which innervates the mystacial pad whisking muscles, followed by cutting at the junction of the temporalis (t) and zygomatico-orbitalis (z) branches (cuts approximately at dashed lines). Whisker and non-mystacial vibrissa movement was measured before and after each cut. (H) Left, example frame capturing movement of left side SO vibrissae, left side A-row whiskers, and right side C-row whiskers. Right, example frame capturing movement of left side G vibrissa, left side C-row whiskers, and right side C-row whiskers. (I) Left, example 1.5 s trace of SO angle (θSO, blue), left whisker (θL, black, middle), and right whisker (θR, black, top) before nerve cut. Middle, same as left, but after left buccolabialis nerve cut. Left whisker movement was abolished, but SO movement persisted. Right, same as middle, but after left temporalis/zygomatico-orbitalis nerve cut. SO movement was abolished. (J) Left, same as (I), but for G angle (θG, red) before nerve cut. Right, same as left, but after buccolabialis cut. G movement was mostly abolished. (K) Summary of nerve cut results, reproduced for convenience from Figure 4G.
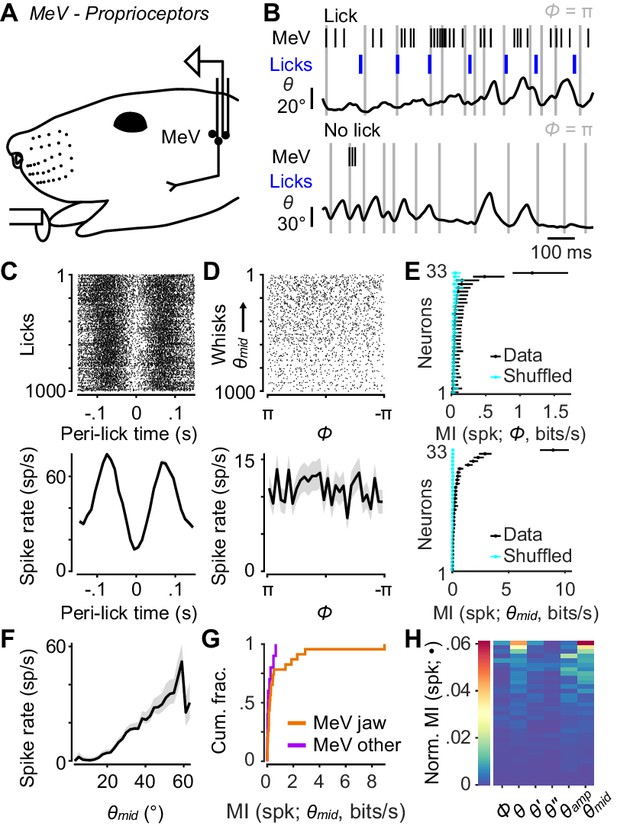
Responses of proprioceptors in the trigeminal mesencephalic nucleus (MeV) during licking and whisking.
(A) Schematic of MeV tetrode recordings during licking and whisking. (B) Example traces for an MeV unit showing spike times (black ticks) and lick times (blue ticks) aligned with position (θ) of a tracked whisker, for one second periods with (top) and without (bottom) licks. (C) Top, spike raster aligned to lick times (n = 1000 random licks) for example unit in (B). Bottom, peri-event time histogram (± SEM) aligned to lick times across all licks. (D) Top, spike raster aligned to whisk cycles (n = 1000 random whisks) for unit in (B). Whisks are ordered by mean θmid. Bottom, phase tuning curve for same unit (mean ± SEM) across all whisk cycles. (E) Top, MI rate between spike count and Φ for each unit (± 95% bootstrap CI). Cyan: results of same calculation but after randomly shuffling spike counts with respect to phase. Bottom, same as top but for MI rate between spike count and θmid. (F) Midpoint tuning curve (mean ± SEM) for unit in (B). (G) Cumulative histograms of MI rate between spike count and θmid for whisking-sensitive MeV units, separately for those that showed modulation by licking or passive jaw movement (orange, n = 23), and others recorded on the same tetrode (purple, n = 10). (H) Heatmap of normalized mutual information for all whisking-sensitive MeV units (n = 33). Conventions as in Figure 2E. Data for panel E are given in Figure 5—source data 1. Data for panel G are given in Figure 5—source data 2. Data for panel H are given in Figure 5—source data 3.
-
Figure 5—source data 1
MATLAB R2016b ‘table’ data structure with MI values and confidence intervals shown in Figure 5E.
- https://doi.org/10.7554/eLife.41535.021
-
Figure 5—source data 2
MATLAB R2016b ‘table’ data structure with MI values shown in Figure 5G.
- https://doi.org/10.7554/eLife.41535.022
-
Figure 5—source data 3
MATLAB R2016b ‘table’ data structure with Normalized MI values shown in Figure 5H.
