A multiphase theory for spreading microbial swarms and films
Figures
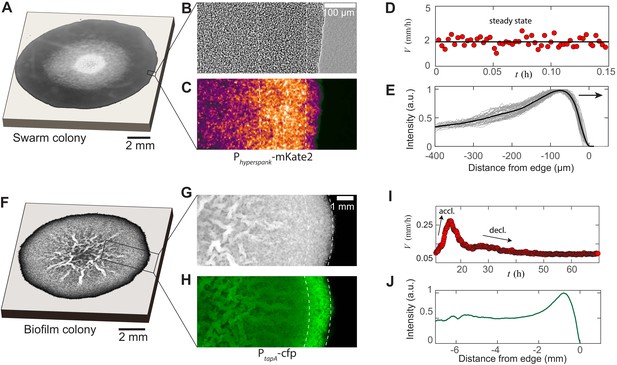
Experimental features of microbial swarms and biofilms.
(A) Snapshot of a Bacillus subtilis swarm expanding on a 0.5 wt% LB/agar gel. (B,C) Brightfield and fluorescent zoom images of the leading swarm edge of a MTC822 strain containing the fluorescent Phyperspank-mKate2 reporter that is expressed constitutively. The dashed white lines indicates the extent of the multi-cellular region. (D) Expansion velocity of the swarm measured at intervals of 10 s over a 10 min period. The solid line corresponds to a mean steady-state velocity of = 2 mm/h. (E) Mean intensity traces of the constitutive fluorophore (mKate2) representing bacterial densities profiles plotted in the moving steady-state frame. The dark grey traces represent separate density profile measurements taken every 10 s in the advancing swarm. The solid line represents the density profile averaged over a period of 30 min. (F) A Bacillus subtilis biofilm colony developing on a 1.5 wt% MSgg/agar gel. (G,H) Brightfield and fluorescent zoom images of the biofilm colony formed by a MTC832 strain harboring the PtapA-cfp fluorescent reporter expressed in cells synthesizing the extracellular polymeric matrix (EPS). The dashed white lines indicates the extent of an active peripheral zone signifying localized EPS production. (I) Expansion velocity of the biofilm colony measured at intervals of 10 mins over a 72 hr period. The peak expansion velocity of = 0.22 mm/h occurs at ∼ 18 h after inoculation. (J) Azimuthally averaged matrix reporter activity (cfp) as a function of spatial distance within the biofilm.
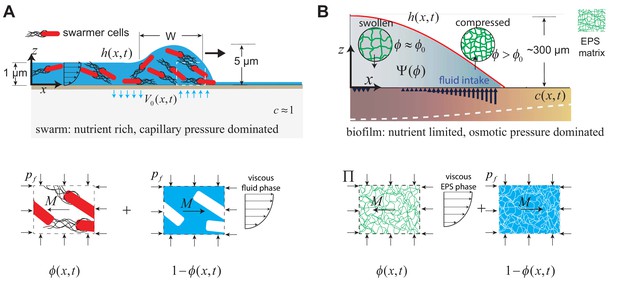
Geometry and variables governing colony expansion in (A) microbial swarms, and (B) bacterial biofilms, respectively.
In both cases, the total thickness of the microbial colony is , the averaged nutrient concentration field is , the volume fraction of the active phase is , the volume fraction of the fluid phase is , and the fluid influx across the agar/colony interface is denoted by . As shown on the bottom panel, the active phase constitutes swarmer cells in the microbial swarm, and secreted EPS polymer matrix in the biofilm. The pressure in the fluid phase is and the effective averaged pressure in the active phase is . In the swarm cell phase, , while the EPS phase effective pressure is , where is the swelling pressure and is related to Flory-Huggins osmotic polymer stress (see Equation A28). The momentum exchange between the two phases is denoted by , which includes the sum of an interfacial drag term and an interphase term as detailed in Equation (A11) in the Appendix.
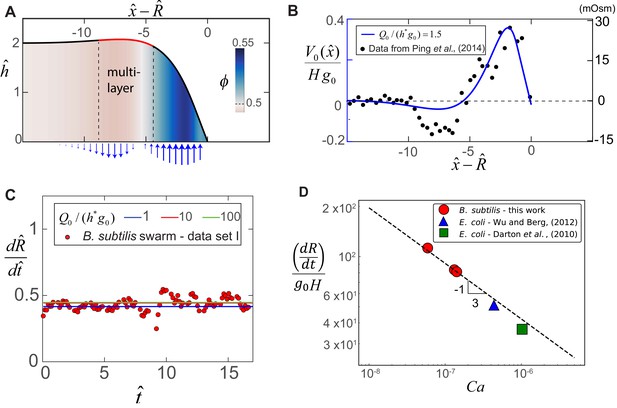
Steady-state morphology and fluid transport in a bacterial swarm obtained by solving (8) for = 0.2, = 1.5 and = 0.5.
(A) Plot of the steady-state thickness against the scaled distance , where and is the radius. The solid red line indicates a region of increased thickness, and the colormap quantifies variations in , the local volume fraction. (B) Plotted on the left-axis is the numerical steady-state fluid uptake profile within the swarm (solid line) calculated from Equation (4). On the right axis are experimental measurements of the steady-state osmotic pressure within an expanding E. coli swarm (filled circles), reproduced from Ping et al. (2014), with the baseline reference value shifted to zero, and with distances normalized by = 50 μm. (C) Predicted steady-state radial colony expansion speeds within the swarm for values of , 10 and 100 respectively. The data points are expansion speeds in B. subtilis swarms measured over 20 min, and scaled using = 1.3 μm and 0.013 s-1. (D) Comparison between the swarm expansion velocities measured for five separate colonies (see Appendix 2) and the estimated capillary number. For each experiment, was obtained by fitting the steady state solution of Equations (7) and (8) to the swarm velocity. The dashed line corresponds to the predicted scaling law in Equation (5).
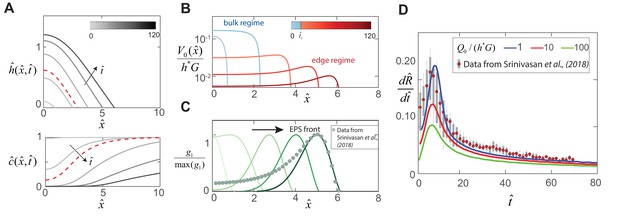
Dynamics of EPS production and biofilm expansion obtained by solving (12 - 14) with , , , and .
(A) On the top are thickness profiles of an expanding biofilm colony, at time intervals of = 1, 5, 20, 50 and 90. The nutrient field at corresponding time intervals is plotted at the bottom. The dotted red line indicates profiles at = 8, the transition point between the bulk and edge expansion regimes. (B) Variation of the vertical fluid uptake profile within the swarm calculated from Equation (9). The light blue lines correspond to the bulk growth regime for = 1, 5 while the red lines correspond to = 20, 50 and 90 in the edge growth regime. (C) Plots of normalized EPS production activity within the biofilm, where is evaluated using the expression in Table 1. The data points are spatial measurements of tapA gene activity in B. subtilis biofilms reproduced from Srinivasan et al. (2018), with distances scaled by = 550 μm. (D) Solid lines indicate transient colony edge expansion velocities for and respectively, and with other parameter values fixed as listed above. The experimental data is reproduced from Srinivasan et al. (2018) and indicates median expansion velocities (filled circles), the 25th to 75th percentile velocities (filled box), and extreme values (vertical lines), where the data has been scaled by m and min.
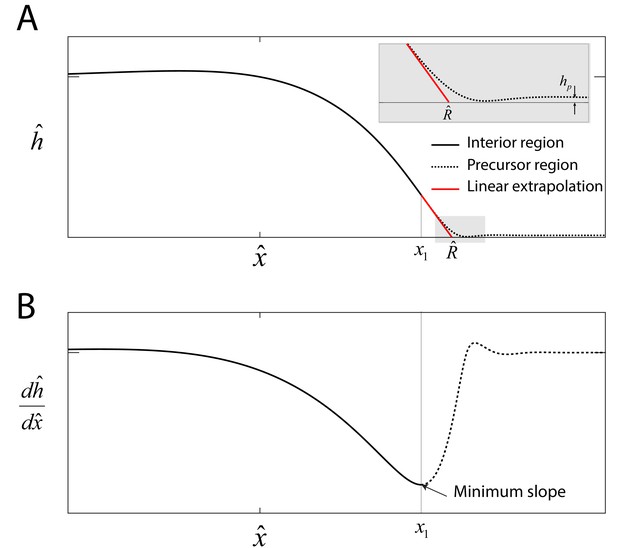
Precursor film.
(A) Numerical solution to Equations (5)-(6) in the main text that represents the swarm profile . The solid black line indicates the swarm profile in the interior region domain . The dashed line beyond denotes the precursor-film region. The solid red line represents a linear extrapolation of the swarm profile in the region , where is the swarm radius. Inset: Magnified view of the transition region, where is the precursor film of thickness. (B) The onset of the precursor film is defined at the point of the numerical profile where the slope is a minimum.