- https://doi.org/10.7554/eLife.41535.023
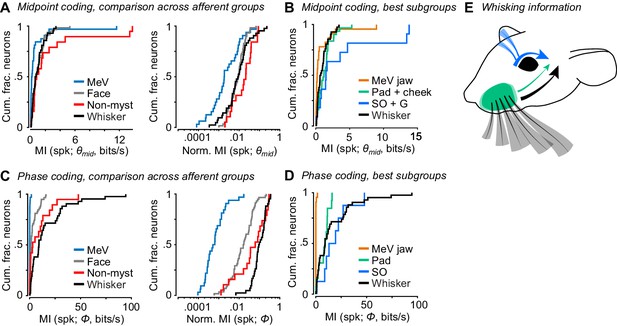
Coding of self-motion by diverse classes of facial mechanoreceptors.
(A) Left, summary cumulative histograms of MI rate between spike count and θmid for whisking-sensitive MeV (n = 33), face (n = 58), non-mystacial vibrissae (n = 19), and whisker units (n = 42). Right, summary histograms of normalized MI between spike count and θmid for same units. (B) Summary histograms of MI rate between spike count and θmid for the best encoding subgroups of whisking-sensitive units: putative jaw proprioceptors in MeV (n = 23), pad and cheek units (n = 31), supraorbital and genal units (n = 11), and whisker units (replotted from A). (C) Left, same as (A) but for MI rate between spike count and Φ. (D) Same as (B) but for the subgroups that best encoded Φ: putative proprioceptors in MeV (n = 23), pad units (n = 13), supraorbital units (n = 8), and whisker units (replotted from C). (E) Schematic depicting flow of information about whisking kinematics from various peripheral mechanoreceptors to the brain: whisker follicle (black), supraorbital vibrissa (blue), and whisker pad hairy skin (green) afferents. (B–D) Panels include data from Figures 2–5 plotted together for comparison. Data for panels A and C are given in Figure 6—source data 1. Data for panel B are given in Figure 6—source data 2. Data for panel D are given in Figure 6—source data 3.
-
Figure 6—source data 1
MATLAB R2016b ‘table’ data structure with MI and Normalized MI values shown in Figure 6A,C.
- https://doi.org/10.7554/eLife.41535.028
-
Figure 6—source data 2
MATLAB R2016b ‘table’ data structure with MI values shown in Figure 6B.
- https://doi.org/10.7554/eLife.41535.029
-
Figure 6—source data 3
MATLAB R2016b ‘table’ data structure with MI values shown in Figure 6D.
- https://doi.org/10.7554/eLife.41535.030
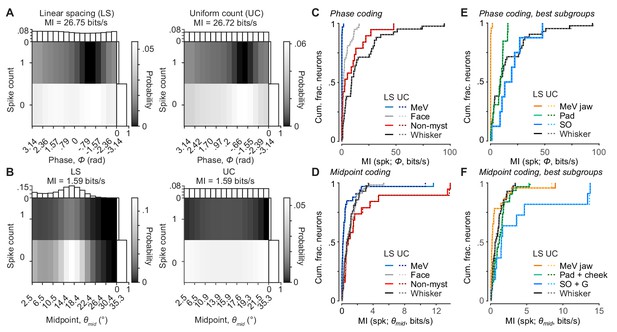
Alternative binning methods for mutual information calculation.
(A) Joint distribution for spike count and Φ comparing binning of Φ with linear spacing (LS, left, same calculation reported throughout paper) and uniform count (UC, right) for an example unit. Marginal probability distributions are plotted for Φ (top) and spike count (right). Note that the LS distribution of Φ is nearly uniform, except fewer bins are observed during retraction phases due to more rapid whisker retraction. MI rate values calculated using each method of binning are reported at top. (B) Same as (A), but for spike count and θmidcomparing LS (left) and UC (right) for an example whisker afferent. (C) Cumulative histograms of MI rate between spike count and Φ, calculated using LS (solid lines) or UC (dotted lines) for the different afferent groups. (D) Same as (C) but for MI rate between spike count and θmid. (E) Same as (C) but for best subgroups. (F) Same as (D) but for best subgroups. (C-F) LS values are repeated from Figure 6 to allow comparison with UC values. For Φ and θmid, MI values calculated with LS or UC binning are almost identical.