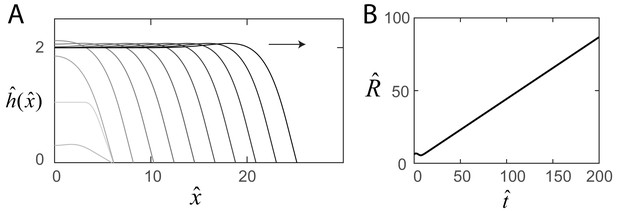
Steady-state swarm solutions.
(A) The evolution of the numerical swarm thickness plotted in the laboratory frame at fixed time intervals. (B) Plot of the swarm radius as a function of time indicating steady-state solutions.
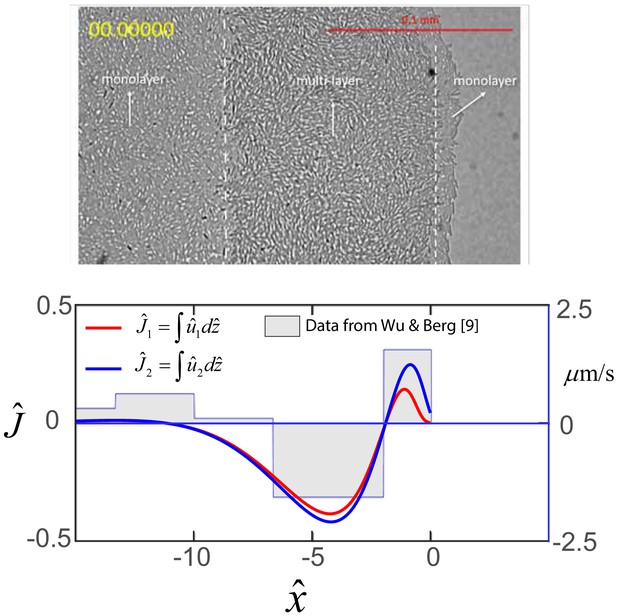
Top: Image of edge of swarm colony.
B. subtilis swarm colony showing the interior monolayer region, the multi-layer front, and the monolayer at the very edge of the colony. Bottom: Horizontal flows in swarms. Steady-state profile of the dimensionless net horizontal velocity in the swarmer cell phase (red) and fluid phase (blue). Expression for and are obtained from Equations (A18) and (A19) as and , where as discussed in the main text. The step function represents experimental flow speeds as a function of the distance form the swarm edge as measured by Wu and Berg (2012), and where the horizontal distance has been scaled by m.
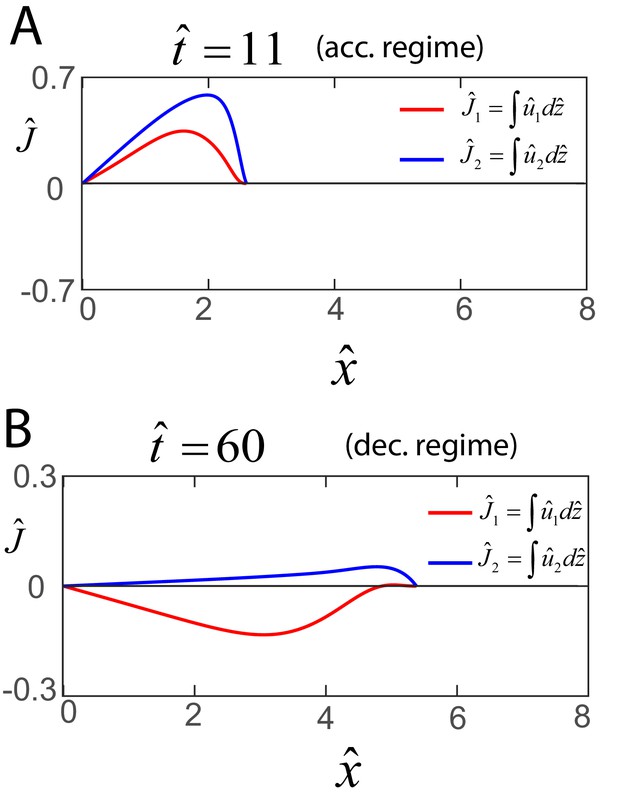
Early and late flows in biofilms.
Profile of the dimensionless net horizontal velocity in the EPS phase (red) and fluid phase (blue). Expression for and are obtained from Equations (A30) and (A31) as and , where and as discussed in the main text.