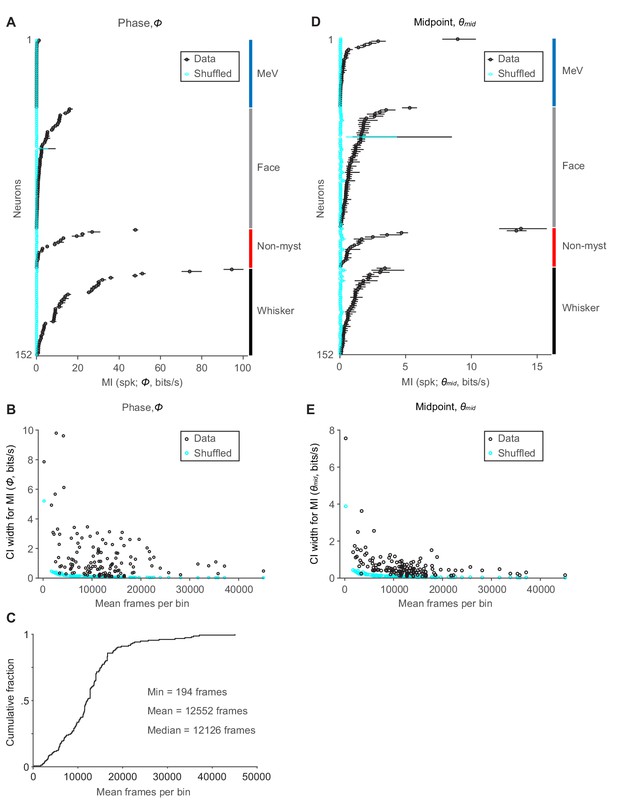
Confidence intervals for mutual information and dependence on sample size.
(A) MI rate (± 95% bootstrap CI) between spike count and Φ for all whisking-sensitive units (n = 152). Cyan: results of same calculation but after randomly shuffling spike counts with respect to Φ. Neurons are sorted by group label (right) and MI rate. (B) Mean number of frames in each bin (n = 16 bins) for marginal distribution of Φ, plotted against the width of the 95% CI for MI rate for Φ. Cyan: results of same calculation after randomly shuffling spike times with respect to Φ. (C) Cumulative histogram showing mean number of frames per bin for all whisking-sensitive units (n = 152). (D) Same as (A), but with MI rate calculated between spike count and θmid. (E) Same as (B), but for θmid. (A,D) MeV data are repeated from Figure 5E.
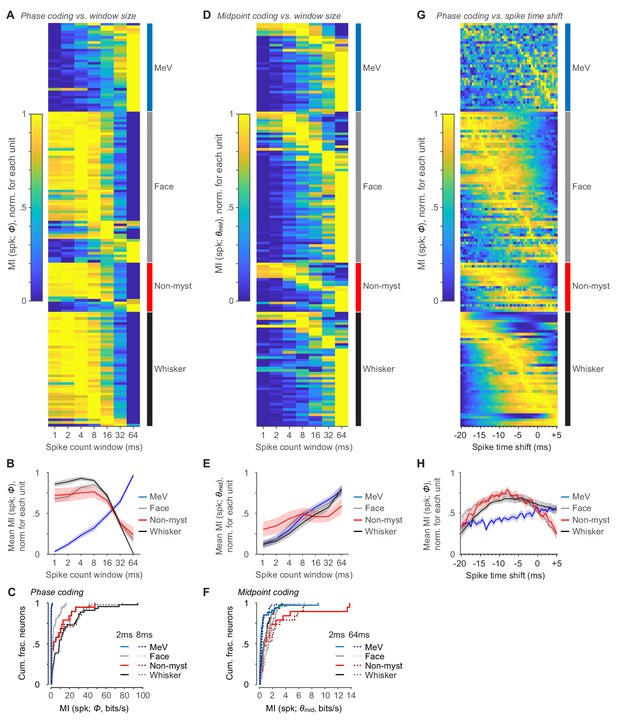
Mutual information calculated using varying windows for spike count.
(A) MI values for spike count and Φ calculated using different spike count window durations, ranging from 1 to 64 ms for all whisking-sensitive units (rows). Values are normalized from min to max MI across time windows for each unit. Rows are sorted by afferent group (labels on right) and within group by index of max. (B) Mean MI values (± SEM) for spike count and Φ, for each afferent group and spike count window, after normalizing each unit as in (A). (C) Summary cumulative histograms of MI rate between spike count and Φ for all whisking-sensitive MeV, face, non-mystacial vibrissae, and whisker units, for 2 ms (solid lines, taken from Figure 6C) and 8 ms (dashed lines) windows. (D–F) Same as (A-C) but for θmid (2 ms window data taken from Figure 6A). (G) MI rates between spike count and Φ, calculated for varying spike time shifts relative to Φ, in 0.5 ms increments for all whisking-sensitive units (rows). Conventions as in (A). (H) Same as (B) but for spike time shifts.
Videos
Whisker mechanoreceptor activity during whisking.
Raw video (slowed 20-fold) showing mouse whiskers during whisking. The tracked γ whisker is indicated with the black overlay, and its angular position (θ) is shown in the trace at bottom. Spike times from a simultaneously recorded afferent responsive to the C2 whisker (third from right) are indicated as black ticks above the θ trace. Audio is the playback of the spike waveform at the corresponding spike time.
Whisking is accompanied by widespread motion of the face.
Raw video (slowed 20-fold) showing side-view of a mouse face during whisking. All whiskers except the A1 and A2 whiskers and much of the facial fur have been trimmed, for a better view of the skin. The skin moves in complex patterns during whisking.
Supraorbital vibrissa movement during whisking.
Raw video (slowed 20-fold) showing mouse whiskers (A1 and A2) and supraorbital (SO) vibrissae during whisking. The A1 whisker (black overlay) and caudal SO vibrissa (blue overlay) are tracked. The angular positions of the whisker (θA1, black trace) and SO vibrissae (θSO, blue trace) are shown at bottom. The SO vibrissae whisk in phase with the whiskers.
Genal vibrissa movement during whisking.
Raw video (slowed 20-fold) showing mouse whiskers (C-row) and genal vibrissa moving in free air. The C1 whisker (black overlay) and genal vibrissa (red overlay) are tracked. The angular positions of the whisker (θC1, black trace) and genal vibrissa (θG, red trace) are shown at bottom. The genal vibrissa whisks in phase with the whiskers.
Microvibrissa movement during whisking.
Raw video (slowed 20-fold) showing mouse whiskers (D2 and D3) and microvibrissae during whisking. The D3 whisker (black overlay) and one of the larger, more dorsal and caudal microvibrissae (green overlay) are tracked. The angular positions of the whisker (θD3, black trace) and microvibrissa (θµ, green trace) are shown at bottom. The microvibrissa does not whisk.
Supraorbital vibrissa movement persists after buccal but not temporal/zygomatic nerve cut.
Raw video (slowed 20-fold) showing split-screen view of mouse whiskers (A1 and A2) and supraorbital (SO) vibrissae on the left side, and whiskers (C-row) on the right side, during whisking. The left A1 whisker (black overlay), left caudal SO vibrissa (blue overlay), and right γ or C1 whisker (black overlay) are tracked. The angular positions of the whiskers (θL and θR, black traces) and SO vibrissae (θSO, blue trace) are shown at bottom. The first episode shows that the SO vibrissae whisk in phase with the ipsilateral whiskers prior to nerve cut. The second episode occurs after cut of the left buccal branch of the facial nerve. Whisking of the left whiskers is abolished, but the SO vibrissae continue to whisk. The third episode occurs after subsequent cut of the left facial motor nerve at the junction of the temporal and zygomatic branches (Figure 4—figure supplement 1G). SO vibrissae whisking is abolished.
Genal vibrissa movement reduced after buccal nerve cut.
Raw video (slowed 20-fold) showing split-screen view of mouse whiskers (C-row) and genal vibrissa on the left side, and whiskers (C-row) on the right side during whisking. The left γ whisker (black overlay), left genal vibrissa (red overlay), and right γ whisker are tracked. The angular positions of the whiskers (θL and θR, black traces) and genal vibrissa (θG, red trace) are shown at bottom. The first episode shows that the genal vibrissa moves in phase with the ipsilateral whiskers prior to nerve cut. The second episode occurs after cut of the left buccal branch of the facial nerve. Whisking of the left whiskers is abolished, and the genal vibrissa motion is greatly reduced. Whisking of the supraorbital vibrissae can be seen in the background.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers |
|---|---|---|---|
| Software, algorithm | WaveSurfer | HHMI Janelia Research Campus | http://wavesurfer.janelia.org/ |
| Software, algorithm | StreamPix 5 or 7 | Norpix | RRID:SCR_015773 |
| Software, algorithm | Janelia Whisker Tracker | Clack et al., 2012 | N/A |
| Software, algorithm | MATLAB 2014a, 2016b, or 2018a | MathWorks | RRID: SCR_001622 |
| Software, algorithm | Adobe Illustrator | Adobe | RRID: SCR_010279 |
| Other | High speed CMOS camera | PhotonFocus | DR1-D1312-200-G2-8 |
| Other | Telecentric lens | Edmund Optics | Cat #: 55–349 |
| Other | Tungsten microelectrode 2 MΩ | World Precision Instruments | Cat #: TM33A20 |
| Other | Tetrode microdrive (custom-built) | Cohen et al., 2012 | N/A |
| Other | Fine 0.3 mm tip water-based marker | Platinum Art Supplies | Cat #: B01FWIE032 |
| Other | Suture thread 8/0 | Fine Science Tools | Cat #: 12051–08 |
Additional files
-
Supplementary file 1
Meta-data and mouse information.
Excel spreadsheet tabulating mouse identifier, genotype, sex, and age, as well as meta-data and figure appearances of data collected from each mouse.
- https://doi.org/10.7554/eLife.41535.031
-
Transparent reporting form
- https://doi.org/10.7554/eLife.41535.032