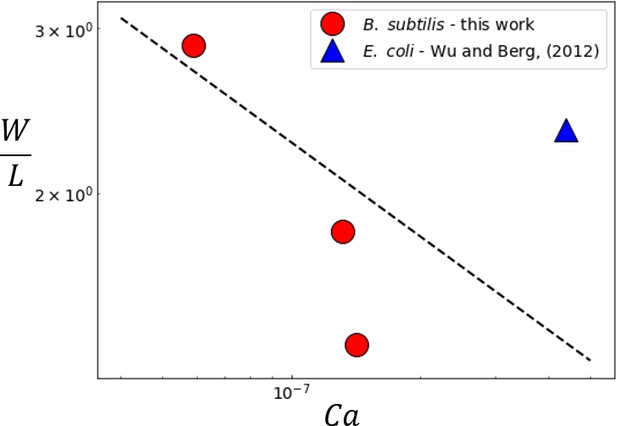
Variation of the experimentally measured multilayer swarm width with .
For B. subtilis swarm experiments, the value of was determined by considering the width of the region where the mean constitutive fluorophore intensity (See Figure 1E). The multilater width in the E. coli swarm was reported as 154 μm ± 27 μm by Wu and Berg (2012). The dashed line corresponds to the predicted scaling from Equation (6).
Tables
Definitions of fluxes for swarms and films
Definitions of the active phase horizontal flux , the fluid phase horizontal flux , active phase growth term , osmotic influx term , and nutrient consumption term for bacterial swarms and films in the generalized thin film evolution equations described by Equations (1–3). Here, is the biofilm viscosity, is the fluid viscosity, is the fluid phase pressure, is the effective pressure in the active phase, is effective swarmer cell growth rate, is the EPS production rate, is the nutrient consumption rate per unit concentration, is the nutrient half-velocity constant and is the thickness of the substrate. For swarms, the active phase corresponds to the swarmer cell phase, and for biofilms, the active phase is the EPS polymer matrix.
| Variables | Swarms | Biofilms | |
|---|---|---|---|
| Flux (Phase I) | |||
| Flux (Phase II) | |||
| Osmotic influx | |||
| Growth term | |||
| Nutrient uptake | - |
List of the symbols, descriptions, and numerical value for each of the parameters used.
https://doi.org/10.7554/eLife.42697.013| Variable | Description | Numerical value |
|---|---|---|
| vertical length scale | swarms - 0.5 μm biofilms - 400 μm | |
| horizontal length scale | swarms - 100 μm biofilms - 1100 μm | |
| horizontal velocity scale | swarms - 1.3 μm/s biofilms - 0.5 μm/s | |
| equilibrium volume fraction of active phase | swarms - 0.5 biofilms - 0.04 | |
| vertical fluid velocity scale | swarms - 10–2 μm/s biofilms - 0.04 | |
| effective swarm cell growth rate | 0.005 — 0.2 s-1 | |
| EPS production rate | 1/40 min-1 | |
| EPS matrix viscosity | 105 Pa.s | |
| fluid viscosity | 10–3 Pa.s | |
| friction coefficient per unit cell volume | 10–2 pN/(μm s-1) | |
| fluid surface tension | 10-2 N/m | |
| osmotic pressure scale | 2100 Pa | |
| Dimensionless parameters: Bacterial swarm | ||
| 0.2 | ||
| 0.1 — 4.3 | ||
| Dimensionless parameters: Bacterial biofilm | ||
| 6.7 | ||
| 0.01 | ||
| 0.02 | ||
| 1 | ||
| 2 | ||
Summary of the comparision between the experimental data and model.
The experimentally measured quantities are the colony expansion speed and multilayer region thickness . The value of is determined by fitting Equations (7)-(8) to the expansion velocity, leading to estimates of the effective growth rate , the horizontal length scale and the capillary number .
| No | Reference | μms-1 (exp) | μm (exp) | (model) | s-1 (model) | μm (model) | × 10-7 (model) |
|---|---|---|---|---|---|---|---|
| 1 | B. subtilis this work - (data set I) | 0.56 | 178 | 1.49 | 0.013 | 98 | 1.32 |
| 2 | B. subtilis this work - (data set II) | 0.26 | 368 | 4.33 | 0.005 | 128 | 0.59 |
| 3 | B. subtilis this work - (data set III) | 0.60 | 132 | 1.35 | 0.015 | 96 | 1.42 |
| 4 | E. coli Wu and Berg, 2012 | 1.7 | 154 | 0.30 | 0.07 | 66 | 4.38 |
| 5 | E. coli Darnton et al., 2010 | 3.8 | - |
Additional files
-
Source code 1
COMSOL file that implements the numerical solutions to Equations (7)-(8) governing bacterial swarm expansion.
- https://doi.org/10.7554/eLife.42697.008
-
Source code 2
COMSOL file that implements the numerical solutions to Equations (12)-(14) governing bacterial biofilm expansion.
- https://doi.org/10.7554/eLife.42697.009
-
Transparent reporting form
- https://doi.org/10.7554/eLife.42697.010





